Carnosine Content and Its Association with Carnosine-Related Gene Expression in Breast Meat of Thai Native and Black-Bone Chicken
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Tissue Collection
2.3. Carnosine Extraction and Purification
2.4. HPLC Analysis
2.5. RNA Extraction
2.6. Quantification of mRNA Expression
2.7. Statistical Analysis
3. Results
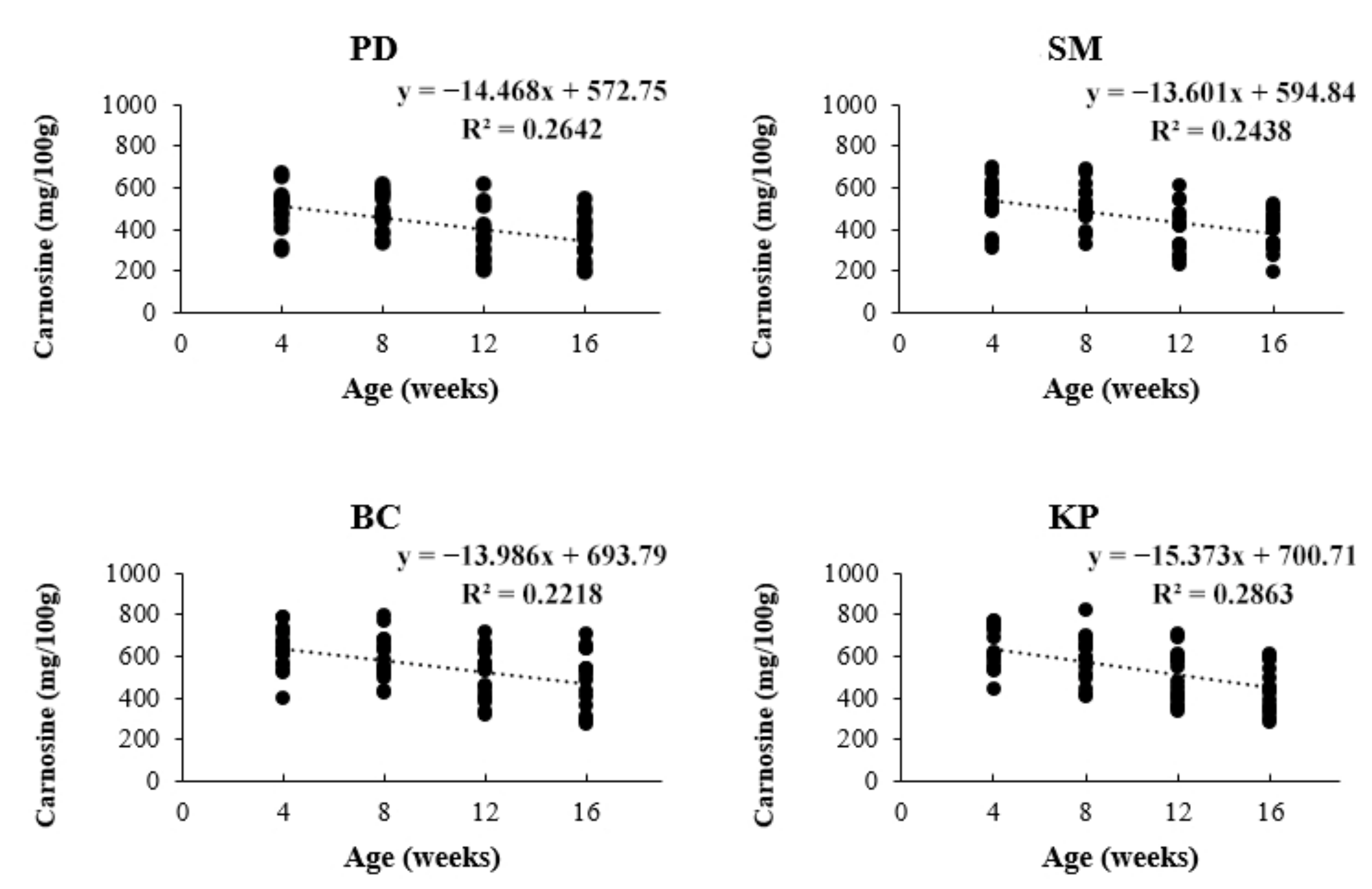
3.1. Carnosine Content in Breast Meat
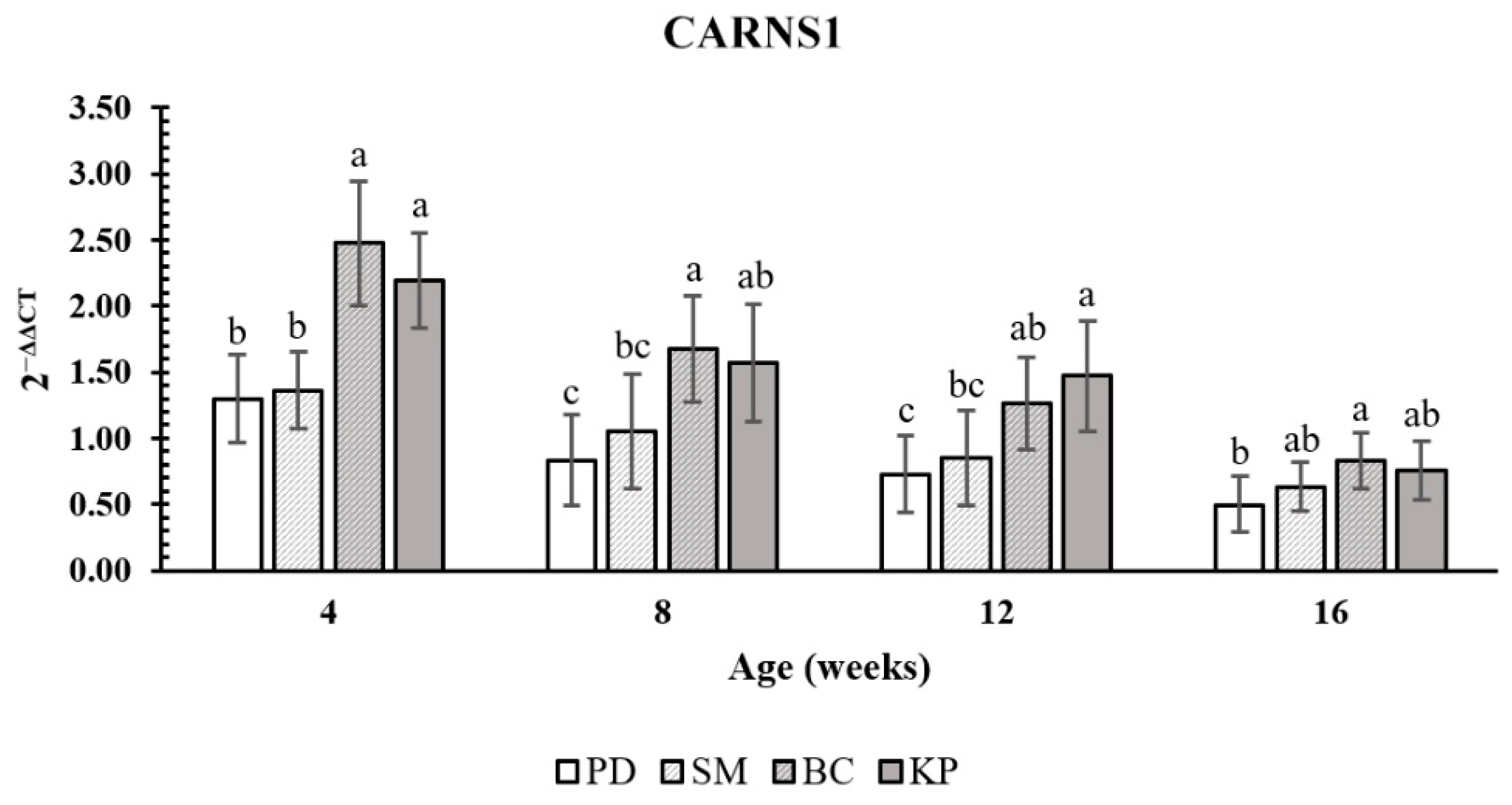
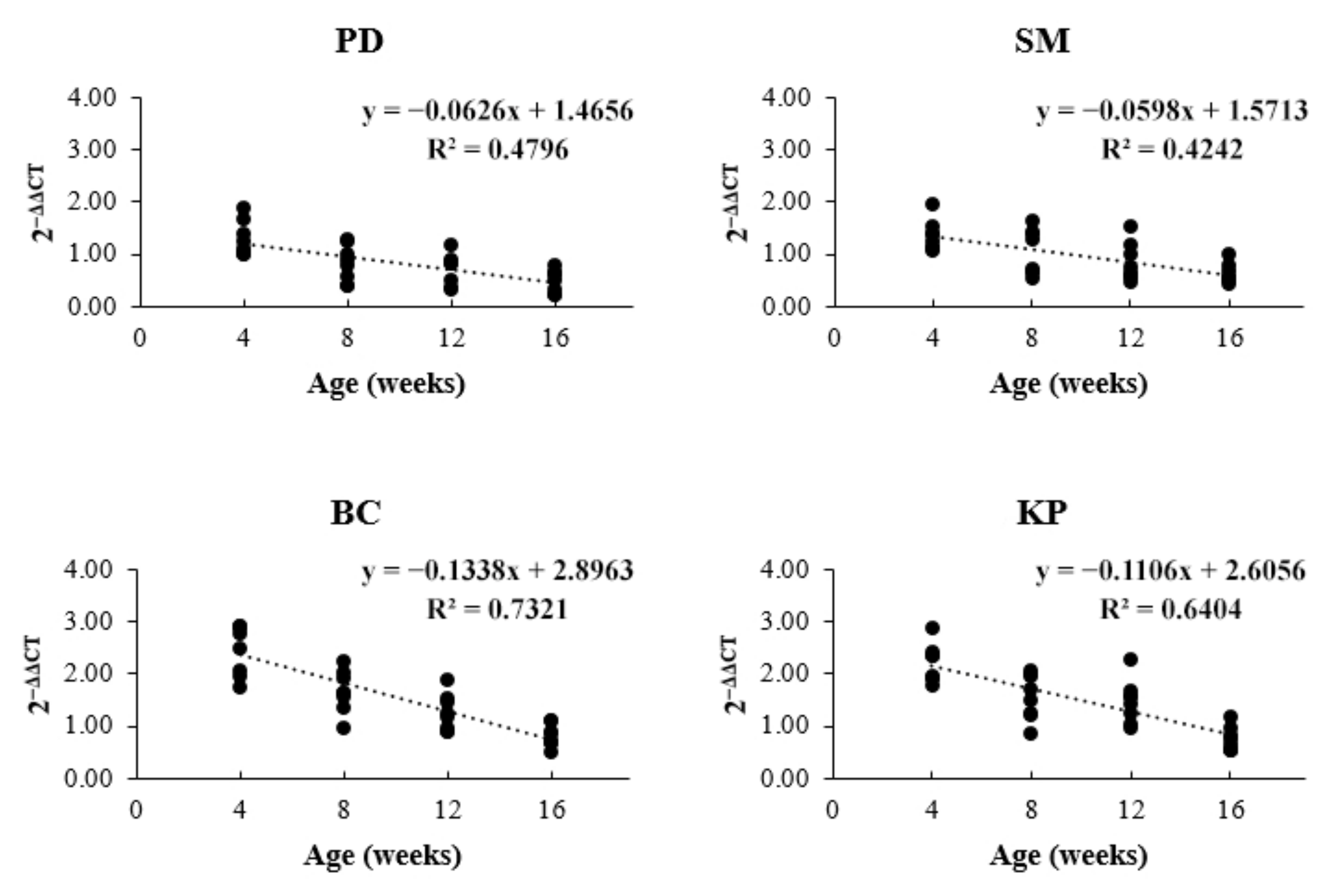
3.2. Expression Level of CARNS1
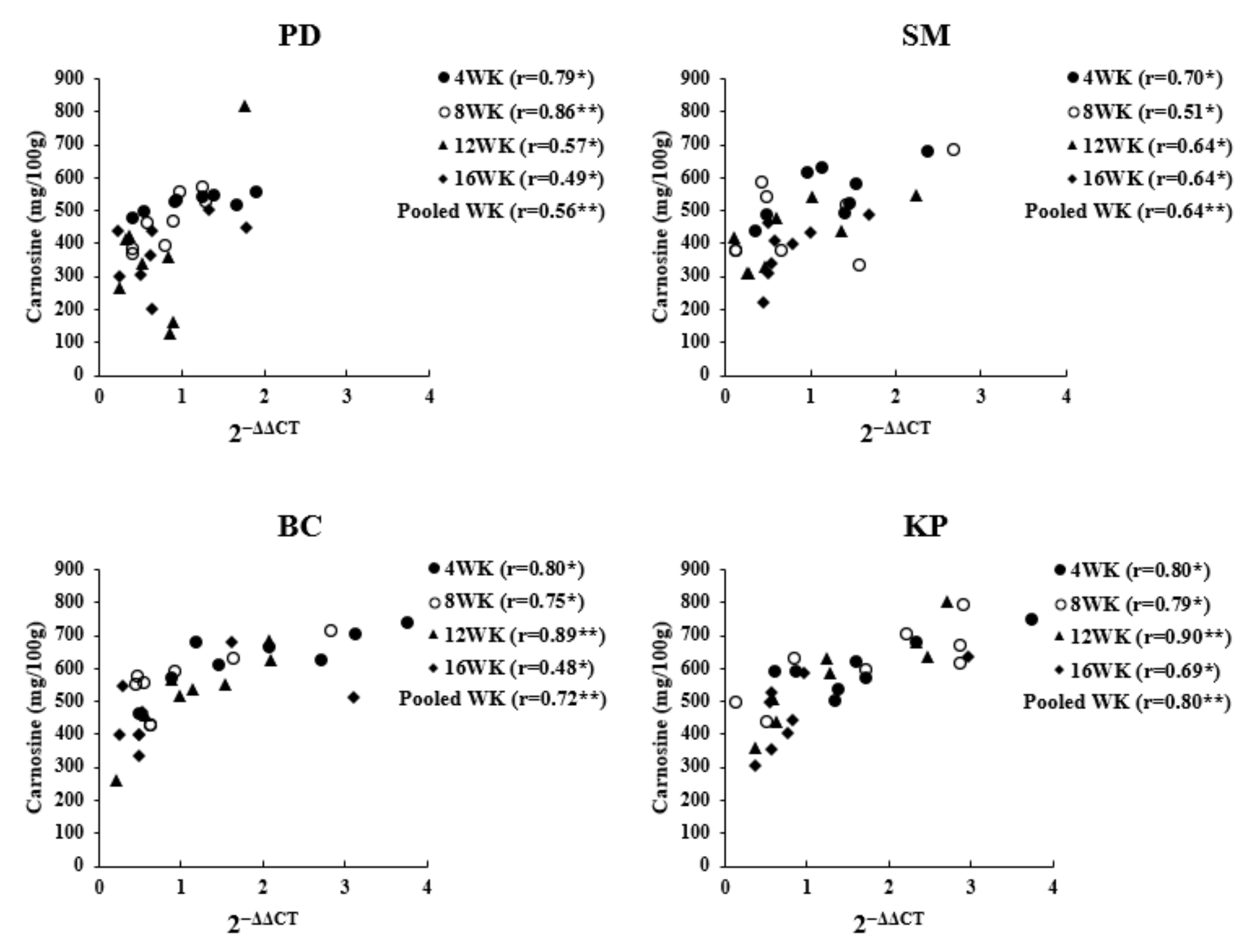
3.3. Correlation between Gene Expression Level and Carnosine Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, anserine, creatine, and inosine 5′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult. Sci. 2003, 92, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van, S.E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, K.; Mousa, A.; de Courten, B. Effects of supplementation with carnosine and other histidine-containing dipeptides on chronic disease risk factors and outcomes: Protocol for a systematic review of randomised controlled trials. BMJ 2018, 8, e020623. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Fresta, C.G.; Musso, N.; Giambirtone, M.; Grasso, M.; Spampinato, S.F.; Merlo, S.; Drago, F.; Lazzarino, G.; Sortino, M.A.; et al. Carnosine Prevents Aβ-Induced Oxidative Stress and Inflammation in Microglial Cells: A Key Role of TGF-β1. Cells 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.K.; Kim, Y.; Baek, I.K.; Auh, J.H. Carnosine and anserine in chicken: Distribution, age-dependency and their anti-glycation activity. Korean J. Food Sci. Anim. Resour. 2012, 32, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Derave, W.; Courten, B.D.; Baba, S.P. An update on carnosine and anserine research. Amino Acids 2019, 51, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguet, A.; Bourgois, J.; Vanhee, L.; Achten, E.; Derave, W. Important role of muscle carnosine in rowing performance. J. Appl. Physiol. 2010, 109, 1096–1101. [Google Scholar] [CrossRef] [Green Version]
- del Favero, S.; Roschel, H.; Solis, M.Y.; Hayashi, A.P.; Artioli, G.G.; Otaduy, M.C.; Benatti, F.B.; Harris, R.C.; Wise, J.A.; Leite, C.C.; et al. Beta-alanine (CarnosynTM) supplementation in elderly subjects (60–80 years): Effects on muscle carnosine content and physical capacity. Amino Acids 2012, 43, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Kai, S.; Watanabe, G.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary histidine on contents of carnosine and anserine in muscles of broilers. Anim. Sci. J. 2015, 86, 541–546. [Google Scholar] [CrossRef]
- Qi, B.; Wang, J.; Ma, Y.; Wu, S.; Qi, G.; Zhang, H. Effect of dietary β-alanine supplementation on growth performance, meat quality, carnosine content, and gene expression of carnosine-related enzymes in broilers. Poult. Sci. 2018, 97, 1220–1228. [Google Scholar] [CrossRef]
- Kojima, S.; Saegusa, H.; Sakata, M. Histidine-containing dipeptide concentration and antioxidant effects of meat extracts from Silky Fowl: Comparison with meat-type chicken breast and thigh meats. Food Sci. Technol. Res. 2014, 20, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Jaturasitha, S.; Chaiwang, N.; Kreuzer, M. Thai native chicken meat: An option to meet the demands for specific meat quality by certain groups of consumers; a review. Anim. Prod. Sci. 2016, 57, 1582–1587. [Google Scholar] [CrossRef]
- Intarapichet, K.O.; Maikhunthod, B. Genotype and gender differences in carnosine extracts and antioxidant activities of chicken breast and thigh meats. Meat Sci. 2005, 71, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xie, M.; Wang, W.; Wu, H.; Fu, Z.; Lin, L. Determination of carnosine in Black-Bone Silky Fowl (Gallus gallus domesticus Brisson) and common chicken by HPLC. Eur. Food Res. Technol. 2007, 226, 311–314. [Google Scholar] [CrossRef]
- Ali, M.; Lee, S.Y.; Park, J.Y.; Jung, S.; Jo, C.; Nam, K.C. Comparison of functional compounds and micronutrients of chicken breast meat by breeds. Food Sci. Anim. Resour. 2019, 39, 632–642. [Google Scholar] [CrossRef]
- Maikhunthod, B.; Intarapichet, K.O. Heat and ultrafiltration extraction of broiler meat carnosine and its antioxidant activity. Meat Sci. 2005, 71, 364–374. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Bae, Y.S.; Kim, S.H.; Lee, S.K.; Lee, J.H.; Jo, C. Changes in endogenous bioactive compounds of Korean native chicken meat at different ages and during cooking. Poult. Sci. 2014, 93, 1842–1849. [Google Scholar] [CrossRef]
- Everaert, I.; Naeyer, H.D.; Taes, Y.; Derave, W. Gene expression of carnosine-related enzymes and transporters in skeletal muscle. Eur. J. Appl. Physiol. 2013, 113, 1169–1179. [Google Scholar] [CrossRef]
- Lokman, I.H.; Jawad, H.S.A.; Goh, Y.M.; Sazili, A.Q.; Noordin, M.M.; Zuki, A.B.Z. Morphology of breast and thigh muscles of Red Jungle Fowl (Gallus gallus spadiceus), Malaysian village chicken (Gallus gallus domesticus) and commercial broiler chicken. Int. J. Poult. Sci. 2016, 15, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Baguet, A.; Everaert, I.; Hespel, P.; Petrovic, M.; Achten, E.; Derave, W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS ONE 2011, 6, e21956. [Google Scholar] [CrossRef] [Green Version]
- Ismail, I.; Joo, S.T. Poultry meat quality in relation to muscle growth and muscle fiber characteristics. Korean J. Food Sci. Anim. Resour. 2017, 37, 873–883. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Kim, S.H.; Kim, H.J.; Alahakoon, A.U.; Lee, J.H.; Jo, C. Endogenous functional compounds in Korean native chicken meat are dependent on sex, thermal processing and meat cut. J. Sci. Food Agri. 2015, 95, 771–775. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Garmyn, A.J.; O’Neil, M.A.; Tait, R.G.; Abuzaid, A.; Mayes, M.S.; Garrick, D.J.; Van Eenennaam, A.L.; VanOverbeke, D.L.; Hilton, G.G.; et al. Genetic parameters for carnitine, creatine, creatinine, carnosine, and anserine concentration in longissimus muscle and their association with palatability traits in Angus cattle. J. Anim. Sci. 2012, 90, 4248–4255. [Google Scholar] [CrossRef] [Green Version]
- Brink, T.C.; Demetrius, L.; Lehrach, H.; Adjaye, J. Age-related transcriptional changes in gene expression in different organs of mice support the metabolic stability theory of aging. Biogerontology 2009, 10, 549–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cararo, J.H.; Streck, E.L.; Schuck, P.F.; Ferreira, G.C. Carnosine and related peptides: Therapeutic potential in age-related disorders. Aging Dis. 2015, 6, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Ren, R.; Ocampo, A.; Liu, G.H.; Izpisua, B.J.C. Regulation of stem cell aging by metabolism and epigenetics. Cell Metab. 2017, 26, 460–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature. 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, J.; Zhang, L.; Li, J.; Wang, S.; Gao, F.; Zhou, G. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 2017, 97, 3733–3741. [Google Scholar] [CrossRef] [PubMed]

| Details | First Period (0–4 Weeks) | Second Period (4–16 Weeks) |
|---|---|---|
| Crude protein (CP) (%) | 21 | 18 |
| Metabolizable energy (ME) (kcal/kg) | 3200 | 3200 |
| Crude fat (%) | 3 | 2 |
| Crude fiber (%) | 6 | 7 |
| Moisture (%) | 13 | 13 |
| Gene | Primer Sequence (5′--> 3′) | Chr | GeneBankID | Product (bp) |
|---|---|---|---|---|
| CARNS1 | F: TGCCCTGGAAGAATTTGTGC | 5 | NM_001172593.1 | 170 |
| R: ACAGCAACCAGCGAGAGAG | ||||
| 18 S rRNA | F: CGGCGACGACCCATTCGAAC | 16 | XR_003078044.1 | 99 |
| R: GAATCGAACCCTGATTCCCCGTC |
| Breeds | Sexes | Ages (Weeks) | AVG (B) 1 | SEM | Effect 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 16 | B | A | S | Intac | ||||
| PD | M | 505.5 ± 140.7 | 490.5 ± 83.6 | 336.4 ± 87.2 | 373.0 ± 85.3 | 428.08 ± 126.9 y | |||||
| F | 510.7 ± 51.6 | 489.8 ± 102.4 | 369.5 ± 156.4 | 349.1 ± 134.1 | |||||||
| SM | M | 537.9 ± 91.4 | 521.0 ± 87.9 | 400.5 ± 135.3 | 401.9 ± 69.4 | 458.82 ± 124.2 y | |||||
| F | 531.5 ± 138.6 | 511.7 ± 117.1 | 375.3 ± 108.0 | 390.6 ± 115.0 | |||||||
| BC | M | 638.6 ± 119.3 | 593.0 ± 104.3 | 506.1 ± 131.8 | 480.2 ± 136.2 | 553.93 ± 139.8 x | |||||
| F | 635.6 ± 94.1 | 594.8 ± 119.5 | 501.5 ± 119.7 | 481.3 ± 159.1 | |||||||
| KP | M | 626.7 ± 75.3 | 592.7 ± 73.9 | 491.4 ± 153.9 | 443.6 ± 126.7 | 546.98 ± 129.5 x | |||||
| F | 635.6 ± 124.6 | 593.4 ± 152.2 | 527.7 ± 83.8 | 464.5 ± 96.4 | |||||||
| AVG (A) 1 | 577.8 ± 117.5 a | 548.4 ± 111.6 a | 438.6 ± 137.5 b | 423.0 ± 121.7 b | 15.8 | ||||||
| SEM | 15.8 | ||||||||||
| p-value | *** | *** | ns | ns | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumpeerawat, P.; Duangjinda, M.; Phasuk, Y. Carnosine Content and Its Association with Carnosine-Related Gene Expression in Breast Meat of Thai Native and Black-Bone Chicken. Animals 2021, 11, 1987. https://doi.org/10.3390/ani11071987
Khumpeerawat P, Duangjinda M, Phasuk Y. Carnosine Content and Its Association with Carnosine-Related Gene Expression in Breast Meat of Thai Native and Black-Bone Chicken. Animals. 2021; 11(7):1987. https://doi.org/10.3390/ani11071987
Chicago/Turabian StyleKhumpeerawat, Panuwat, Monchai Duangjinda, and Yupin Phasuk. 2021. "Carnosine Content and Its Association with Carnosine-Related Gene Expression in Breast Meat of Thai Native and Black-Bone Chicken" Animals 11, no. 7: 1987. https://doi.org/10.3390/ani11071987






