Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation and Catheterization
2.2. The Study Protocol
2.3. Induction of Acute Pulmonary Embolism
2.4. Adminstration of Vasodilator
2.5. Measurement of the Pulmonary Arterial Wave Reflection
2.6. Right Heart Functional Evaluation by Echocardiography
2.7. Statistical Analysis
3. Results
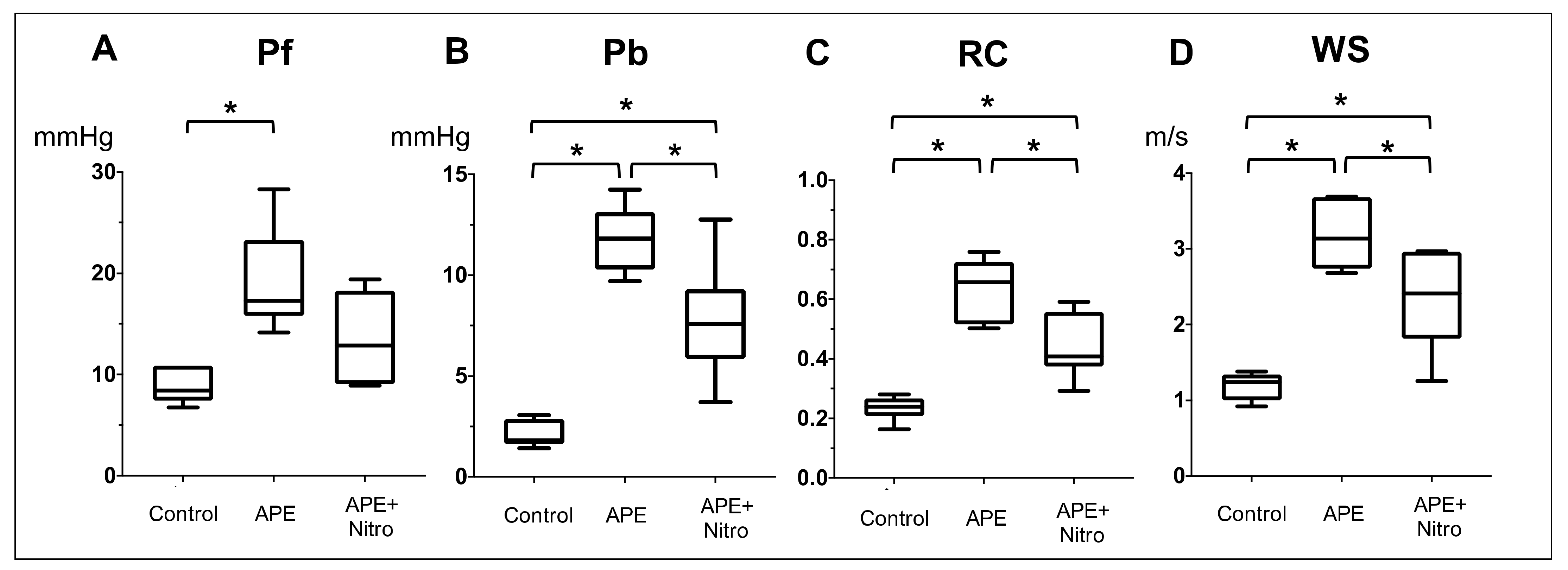
3.1. Effect of APE on Hemodynamics, Echocardiographic and PAWR Variables
3.2. Changes in Hemodynamics, Echocardiographic and PAWR Variables after Nitroprusside Administration
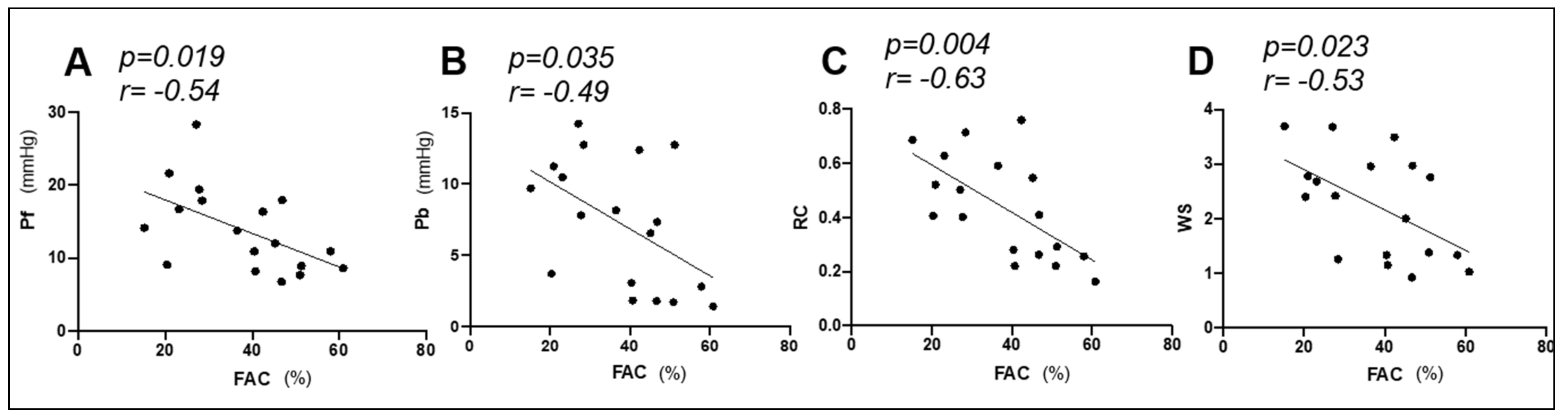
3.3. Correlation between PAWR Variables and Hemodynamic and Echocardiographic Right Heart Functional Parameters
3.4. Effect of PAWR Variables on Hemodynamic and Echocardiographic Right Heart Functional Parameters
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and Diagnosis of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Sanchez, M.A.G.; Kumar, R.K.; Landzberg, M.; Machado, R.F.; et al. Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part One. Am. J. Respir. Crit. Care Med. 1994, 150, 833–852. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Kim, N.H.S.; Rubin, L.J. The right ventricle in pulmonary hypertension. Coron. Artery Dis. 2005, 16, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.M.; Kaminski, K.A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2015, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.S.; Lee, P.-F.; Lanning, C.J.; Ivy, D.D.; Kirby, K.S.; Claussen, L.R.; Chan, K.C.; Shandas, R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am. Heart J. 2008, 155, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragu, R.; Rispler, S.; Habib, M.; Sholy, H.; Hammerman, H.; Galie, N.; Aronson, D. Pulmonary arterial capacitance in patients with heart failure and reactive pulmonary hypertension. Eur. J. Heart Fail. 2015, 17, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Nishimura, R.A.; Sorajja, P.; Cha, S.; McGoon, M.D. Relationship of Pulmonary Arterial Capacitance and Mortality in Idiopathic Pulmonary Arterial Hypertension. J. Am. Coll. Cardiol. 2006, 47, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ohtani, T.; Kioka, H.; Onishi, T.; Tsukamoto, Y.; Nakamoto, K.; Taniguchi, T.; Nakatani, S.; Hirayama, A.; Sakata, Y. Clinical Significance of Pulmonary Arterial Capacitance Calculated by Echocardiography in Patients With Advanced Heart Failure. Circ. J. 2017, 81, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Thenappan, T.; Prins, K.W.; Pritzker, M.R.; Scandurra, J.; Volmers, K.; Weir, E.K. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann. Am. Thorac. Soc. 2016, 13, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champion, H.C.; Michelakis, E.D.; Hassoun, P.M. Comprehensive Invasive and Noninvasive Approach to the Right Ventricle–Pulmonary Circulation Unit. Circulation 2009, 120, 992–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Manisty, C.; Parker, K.H.; Simonsen, U.; Nielsen-Kudsk, J.E.; Mellemkjær, S.; Connolly, S.; Lim, P.B.; Whinnett, Z.I.; Malik, I.S.; et al. Wave Intensity Analysis Provides Novel Insights Into Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2017, 6, e006679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Manisty, C.; Simonsen, U.; Howard, L.S.; Parker, K.H.; Hughes, A.D. Pulmonary artery wave propagation and reservoir function in conscious man: Impact of pulmonary vascular disease, respiration and dynamic stress tests. J. Physiol. 2017, 595, 6463–6476. [Google Scholar] [CrossRef] [PubMed]
- Ghiadoni, L.; Bruno, R.M.; Stea, F.; Virdis, A.; Taddei, S. Central blood pressure, arterial stiffness, and wave reflection: New targets of treatment in essential hypertension. Curr. Hypertens. Rep. 2009, 11, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.H.; Wang, J.-J.; Dobson, G.M.; Parker, K.H.; Tyberg, J.V. Negative wave reflections in pulmonary arteries. Am. J. Physiol. Circ. Physiol. 2001, 281, H895–H902. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Kobayashi, H.; Sugawara, M.; Tomita, T.; Ohara, K.; Yoshimura, H. Helium inhalation enhances vasodilator effect of inhaled nitric oxide on pulmonary vessels in hypoxic dogs. Am. J. Physiol. Circ. Physiol. 2001, 280, H1875–H1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, A.D.; Parker, K.H. Forward and backward waves in the arterial system: Impedance or wave intensity analysis? Med. Biol. Eng. Comput. 2009, 47, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Castelain, V.; Hervé, P.; Lecarpentier, Y.; Duroux, P.; Simonneau, G.; Chemla, D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J. Am. Coll. Cardiol. 2001, 37, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Dias-Junior, C.A.; Souza-Costa, D.C.; Zerbini, T.; Da Rocha, J.B.T.; Gerlach, R.F.; Tanus-Santos, J.E.; Da Rocha, J.B.T. The Effect of Sildenafil on Pulmonary Embolism-Induced Oxidative Stress and Pulmonary Hypertension. Anesthesia Analg. 2005, 101, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Light, R.B. Effect of sodium nitroprusside and diethylcarbamazine on hypoxic pulmonary vasoconstriction and regional distribution of pulmonary blood flow in experimental pneumonia. Am. J. Respir. Crit. Care Med. 1996, 153, 325–330. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, G.E.; Barst, R.J.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M.; Fishman, A.P.; Goldring, R.M.; Groves, B.M.; Kernis, J.T.; et al. Survival in Patients with Primary Pulmonary Hypertension. Ann. Intern. Med. 1991, 115, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.H. An introduction to wave intensity analysis. Med. Biol. Eng. Comput. 2009, 47, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Khir, A.; O’Brien, A.; Gibbs, J.; Parker, K. Determination of wave speed and wave separation in the arteries. J. Biomech. 2001, 34, 1145–1155. [Google Scholar] [CrossRef]
- Poser, H.; Berlanda, M.; Monacolli, M.; Contiero, B.; Coltro, A.; Guglielmini, C. Tricuspid annular plane systolic excursion in dogs with myxomatous mitral valve disease with and without pulmonary hypertension. J. Vet. Cardiol. 2017, 19, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Vezzosi, T.; Domenech, O.; Costa, G.; Marchesotti, F.; Venco, L.; Zini, E.; Del Palacio, M.J.F.; Tognetti, R. Echocardiographic evaluation of the right ventricular dimension and systolic function in dogs with pulmonary hypertension. J. Vet. Intern. Med. 2018, 32, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Serres, F.; Chetboul, V.; Gouni, V.; Tissier, R.; Sampedrano, C.C.; Pouchelon, J.-L. Diagnostic Value of Echo-Doppler and Tissue Doppler Imaging in Dogs with Pulmonary Arterial Hypertension. J. Vet. Intern. Med. 2007, 21, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Boon, J.A. Veterinary Echocardiography; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Su, J.; Hughes, A.D.; Simonsen, U.; Howard, L.S. Nitric Oxide Attenuates Arterial Pulse Wave Reflection in a Vasodilator Responding Pulmonary Arterial Hypertension Patient. Circ. Cardiovasc. Interv. 2018, 11, e006242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamanoglu, M.; McGoon, M.; Frantz, R.P.; Benza, R.L.; Bourge, R.C.; Barst, R.J.; Kjellström, B.; Bennett, T.D. Right Ventricular Pressure Waveform and Wave Reflection Analysis in Patients With Pulmonary Arterial Hypertension. Chest 2007, 132, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Quail, M.A.; Knight, D.S.; Steeden, J.A.; Taelman, L.; Moledina, S.; Taylor, A.M.; Segers, P.; Coghlan, G.J.; Muthurangu, V. Noninvasive pulmonary artery wave intensity analysis in pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 2015, 308, H1603–H1611. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Huez, S. Reflections on wave reflections in chronic thromboembolic pulmonary hypertension. Eur. Heart J. 2007, 28, 785–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, Y.; Nakanishi, N.; Hayashi, T.; Nagaya, N.; Sakamaki, F.; Satoh, N.; Ohya, H.; Kyotani, S. Pulmonary artery reflection for differentially diagnosing primary pulmonary hypertension and chronic pulmonary thromboembolism. J. Am. Coll. Cardiol. 2001, 38, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Tedford, R.J. Determinants of Right Ventricular Afterload (2013 Grover Conference Series). Pulm. Circ. 2014, 4, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thenappan, T.; Shah, S.J.; Rich, S.; Gomberg-Maitland, M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur. Respir. J. 2007, 30, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Hughes, A.D.; Simonsen, U.; Nielsen-Kudsk, J.E.; Parker, K.H.; Howard, L.S.; Mellemkjaer, S. Impact of pulmonary endarterectomy on pulmonary arterial wave propagation and reservoir function. Am. J. Physiol. Circ. Physiol. 2019, 317, H505–H516. [Google Scholar] [CrossRef] [PubMed]
- Dias-Junior, C.A.; Tanus-Santos, J.E. Hemodynamic effects of sildenafil interaction with a nitric oxide donor compound in a dog model of acute pulmonary embolism. Life Sci. 2006, 79, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Chun, Y.-G.; Lee, I.-C.; Tuder, R.M.; Hong, S.-B.; Shim, T.-S.; Lim, C.-M.; Koh, Y.; Kim, W.-S.; Kim, D.-S.; et al. Pathogenic Role of Endothelin 1 in Hemodynamic Dysfunction in Experimental Acute Pulmonary Thromboembolism. Am. J. Respir. Crit. Care Med. 2001, 164, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Shelub, I.; Van Grondelle, A.; McCullough, R.; Hofmeister, S.; Reeves, J.T. A model of embolic chronic pulmonary hypertension in the dog. J. Appl. Physiol. 1984, 56, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Andersen, A.; Lyhne, M.D.; Arcanjo, D.D.R.; Kjaergaard, B.; Simonsen, U.; Nielsen-Kudsk, J.E. Terlipressin Increases Systemic and Lowers Pulmonary Arterial Pressure in Experimental Acute Pulmonary Embolism. Crit. Care Med. 2020, 48, e308–e315. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, M.; Westerhof, B.E.; Ruigrok, D.; Braams, N.J.; Groeneveldt, J.A.; Bayoumy, A.A.; Marcus, J.T.; Meijboom, L.J.; De Man, F.S.; Westerhof, N.; et al. Early return of reflected waves increases right ventricular wall stress in chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 2020, 319, H1438–H1450. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An Evaluation of Long-term Survival From Time of Diagnosis in Pulmonary Arterial Hypertension From the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Liu, S.F. Regulation of pulmonary vascular tone. Pharmacol. Rev. 1995, 47, 87–131. [Google Scholar] [PubMed]
- Lau, E.M.; Manes, A.; Celermajer, D.S.; Galiè, N. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: Time to move forward. Eur. Heart J. 2011, 32, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Shiraishi, K.; Mandour, A.S.; Sato, K.; Shimada, K.; Goya, S.; Yoshida, T.; Kitpipatkun, P.; Hamabe, L.; Uemura, A.; et al. The Utility of Intraventricular Pressure Gradient for Early Detection of Chemotherapy-Induced Subclinical Cardiac Dysfunction in Dogs. Animals 2021, 11, 1122. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Andersen, A.; Gade, I.L.; Ringgaard, S.; Kjaergaard, B.; Nielsen-Kudsk, J.E. A porcine in-vivo model of acute pulmonary embolism. Pulm. Circ. 2018, 8, 2045893217738217. [Google Scholar] [CrossRef] [PubMed] [Green Version]

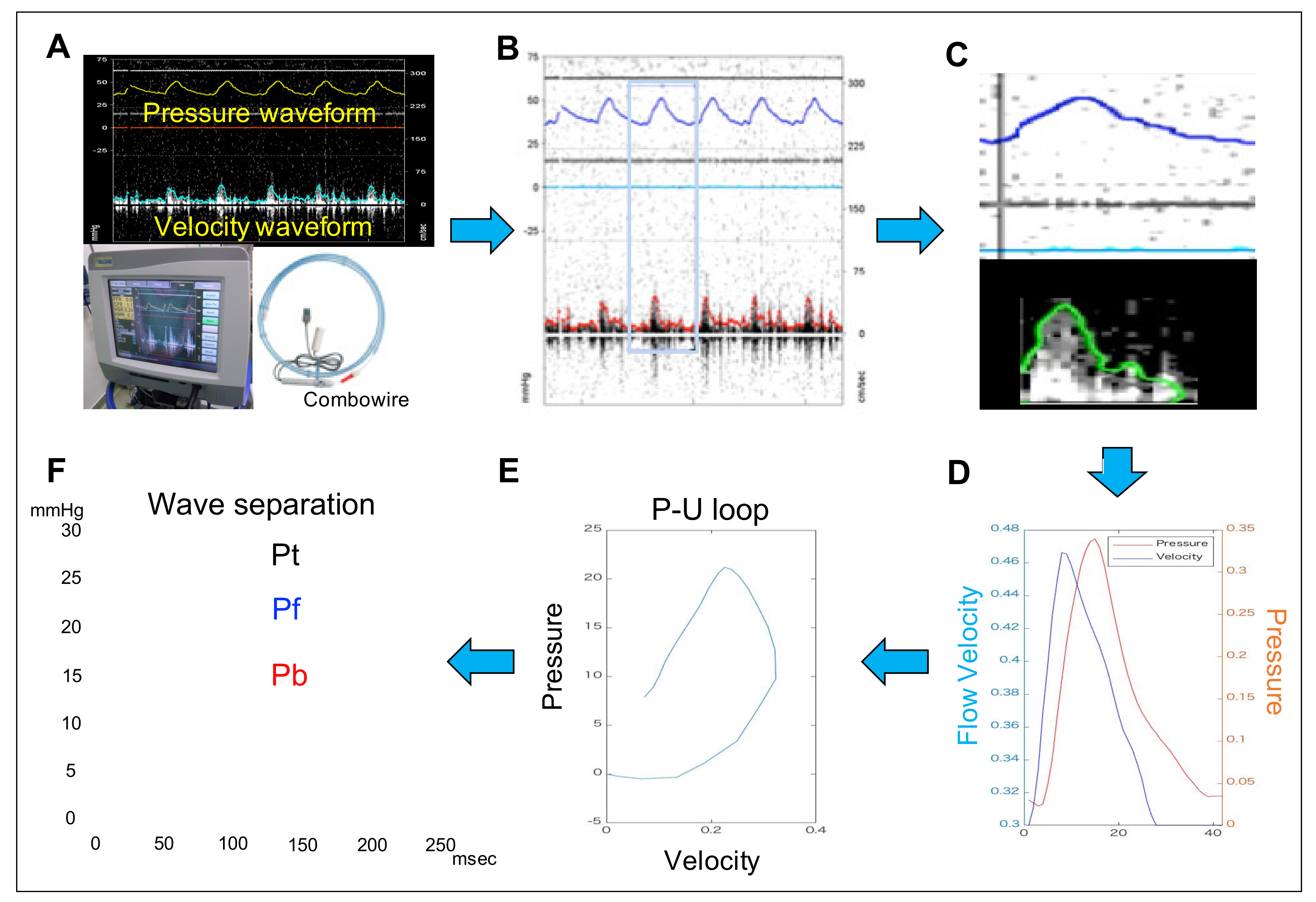


| Hemodynamic Variables | Control | APE | APE + Nitro |
|---|---|---|---|
| HR, /min | 128 ± 9 | 138 ± 11 | 132 ± 13 |
| BT, °C | 35.5 ± 1.0 | 36 ± 1 | 36 ± 1 |
| sPO2, % | 99 ± 1 | 98 ± 1 | 99 ± 1.4 |
| EtCO2, mmHg | 43 ± 4.0 | 35 ± 2 * | 36 ± 2 ** |
| SAP, mmHg | 109 ± 9 | 105 ± 17 | 91 ± 12 **† |
| MAP, mmHg | 95 ± 13.0 | 91 ± 21 | 75 ± 12 **† |
| DAP, mmHg | 86 ± 17 | 82 ± 22 | 68 ± 12 **† |
| RAP, mmHg | 4 ± 1 | 6 ± 3 | 6 ± 3 |
| LAP, mmHg | 7 ± 1 | 7 ± 2 | 7 ± 2 |
| SPAP, mmHg | 20 ± 4 | 59 ± 13 * | 41 ± 13 **† |
| mPAP, mmHg | 13 ± 4 | 42 ± 15 * | 30 ± 12 |
| DPAP, mmHg | 10 ± 4 | 33 ± 16 * | 25 ± 12 |
| CO, L/min | 2.75 ± 0.1 | 2.2 ± 0.4 * | 2.4 ± 0.6 |
| SV, mL | 21.5 ± 1.4 | 16 ± 2.5 * | 18.5 ± 10.1 |
| PVR, wood unit | 2.5 ± 1.2 | 16 ± 5.0 * | 9.6 ± 5 **† |
| Echocardiographic Variables | |||
| RVOT flow peak velocity, cm/s | 82.0 ± 12.7 | 74 ± 15 | 77 ± 15 |
| Pulmonary ET, ms | 203 ± 26 | 187 ± 27 | 178 ± 10 |
| Pulmonary ACT, ms | 96.7 ± 26.5 | 67 ± 14 | 82 ± 11 |
| Pulmonary ACT/ET | 0.47 ± 0.10 | 0.37 ± 0.1 | 0.46 ± 0.08 |
| LVFS, % | 33.6 ± 7.5 | 43.9 ± 12.5 | 39 ± 8.5 |
| LVIDd, mm | 26.4 ± 3.3 | 22.8 ± 2.36 | 24.9 ± 3.2 |
| TAPSE, mm | 11.4 ± 2.0 | 9.5 ± 1.0 * | 9.6 ± 2.2 |
| Tricuspid annular velocity Fw S’, cm/s | 7.9 ± 2.6 | 8.7 ± 2.2 | 7.7 ± 3.0 |
| Tricuspid annular velocity Fw E’, cm/s | 7.7 ± 1.8 | 6.9 ± 1.8 | 6.9 ± 3.0 |
| Tricuspid annular velocity Fw A’, cm/s | 7.7 ± 1.7 | 9.6 ± 3.2 | 9.6 ± 3.4 |
| LVOT peak velocity, cm/s | 81.5 ± 18.0 | 86.3 ± 22.2 | 86 ± 21 |
| FAC, % | 45.4 ± 14.3 | 28.2 ± 11 * | 38 ± 12 |
| TR, cm/s | 217.7 ± 32.2 | 381 ± 63 * | 295 ± 40 ** |
| PAWR variables | |||
| Pf, mmHg | 8.8 ± 1.7 | 19.2 ± 5.1 * | 13.5 ± 4.4 |
| Pb, mmHg | 2.1 ± 0.67 | 11.8 ± 1.7 * | 6.0 ± 2.3 **† |
| RC (Pb/Pf) | 0.23 ± 0.04 | 0.64 ± 0.1 * | 0.44 ± 0.11 **† |
| WS, m/s | 1.2 ± 0.2 | 3.18 ± 0.49 * | 2.33 ± 0.65 **† |
| Variables | Pf | Pb | RC | WS | ||||
|---|---|---|---|---|---|---|---|---|
| R | p-Value | R | p-Value | R | p-Value | R | p-Value | |
| PVR | 0.53 * | 0.022 | 0.6 * | 0.0001 | 0.59 * | 0.0002 | 0.62 * | 0.0002 |
| mPAP | 0.47 * | 0.048 | 0.57 * | 0.013 | 0.65 * | 0.004 | 0.69 * | 0.002 |
| CO | −0.56 * | 0.015 * | −0.31 | 0.2 | −0.41 | 0.089 | −0.3 | 0.23 |
| RAP | 0.015 | 0.95 | 0.078 | 0.79 | 0.27 | 0.28 | 0.186 | 0.46 |
| LAP | −0.053 | 0.83 | −0.15 | 0.54 | −0.221 | 0.38 | −0.049 | 0.85 |
| MAP | 0.063 | 0.81 | −0.06 | 0.8 | −0.012 | 0.96 | 0.034 | 0.89 |
| FAC | −0.54 * | 0.019 | −0.49 * | 0.035 | −0.63 * | 0.004 | −0.53 * | 0.023 |
| TAPSE | −0.35 | 0.15 | −0.21 | 0.4 | −0.63 | 0.17 | −0.39 | 0.11 |
| Variables | Pf | Pb | RC | WS | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | R2 | p-Value | R2 | p-Value | |
| PVR | 0.29 * | 0.022 | 0.42 * | 0.004 | 0.36 * | 0.008 | 0.59 * | <0.001 |
| mPAP | 0.22 * | 0.048 | 0.33 * | 0.013 | 0.41 * | 0.004 | 0.48 * | 0.002 |
| CO | 0.31 * | 0.015 | 0.1 | 0.2 | 0.17 | 0.089 | 0.089 | 0.23 |
| RAP | 0.00023 | 0.95 | 0.006 | 0.75 | 0.072 | 0.28 | 0.034 | 0.46 |
| LAP | 0.0028 | 0.83 | 0.024 | 0.53 | 0.049 | 0.38 | 0.0023 | 0.85 |
| MAP | 0.004 | 0.8 | 0.004 | 0.8 | 0.00015 | 0.96 | 0.0011 | 0.89 |
| FAC | 0.3 * | 0.02 | 0.25 * | 0.035 | 0.4 * | 0.005 | 0.28 * | 0.02 |
| TAPSE | 0.12 | 0.15 | 0.044 | 0.4 | 0.15 | 0.1163 | 0.15 | 0.1105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, T.; Mandour, A.S.; Matsuura, K.; Shimada, K.; El-Husseiny, H.M.; Hamabe, L.; Yilmaz, Z.; Uemura, A.; Tanaka, R. Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator. Animals 2021, 11, 1977. https://doi.org/10.3390/ani11071977
Yoshida T, Mandour AS, Matsuura K, Shimada K, El-Husseiny HM, Hamabe L, Yilmaz Z, Uemura A, Tanaka R. Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator. Animals. 2021; 11(7):1977. https://doi.org/10.3390/ani11071977
Chicago/Turabian StyleYoshida, Tomohiko, Ahmed S. Mandour, Katsuhiro Matsuura, Kazumi Shimada, Hussein M. El-Husseiny, Lina Hamabe, Zeki Yilmaz, Akiko Uemura, and Ryou Tanaka. 2021. "Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator" Animals 11, no. 7: 1977. https://doi.org/10.3390/ani11071977
APA StyleYoshida, T., Mandour, A. S., Matsuura, K., Shimada, K., El-Husseiny, H. M., Hamabe, L., Yilmaz, Z., Uemura, A., & Tanaka, R. (2021). Changes in the Pulmonary Artery Wave Reflection in Dogs with Experimentally-Induced Acute Pulmonary Embolism and the Effect of Vasodilator. Animals, 11(7), 1977. https://doi.org/10.3390/ani11071977









