Simple Summary
The information about the risks related to the use of medication during breastfeeding is lacking for most commonly used drugs. The ConcePTION project aims to fill this gap using multiple approaches. Within the project, the pig has been selected as the most appropriate in vivo animal model. In agreement with the application of the “3Rs” principle (Replacement, Reduction and Refinement) and international legislations, the present paper reports the establishment of cellular lines of porcine mammary epithelial cells as a valid tool to study the mammary epithelial barrier function in vitro.
Abstract
The ConcePTION project aims at generating further knowledge about the risks related to the use of medication during breastfeeding, as this information is lacking for most commonly used drugs. Taking into consideration multiple aspects, the pig model has been considered by the consortium as the most appropriate choice. The present research was planned to develop an efficient method for the isolation and culture of porcine Mammary Epithelial Cells (pMECs) to study the mammary epithelial barrier in vitro. Mammary gland tissues were collected at a local slaughterhouse, dissociated and the selected cellular population was cultured, expanded and characterized by morphology, cell cycle analysis and immunophenotyping. Their ability to create a barrier was tested by TEER measurement and sodium fluorescein transport activity. Expression of 84 genes related to drug transporters was evaluated by a PCR array. Our results show that primary cells express epithelial cell markers: CKs, CK18, E-Cad and tight junctions molecules ZO-1 and OCL. All the three pMEC cellular lines were able to create a tight barrier, although with different strengths and kinetics, and express the main ABC and SLC drug transporters. In conclusion, in the present paper we have reported an efficient method to obtain primary pMEC lines to study epithelial barrier function in the pig model.
1. Introduction
ConcePTION is a European IMI (Innovative Medicines Initiative) project, approved in April 2019, with the aim of generating accurate knowledge about the use of medication during pregnancy and breastfeeding. At the present moment, information on the safety of maternal medication during breastfeeding is lacking for most drugs [1].
The Work Package 3 (WP3) of ConcePTION aims to generate a non-clinical testing platform to establish drug transfer into human breast milk, and subsequent neonatal exposure. To achieve this goal, different approaches will be followed including in vivo and in vitro studies with animal models. Animal models have been used successfully in the past to predict the safety of maternal medication during breastfeeding, but caution is necessary due to species specific differences (e.g., in transporters, enzymes and milk composition) [2]. Among different animal species considered, the pig has been chosen as the most fit for purpose model within the ConcePTION project [3,4]. In fact, even though several macroscopic differences exist between pig and human mammary gland anatomy, many similitudes have been reported at the molecular level. Within the complex structure of a functional mammary gland, mammary epithelial cells (MECs) are engaged not only in the secretory function but also in xenobiotic transfer from mother to newborn [5,6]. Given that, in vitro studies with MECs have been proposed as a helpful approach to determine the possibility of transepithelial drug transport and rate [7,8,9,10]. Therefore, given the importance of the model, MECs were isolated from different species and by different methods, with variable results in terms of both quality and quantity [11]. In 2001, Kumura et al. [12] first described the isolation of porcine mammary epithelial cell (pMECs) from mammary tissue of a 180-day-old sow using a floating collagen culture system. Then, Zheng & He [13] isolated pMECs from a three-year old lactating Guanzhong pig utilizing the technique of explant migration. Sun et al. [14] and Dahanayaka et al. [15] reported the isolation of pMECs by associating manual mechanical and enzymatic dissociation. However, a fully-characterized pMECs in vitro model to study the mammary epithelial barrier is still lacking. The present research was planned to develop an efficient method of pMECs isolation from tissue collected from slaughterhouses, aiming to evaluate the external validity of the in vivo porcine animal model and determine its accuracy as a solid translational model for the study of mammary epithelial barrier in the full respect of the Reduction principles [16].
2. Materials and Methods
2.1. Chemicals and Reagents
Dulbecco Phosphate Buffered Saline (DPBS), Phosphate Buffered Saline (PBS), DPBS without calcium and magnesium, Fetal Bovine Serum (FBS), trypsin-EDTA, Antibiotic-Antimycotic (anti-anti) gentamicin, recombinant human epidermal growth factor (hEGF), Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12), RNaseA/T1 and glucose were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Propidium iodide (PI) and Multi Tissue Dissociation Kit1 were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). TRI Reagent was purchased from Molecular Research Center In, OH, USA and NucleoSpin RNA II kit from Macherey-Nagel GmbH & Co. KG, Düren, Germany. RT2 First Stand Kit, RT2 SYBR Green qPCR Mastermix and RT² Profiler™ PCR Array Pig Drug Transporters (Cat. No. PASS-070ZD) were purchased from Qiagen Hilden, Germany. Dimethyl sulfoxide (DMSO), Diaminobenzidine and FluoroshieldTM with DAPI histology mounting medium were purchased from Sigma-Aldrich (St. Louis, MO, USA). Betadine 10% cutaneous solution was purchased from Meda Pharma Spa (Milan, Italy). EZ-PCR Mycoplasma Detection kit was purchased from Biological Industries (Kibbutz Beit-Haemek, Israel). Avidin-biotin-peroxidase kit, ABC Kit Elite, was purchased from Vector, Burlingame, CA, USA). Tissue embedding medium OCT was obtained from Sakura (Finetek, Torrance, CA USA). Sodium fluorescein was purchased from Siegfield Zofingen, (Switzerland). All plastic supports for cell culture were purchased from Corning-Beckton-Dickinson (Franklin Lakes, NJ, USA). Hanks Balanced Salt Solution (HBSS) was purchased from Lonza (Walkersville, MD USA) 2-[4-(2-hydroxyethyl)piperazin-1-yl] ethanesulfonic acid (HEPES) was purchased from VWR International (Milan, Italy). Fluorescein sodium was purchased from Siegfield (Zofingen Switzerland). The culture medium used for cell isolation, named the Isolation Medium (Im), was the Epithelial Mammary Cell Medium supplemented with 0.004 mL/mL Bovine Pituitary Extract (BPE), 10 ng/mL hEGF, 5 μg/mL insulin, 0.5 μg/mL hydrocortisone purchased from Promo Cell (Heidelberg, Germany), 1% anti-anti and 50 µg/mL gentamicin. The medium used for cell expansion named the Expansion Medium (Em) was composed of DMEM/F12 with 10% FBS, 5 μg/mL insulin and 0.5 μg/mL hydrocortisone (both provided by Promo Cell), 5 ng/mL hEGF (provided by Thermo Fisher) and 1% anti-anti. Transport buffer (Tb) for transepithelial electrical resistance (TEER) measurement, consisted of HBSS (Lonza) (with addition of 10 mM HEPES (VWR International) and 25 mM glucose (Thermo Fisher Scientific). The antibodies used in the different application are reported in Table 1.

Table 1.
Antibodies used for the immunofluorescence (IF), immunohistochemical (IHC) and flow cytometry (FC) analysis.
2.2. Isolation and Culture of Porcine Mammary Epithelial Cells (pMECs)
Porcine mammary gland (MG) tissues were collected from sows (n = 3), namely MG2, MG3 and MG8, at a local slaughterhouse, in order to obtain three primary mammary epithelial cell lines. After collection an entire mammary line from each sow was immediately transferred to the laboratory in a temperature-controlled container (+4 °C); the tissue surfaces were firstly disinfected with Betadine (10% cutaneous solution) and ethanol 70%. The abdominal breasts were selected, transferred under a laminar flux hood than processed in aseptic conditions. Portions of mammary glands were isolated with sterile scissors and scalpels, removing cutaneous tissue, nipple and excess adipose tissues. Samples were collected for further histological and immunohistochemical analysis. The tissue was cut into pieces of approximately 1 g then minced with the scalpel into smaller pieces of 1–2 mm3.
The digestion of mammary tissue was performed by combining enzymatic and mechanical dissociation using the GentleMACS Octo Dissociator (Miltenyi Biotec). Mammary tissue was transferred into GentleMACS C Tubes (Miltenyi Biotec) containing the mix enzyme solution prepared following the Multi Tissue Dissociation Kit1 instructions (1 g of tissue was suspended in 3.3 mL enzymatic solution) and using the 37C_Multi_B program for 1 h. Once the run program finished, C Tubes were centrifuged at 100× g for 2 min to separate and subsequently eliminate the fat portion. Then, the suspension was filtered through a 100 μm and a 70 μm MACS Smart Strainers (Miltenyi Biotec). The last filter was washed with 10 mL of Im. The filtrate suspension was centrifuged at 800× g for 10 min, and the cellular pellet was washed once with 10 mL of Im. As indicated by [17,18] gentamicin treatment induces fibroblast apoptosis while MECs are highly resistant, therefore 50 μg/mL gentamicin was present in Im. Isolated cells were seeded in a well of a primary 6-multiwell plate in 3 mL of Im. The cells were incubated overnight at 38.5 °C in a humidified atmosphere containing 5% CO2. For better selection of pMECs, considering that under serum-free conditions MEC progenitors form spherical structures [19,20,21], we chose to grow cells in suspension for 24 h. Therefore, the day after, the non-adherent spheres were collected and transferred in a new well of primary 6-multiwell plate in Im added with 20% FBS. The serum allowed the spheres to adhere and cell sprouting, then the amount of FBS was reduced every 24 h, from 20% to 10% until reaching serum-free condition again to avoid growth of eventually residual fibroblasts. At ~70% confluence, cells were detached with trypsin-EDTA 1X solution, counted and seeded into a T-25 primary culture flask until 70–75% confluence was reached (first passage P). Cells were then expanded to P 10 in the Em in T25 or T75 primary culture flasks.
Cell doubling time among passages was calculated as previously described [22] for each primary cell line. For the cryopreservation, aliquots of 1 × 106 cells were suspended in 1 mL of freezing medium (90% FBS and 10% DMSO). Aliquots of cell culture medium were periodically collected and analyzed by the EZ-PCR Mycoplasma Detection kit to ascertain the possible mycoplasma contamination.
2.3. Histological and Immunohistochemical Examination of Mammary Gland Tissues
Samples of mammary gland of 1 cm3 were embedded in OCT and frozen in isopentane cooled in liquid nitrogen. Seven micrometers-thick sections were cut with a Leica CM1950 cryostat (Leica, Wetzlar, Germany), then left to adhere to a microscope slide and stained with hematoxylin and eosin (H&E) according to standard procedure. Images were obtained using a Leika Aristoplan microscope equipped with a DFK 33UX264 camera (The Imaging Source Europe GmbH, Bremen, Germany). On serial sections, an immunohistochemical protocol for E-Cadherin and pan-cytokeratin (CK) was applied (Table 1). Briefly, sections were immersed in PBS, endogenous peroxidase was blocked with hydrogen peroxide 3% in methanol for 30 min at room temperature (RT). After preincubating for 30 min with 10% normal goat serum in PBS, sections were allowed to react for 1 h at room temperature with the primary antibodies (Table 1). Binding sites were revealed by secondary biotinylated antibody and amplified using an avidin–biotin–peroxidase system. Diaminobenzidine (0.04%, 10 min at room temperature) was used as the chromogen and the sections were then counterstained with Mayer’s hematoxylin for 1 min, rinsed in tap water, and mounted with glycerin jelly. In the control sections an isotype matched primary antibody of irrelevant specificity replaced the primary antibody.
2.4. Cell Cycle Analysis
The cells were seeded in a T25-flask and cultured until confluence, then harvested and counted. Aliquots of 8 × 105 cells were washed twice in 5 mL of DPBS without calcium and magnesium and fixed overnight in 70% ice-cold ethanol (800 µL) added drop-by-drop with continuous vertexing. Then, the cells were washed with 10 mL of DPBS without calcium and magnesium and the cellular pellet was incubated with 800 µL of staining solution containing 50 µg/mL of PI and 100 µg/mL RNaseA/T1 in DPBS without calcium and magnesium for 30 min in the dark at room temperature. Cell distribution in cell cycle phases was analyzed by MACSQuant® Analyzer10 (equipped with three lasers: 405, 488 and 638 nm) and Flowlogic ™ software (Inivai Technologies, Australia) as previously described [23]. FoxSynchronous Model was used to determinate the percentage of cells across the cell cycle. DNA index (DI) was calculated as the ratio between the mean G1 fluorescence level in MG2, MG3 and MG8 cells and that of the neither tumorigenic nor transformed porcine cell line, IPEC-J2 (DSMZ Leibniz Institute, Germany). A DI of 1.0 represents a normal diploid DNA content.
2.5. Immunofluorescence of Cytokeratins (CKs), Zonula Occludens-1 (ZO-1), Occludin (OCL)
The cells were seeded on an 8-well slide chamber (BD Falcon Bedford, Franklin Lakes, NJ, USA) at a concentration of 5 × 104 cells/well. After 24 h, the cells were washed in DPBS and fixed in 4% paraformaldehyde for 15 min at RT. Subsequently, fixed cells were permeabilized with 0.5%Triton-X 100 in DPBS for 15 min at RT and then blocked with 0.5%Triton-X 100, 10% FBS in DPBS (blocking solution) for 1 h at room temperature. Then, cells were incubated ON at 4 °C in a humid atmosphere with the primary antibodies diluted in blocking solution (Table 1). Negative controls were carried out by primary antibody omission. After being rinsed in PBS (3 times 10 min each), the cells were incubated with fluorochrome-labeled secondary antisera diluted in DPBS 1 h at RT (see Table 1). After 3 washes (10 min each) in PBS, slides were counterstained by DAPI and ultimately mounted. Images were obtained using a Nikon digital camera (DS-Qi2 Monochrome Digital Microscope Camera), installed on a Nikon epifluorescence microscope Eclipse E600 and analyzed with digital image software NIS-Elements BR Ver5.30.00 (Nikon, Tokyo, Japan).
2.6. Epithelial Cadherin (E-Cad) and Cytokeratin18 (Ck18) Analysis by Flow Cytometry
To confirm the epithelial immunophenotype of the obtained primary cell lines, epithelial cadherin and cytokeratin 18 Flow Cytometry analysis was performed. Briefly, 3 × 105 cells were fixed in 4% paraformaldehyde for 20 min at RT (300 µL) and permeabilized in absolute methanol, previously cooled at −20 °C, for 20 min at 4 °C (500 µL). Then, cells were washed in DPBS, suspended in 100 µL DPBS and incubated overnight at 4 °C in the dark with anti-E-cadherin or anti-cytokeratin 18 antibodies (Table 1). Negative controls, to evaluate inherent background or auto fluorescence, were obtained omitting primary antibodies. After incubation, cells were washed once and suspended in 300 µL of DPBS then analyzed with the MacsQuant Analyzer10. Data were analyzed using the Flowlogic™ software. To start, cellular events were discriminated from debris using forward (FSC-A) vs. side scatter (SSC-A). Doublets’ exclusion was obtained by plotting FSC-area vs height (FSC-A/FSC-H). The fluorescent staining intensity was determined comparing the median intensity fluorescence (MFI) of the negative control and the MFI of single stained cells. Positive staining was categorized as dim (slightly increased when compared to the negative control) or bright (considerable increased when compared to the negative control).
2.7. Barrier Study: TEER Measure and Sodium Fluorescein Transport
The monolayer formation and integrity were first assessed through the measurement of trans-epithelial electrical resistance (TEER) as previously reported for another pig epithelial cell line [24], with some important modifications. Briefly, pMECs at P10 were seeded on polyester permeable supports (pore size 0.4 µm; membrane area 0.3 cm2) at a density of 3.3 × 105 cells/cm2. The transwells were incubated at 37 °C, 5% CO2 in Em. Medium in the apical and basal compartment was changed every 48 h. The barrier function was evaluated measuring TEER in function of time and sodium fluorescein transport every other day. Resistance (R) was measured in Tb at 37 °C, using an Epithelial Volt/Ohm Meter 2 (EVOM2, World Precision Instruments). TEER was calculated using Equation (1), where Rtotal is the measured resistance, Rmean blank is the mean resistance of the blank insert for each day and Marea is the surface area of the membrane.
After TEER measurements, sodium fluorescein leakage was determined. 0.5 mL sodium fluorescein (2.66 mM) and 1.2 mL of Tb, respectively, were added to the apical and basal compartment. After 1 h incubation at 37 °C under gentle shaking, samples were taken from the basal compartment. The concentration of sodium fluorescein in the samples was measured via fluorescence intensity (λ = 490/524 nm) using a Tecan Infinite M200 plate reader (Tecan Group Ltd., Männedorf, Austria).
The percentage of sodium fluorescein transport was calculated using Equation (2), where Csample is the concentration of sodium fluorescein in the sample, C0 is the donor concentration of sodium fluorescein, Vb is the volume of the basal compartment and Va is the volume of the apical compartment.
2.8. pMECs RNA Extraction and Pig Drug Transporters Analysis by RT2 Profilertm PCR Array
RNA extraction was performed from pMECs (0.5 × 106 cells) at P10 using TRI Reagent and the NucleoSpin RNA II kit, as previously described [25]. After spectrophotometric quantification (DeNovix DS-11, DeNovix Inc., Wilmington, NC, USA) total RNA (500 ng) was reverse-transcribed to cDNA using the RT2 First Stand Kit. For each cell line, RT² Profiler™ PCR Array Pig Drug Transporters were performed according to the manufacturer’s instructions, using RT2 SYBR Green qPCR Mastermix and CFX 96 Touch (Bio-Rad Laboratories, Hercules, CA, USA). The array contained primers for 84 transepithelial transporter pathway-related genes and five housekeeping genes, 7 wells included reverse-transcription controls, positive PCR controls, and a genomic DNA contamination control. Gene expression was evaluated using the ΔCt method (mean reference genes Ct—interest gene Ct), according to the RT2 Profiler PCR Array Handbook.
2.9. Statistical Analysis
Doubling time, cell cycle, qPCR, TEER and fluorescein sodium transport data were analyzed by one-way analysis of variance (ANOVA) followed by the post hoc Tukey comparison test (p < 0.05) (GraphPad Prism 5 software). E-Cad and CK18 quantitative expression data by Flow Cytometry were analyzed with Student’s t test comparing MFI of the negative control and the MFI of single stained cells (p < 0.05) in each cell lines.
3. Results
3.1. Histological and Immunohistochemical Characteristics of the Mammary Glands
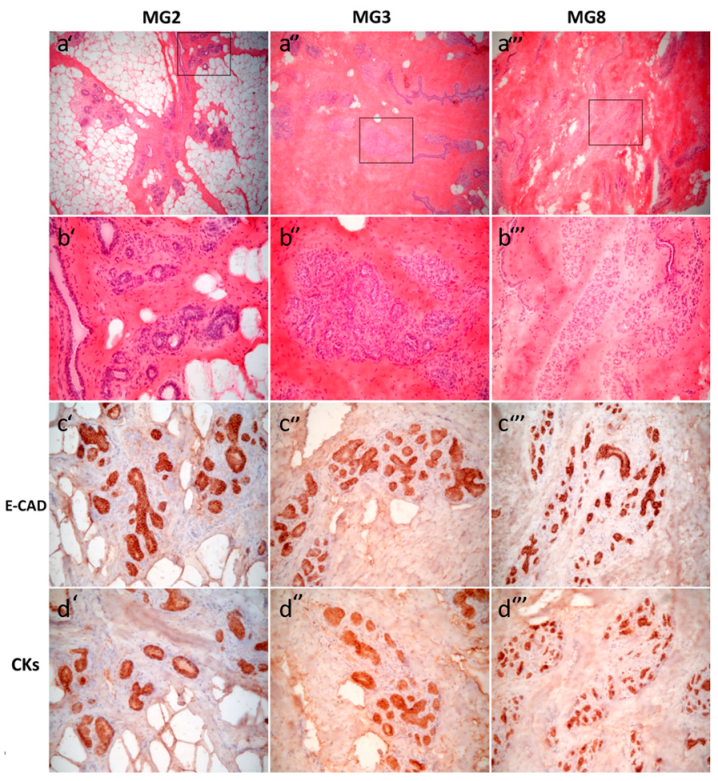
All samples obtained from the three sows showed resting mammary gland with a mixture of adipose tissue and dense collagen stroma, embedding mammary interlobular and intralobular ducts. The latter had variable amounts of branching ducts, more abundant in sections from gilt 3 and 8 (MG3 and MG8) than in samples from gilt 2 (Figure 1). True functional alveoli with lumen containing secreted material were absent in all the samples available. Immunohistochemical results in anti-E-Cadherin-stained sections highlighted a uniform cytoplasmic staining in both interlobular and intralobular ducts, and showed diffuse staining, both cytoplasmic and, with a stronger intensity, in the plasma membrane, mainly in epithelial cells of interlobular than intralobular branching ducts.
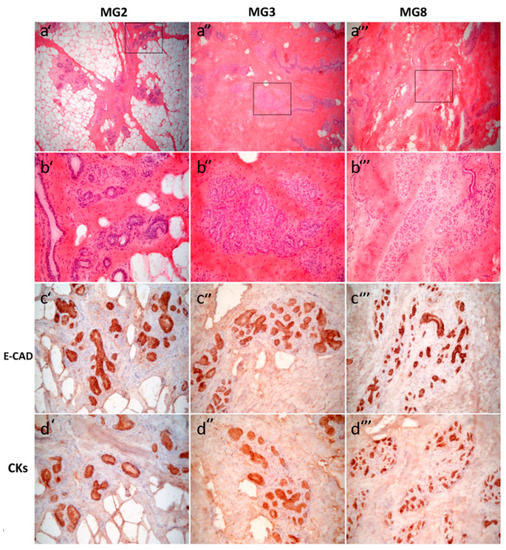
Figure 1.
Histological and immunohistochemical features of abdominal porcine mammary glands MG2 (a’, b’, c’, d’), MG3 (a’’, b’’, c’’, d’’) and MG8 (a’’’, b’’’, c’’’, d’’’). Hematoxylin and eosin (H&E) (a’, a’’, a’’’) 6.3× objective and (b’, b’’, b’’’) 25× objective. Rectangles in (a’, a’’, a’’’) indicate areas magnified in (b’, b’’, b’’’, respectively). All three tissue samples show resting mammary gland with glandular parenchyma showing interlobular and intralobular ducts and, mainly in MG3 (a’’, b’’), intralobular branching ducts lacking in MG2 (a’, b’). There is a variable extent of adipose tissue among samples (MG2, a’) or dense collagen stroma (MG3 and MG8, a’’ and a’’’, respectively). By immunohistochemistry, an epithelial E-Cad/panCK positive phenotype is evident in all the three MG samples (c’, c’’, c’’’ and d’, d’’, d’’’, respectively).
3.2. pMECs Morphology, Expansion and Epithelial Marker Expression
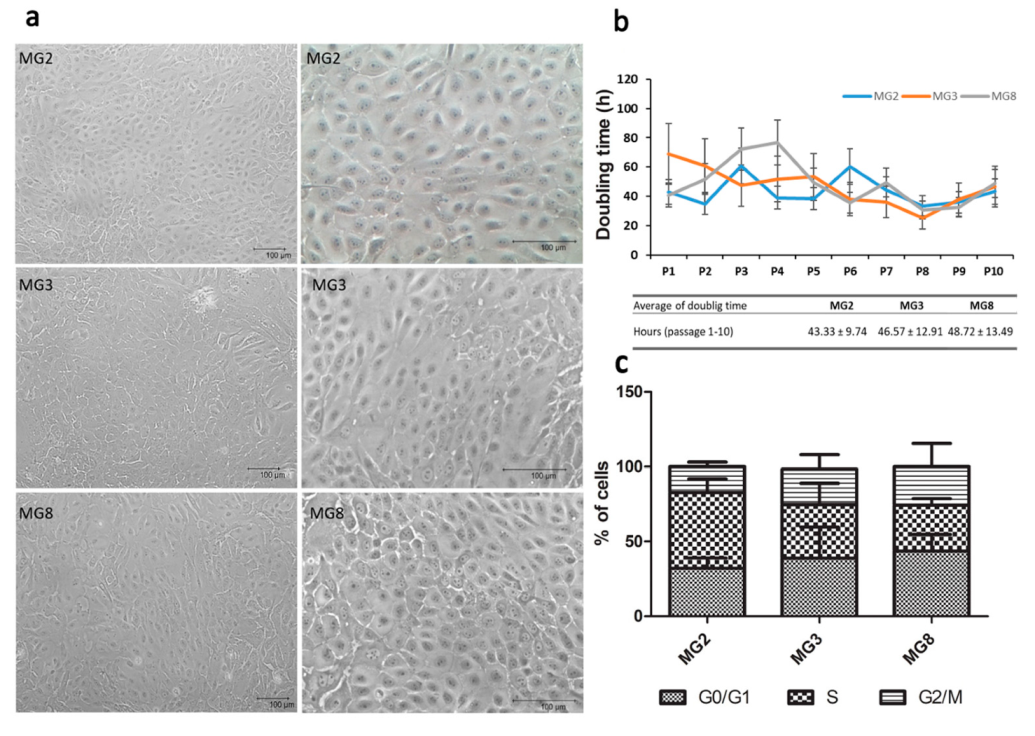
Primary cells were obtained from all three animals with different quantitative rates: 1.1 × 105 cells/g of tissue from MG2 and MG8; 2.1 × 105 cells/g of tissue from MG3. Cells were isolated and expanded until P10 showed a typical cobblestone morphology (Figure 2a) that was maintained through the different passages. However, in higher passages “lumen like structures” were present in all three primary cell lines, more frequently in MG8 pMECs (Figure S1). All three primary cell lines were detected as negative for mycoplasma contamination during expansion. Doubling time data indicated that cells grew with a similar rate (Figure 2b). The DNA index was normal for all three cell populations, with a mean value of 0.996 ± 0.047. Regarding cell cycle, selected single populations show the three distinct phases that could be recognized in a proliferating cell population. Statistically significant difference was not observed between the three primary lines regarding cellular distribution in the different phases of the cell cycle (Figure 2c).
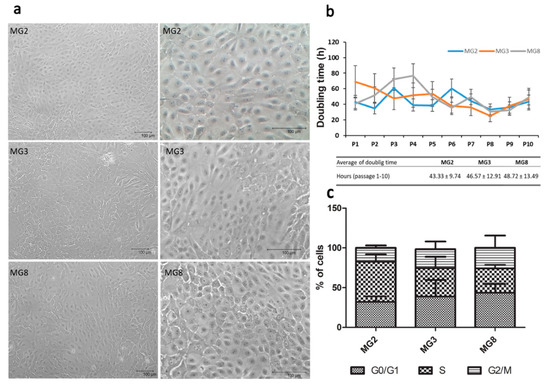
Figure 2.
pMECs morphology, doubling time and distribution across cell cycle phases. (a) Representative images of cell morphology after expansion, scale bar = 100 μm; (b) pMEC growth curve (passage 1 to 10) for each cell line is represented, doubling time is expressed in hours, table data represent the mean ± SEM of the duplication time from P1 to P10 for each cell line. (c) Stacked bar chart shows cellular distribution across cell cycle phases (G0/G1, S and G2/M). Data were analyzed with a one-way analysis of variance (ANOVA) followed by the post hoc Tukey’s multiple comparison test (p < 0.05) (n = 3).
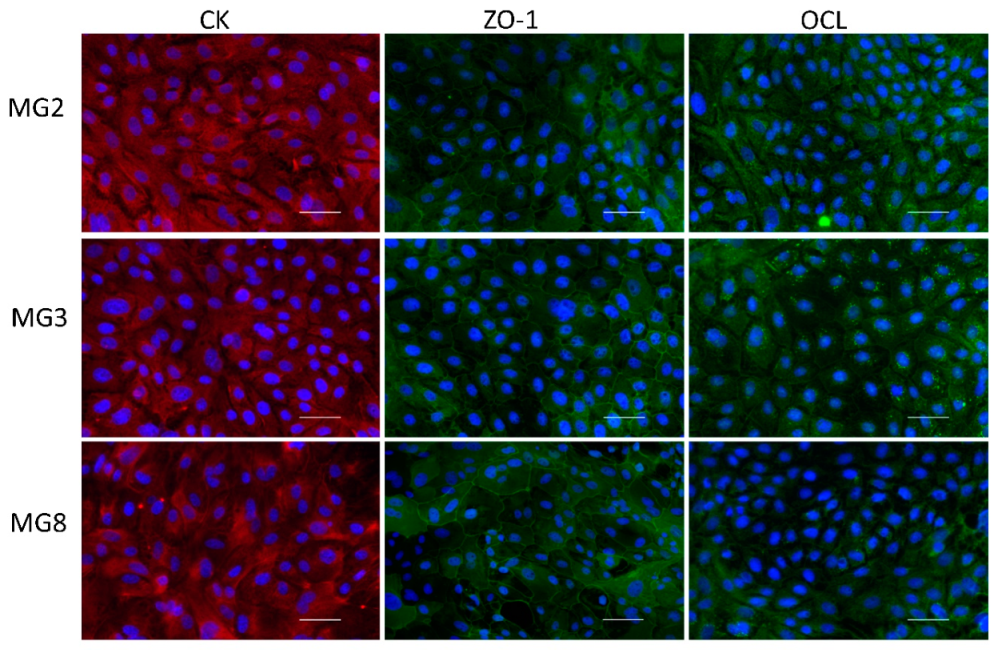
Immunofluorescence analysis of typical epithelial markers indicated that pMECs from the three animals showed a diffuse positivity to the pan-cytokeratin antibody with correct cytoplasmatic localization. Moreover, immunofluorescence of the two characteristic tight junctions’ molecules ZO-1 and Occludin showed a positive signal, correctly distributed partly in the cytoplasm and partly on the membrane at intercellular junction points (Figure 3).
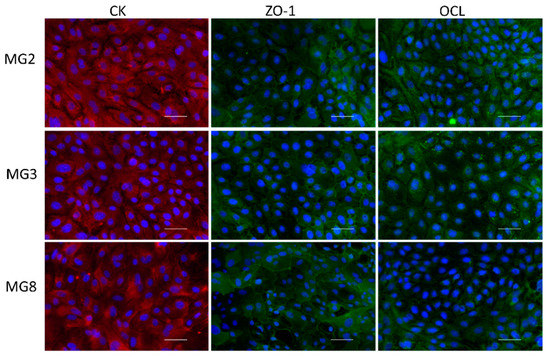
Figure 3.
Immunofluorescence analysis of Citokeratins (CKs), Zonula Occludens-1 (ZO-1), Occludin (OCL) for MG2, MG3 and MG8 pMEC lines. Images of pMECs in the first column are representative of cells marked with the mammary epithelial CK (red); images in the other two panel columns are representative of cells marked with the tight junction proteins ZO-1 and OCL respectively (green). Nuclei are stained with DAPI (blue), scale bar = 100 µm.
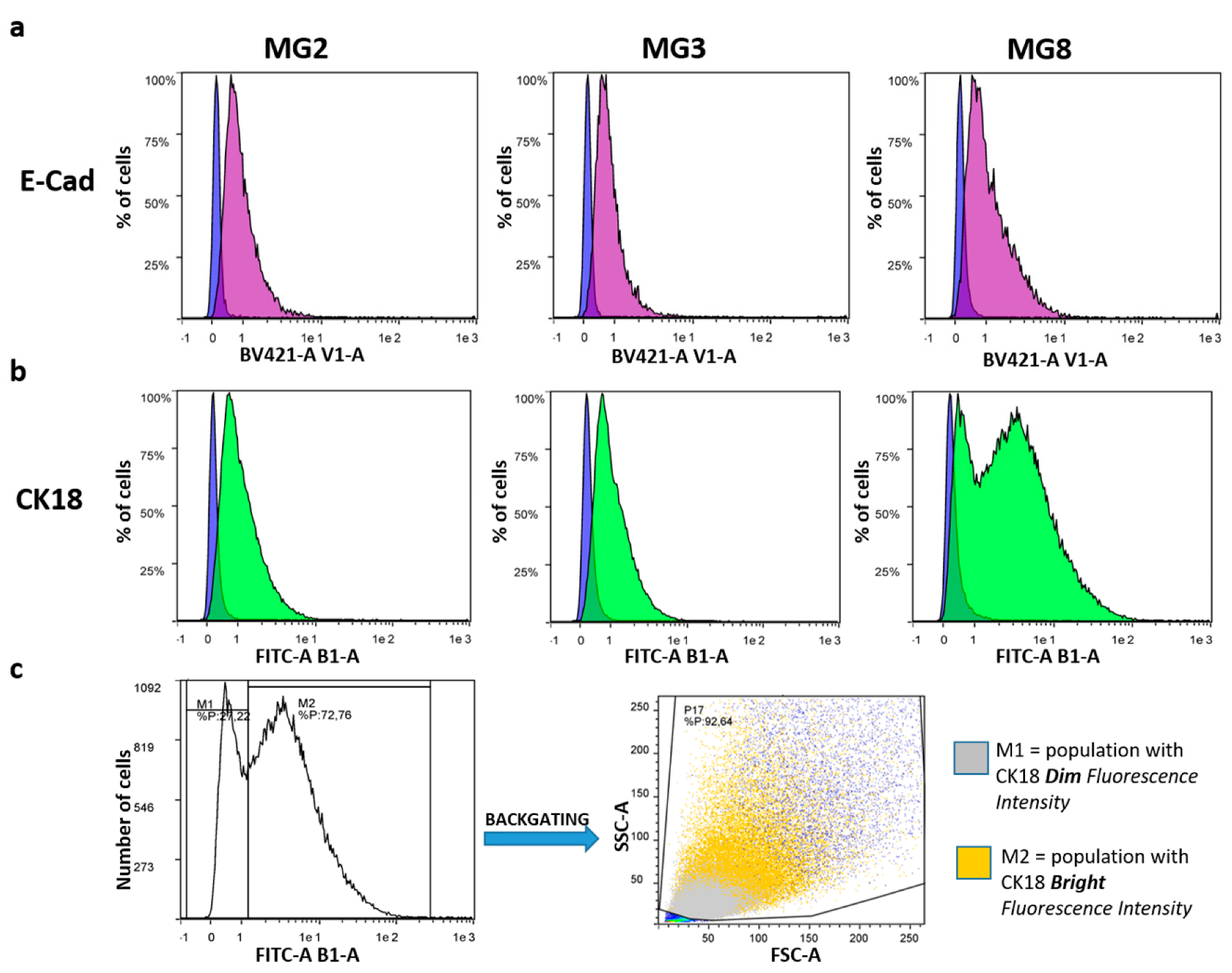
Furthermore, quantitative flow cytometry analysis showed that all three cell cultures expressed epithelial markers, such as the cell adhesion protein E-Cad and the specific intermediate filament protein CK18. Specifically concerning E-Cad, the positive peaks had a dim fluorescence intensity but appeared unimodal, indicating weak positivity of the entire cellular population (Figure 4a). On the other hand, CK18 showed a dim fluorescence intensity peak in all cellular samples (Figure 4b). However, in MG8 pMECs, the fluorescence peak appeared bimodal, with one population having a dim fluorescence intensity and another one with a bright fluorescence intensity. Analyzing the peak with a back-gating strategy, it was evident that MG8 pMECs consisted of two distinct populations: one with a dim CK18 fluorescence intensity, composed of smaller and less complex cells, and the other with a bright CK18 fluorescence intensity, composed of larger and more complex cells (Figure 4c).
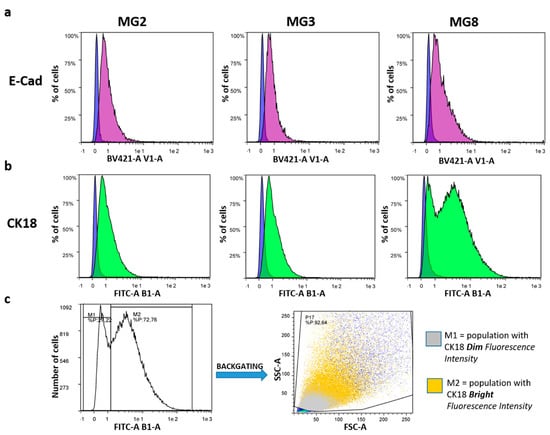
Figure 4.
Flow cytometric analysis of porcine epithelial markers, E-cad and CK18. (a) Each graph shows the percentage of cells expressing E-Cad (purple Area Under the Curve—AUC) and the relative negative control (blue AUC, cells not incubated with any antibodies). (b) Each graph shows the percentage of cells expressing CK18 (green AUC) and the relative negative control (blue AUC, cells not incubated with any antibodies). (c) CK18 bimodal fluorescence peak in MG8: M1 is the fraction with a dim fluorescence intensity, and M2 the fraction with a bright fluorescence intensity. Back-gating strategy allows the visualization of M1 and M2 in the cytogram (FSC-A vs SSC-A) of MG8 cells.
3.3. pMECs Barrier Function
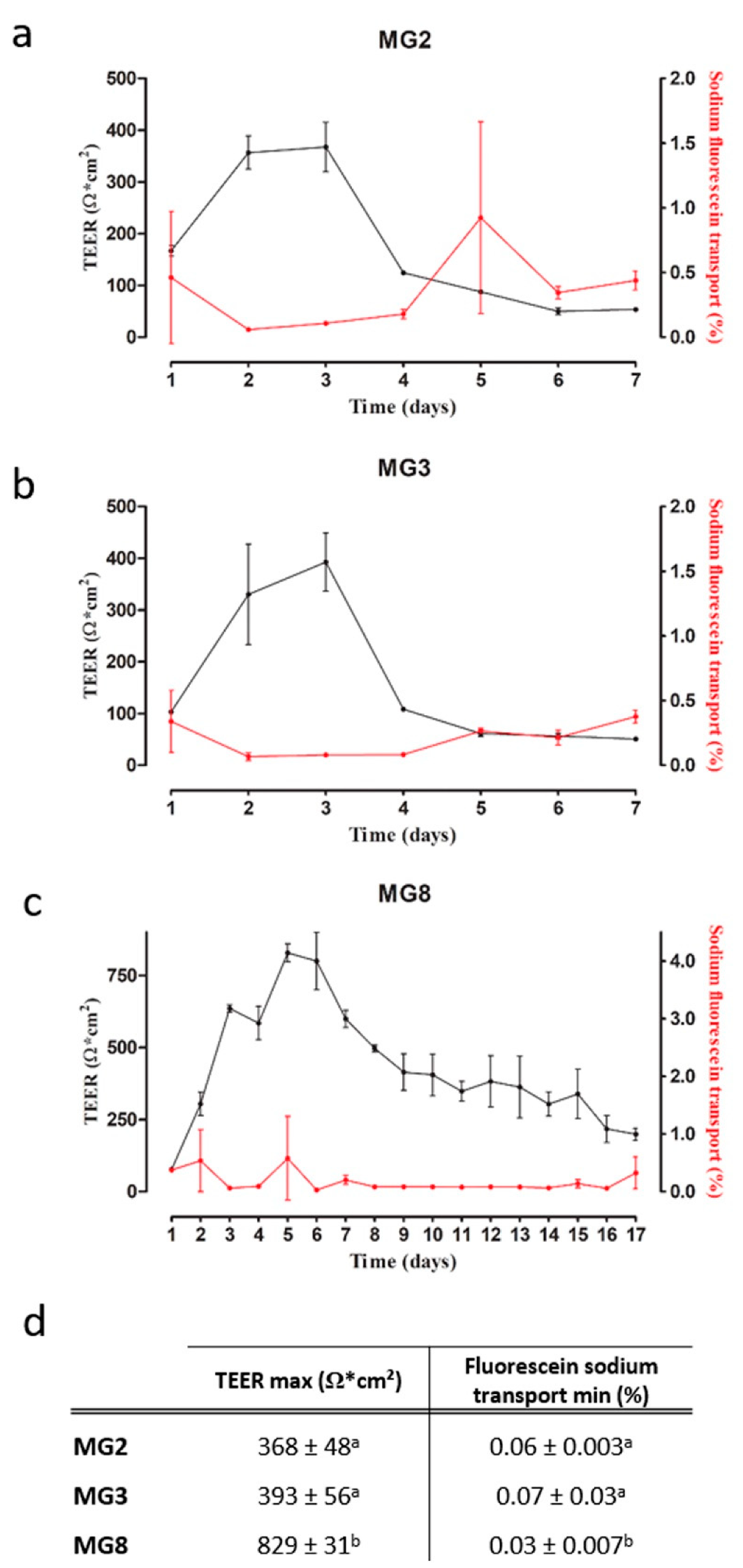
The barrier function of pMECs was evaluated via TEER and fluorescein sodium transport. TEER is a well-established method to evaluate the integrity of epithelial monolayers via measurement of the ionic conductance of the paracellular pathway [26], whereas fluorescein sodium is used to measure the passive paracellular transport [27]. For MG2 and MG3 pMECs, a plateau in TEER values (+/− 350–400 Ω × cm2) was already reached after two to three days, followed by a quick drop in TEER values on day four. Similarly, fluorescein sodium transport reached values around 0.06% at day two, and increased again after day four (Figure 5a,b). MG8 pMECs showed a different pattern in both TEER and fluorescein sodium transport: the initial increase in TEER was slower, a maximum of 829 Ω × cm2 was reached at the day 5 (Figure 5c). Afterwards, TEER values also decreased, but were still higher than the maximal values reached in MG2 (368 Ω × cm2) and MG3 (393 Ω × cm2) for up to 15 days. Similarly, fluorescein sodium transport was low in a period starting from day 3 up to day 16 (Figure 5c). Furthermore, statistically significant differences are evident in TEER maximum values and in fluorescein sodium transport minimum values reached between MG2, MG3 and MG8 (Figure 5d).
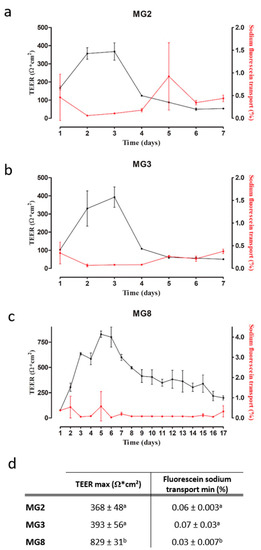
Figure 5.
pMECs barrier function analysis via TEER and fluorescein sodium transport. (a–c) TEER (black) and sodium fluorescein transport (red) trends over the time (days) of MG2, MG3 and MG8, respectively. (d) TEER max and fluorescein sodium transport min data of MG2, MG3 and MG8 represented as the mean ± SD. The letters above indicate the differences between the three cell lines. Data were analyzed with a one-way analysis of variance (ANOVA) followed by the post hoc Tukey’s multiple comparison test (p < 0.05) (n = 3).
3.4. pMECs Drug Transporter Expression
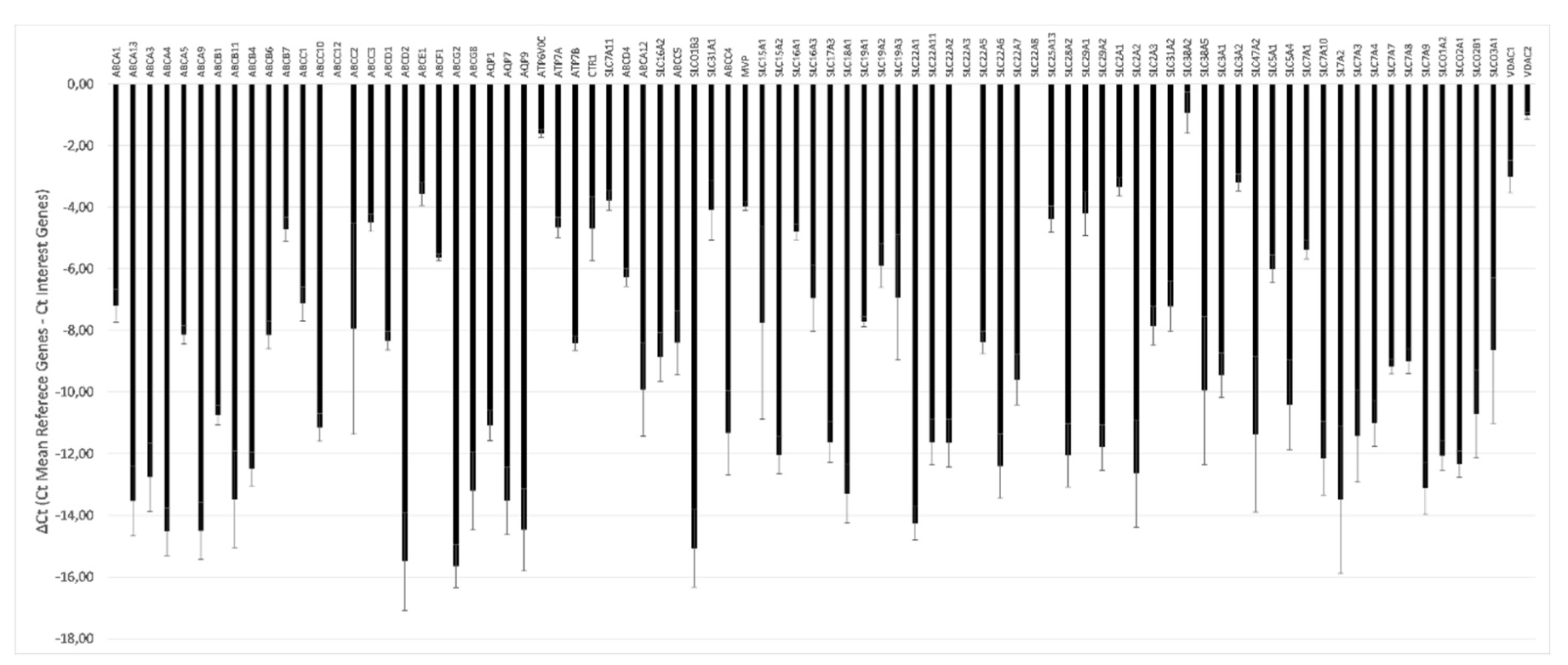
The transcriptional profile of drug transporters showed a differential level of gene expression, ranging from ΔCt values that were very negative (lower expression) to ΔCt values that were less negative (higher expression) (Figure 6).
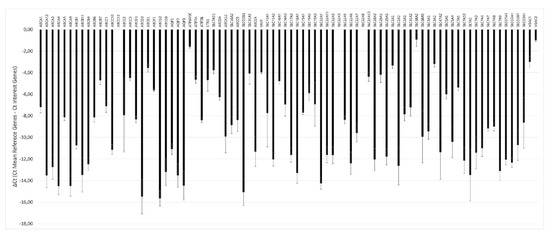
Figure 6.
Transcriptional profile of drug transporters in the pMECs lines. Eighty-four genes of the RT2 Profiler™ PCR Array Pig Drug Transporters were represented. The relative gene expression was calculated as ΔCt (mean reference genes Ct- interest genes Ct) ± SD (n = 3).
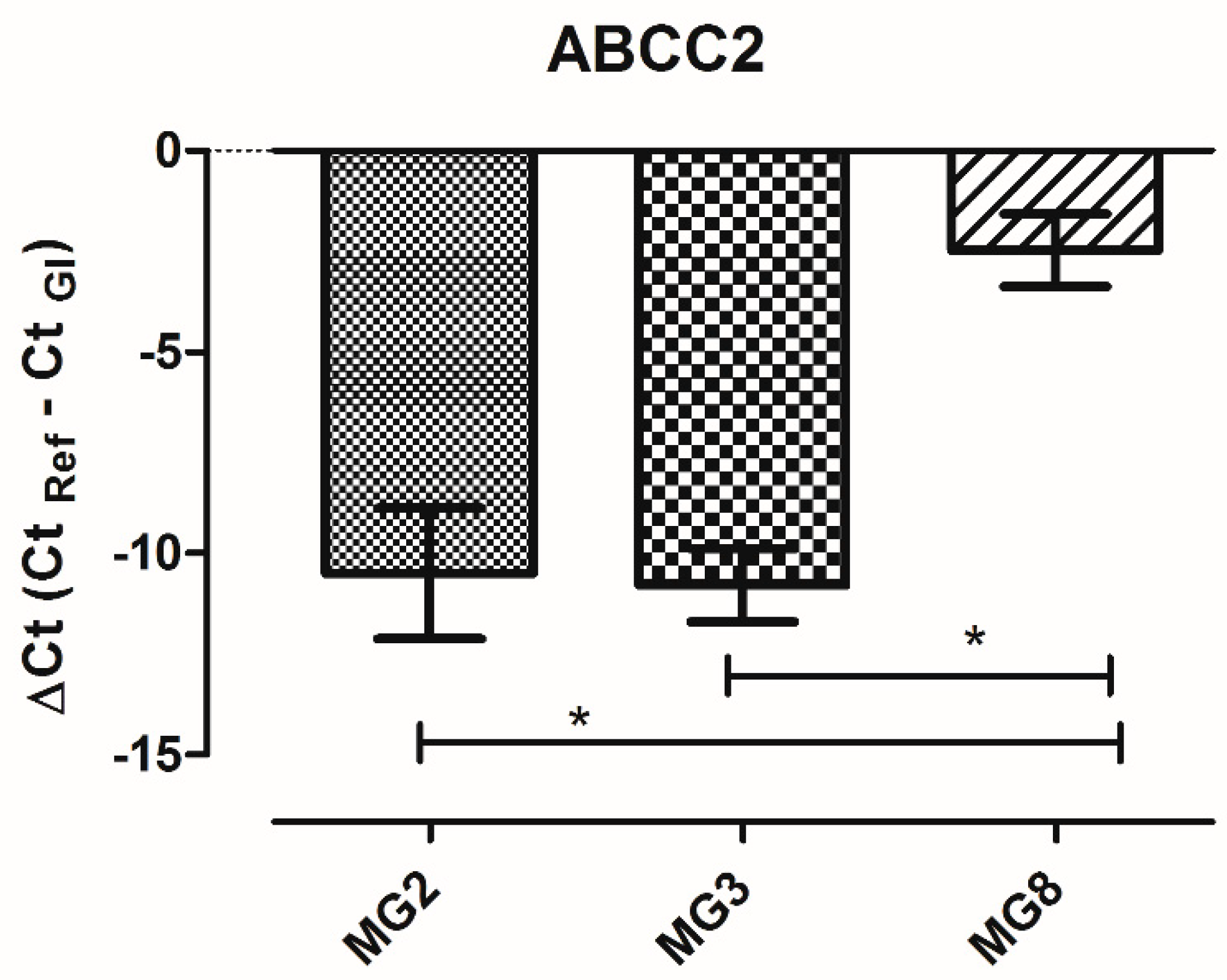
Among the 84 analyzed genes, only three were not detectable (ABCC12; SLC22A3; SLC22A8). The level of expression of each gene is similar among the three animals. The only gene that shows a variation in ΔCt higher than five cycles among animals is ABCC2. A single qPCR reaction confirmed the statistically significant higher expression of ABCC2 gene in MG8 pMECs respect to the other cell lines (Figure 7).
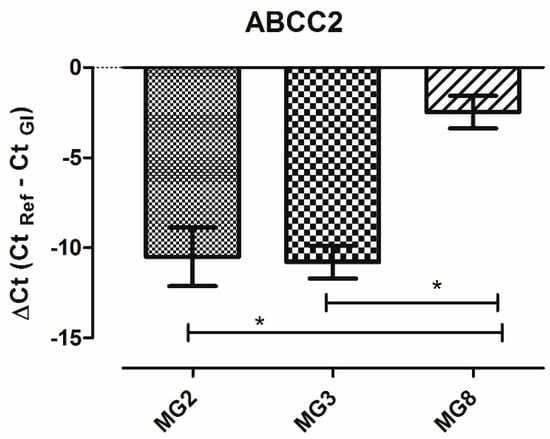
Figure 7.
ABCC2 gene expression in pMECs data represents the mean (ΔCt) ± SD. The data were analyzed using one-way ANOVA. Significant differences are indicated by * p < 0.05 (n = 3).
4. Discussion
In the present study, we successfully developed a new method to produce pig mammary cell cultures for studying the epithelial barrier. This work is part of the IMI funded ConcePTION Project, which aims at generating further knowledge about the use of medication during pregnancy and breastfeeding, as this information is lacking for most commonly used drugs [1].
The ConcePTION consortium, taking into account multiple factors, considered the pig as the most appropriate choice of translational animal model for project purposes [3] because of physiological, anatomical and metabolic similarities with humans [2].
Currently, no companies produce primary mammary cell lines of porcine species, therefore the first goal of the present study was to develop a reproducible method of pMEC isolation and culture. To be compliant with both international legislations and the 3Rs ethical principles [28], the protocol was based on mammary tissue collected from a local slaughterhouse, which implies that the starting material could be variable. Histological investigation showed that all three tissue samples presented resting mammary glands: the secretive component (alveoli) of the gland was absent, but its potential production depends on the amount of branching ducts available [29,30]. Immunohistochemistry provided objective evidence that the epithelial component of the samples was mainly represented by epithelial cells of ducts (E-cad and pan-CK positive) instead of cells with obvious secretive phenotypes (E-cad positive but pan-CK positive associated with an intraluminal secretory component).
Regardless of tissue variability, the method that we have developed has allowed us to obtain primary cell cultures from three different animals, albeit with different quantitative percentage. The highest number of cells was obtained from MG3 and this data agrees with the indications of histology and immunohistochemistry, evidencing the highest amounts of branching intralobular ducts in this sample. The three cell line populations were successfully expanded till the 10th passage, overall data obtained from doubling time and cell cycle analysis indicated that these cells are compatible with a normal diploid proliferating population. All the three pMEC populations grew in a monolayer and exhibited typical epithelial-like morphology, in agreement with those previously obtained by Dahanayaka et al., [15] and Zheng [13].
Our results indicated that the three populations express markers typical of mammary epithelial cells: cytokeratins and epithelial cadherin. Furthermore, the flow cytometric investigation of cytokeratin 18 has allowed us to identify two subpopulations in MG8 pMECs, expressing a different intensity of cytokeratin 18. This molecule has been recognized by many authors as a specific marker for mammary epithelial cells with a highly elevated secretory profile, in many different species [21,31,32] including the pig [15]. Further investigations using final hormonal stimulation will be necessary to clarify this intriguing aspect. Overall, these results indicate that the present pMECs isolation method allows us to efficiently obtain a pure population of mammary epithelial cells. The use of gentamycin from the early stages of culture permitted the induction of fibroblast apoptosis, while MECs showed an intrinsic resistance [17,18] and the choice of culturing MECs in suspension [19,20,21] for the first day turned out to obtain the purity required.
A tight monolayer is required to evaluate transport across biological barrier including the mammary one [8]. A fundamental point supporting our model is that all three lines expressed the two important proteins Occludin and ZO-1, which make up the tight junctions that are foundational for the barrier role [33,34]. Then, the three primary cell lines obtained were tested for the ability to create a tight monolayer by TEER measurement [26]. The cells grew on PET transwell and created a tight barrier, although quantitative and kinetically different patterns existed among the three cell lines. In particular, MG2 and MG3 pMECs showed similar behavior while MG8 seemed to form a more compact barrier which remained undamaged for a longer time. These data were also confirmed by the fluorescein transport evaluation.
To our knowledge, there are no other published data relative to the TEER measurement of pMECs in vitro culture, however we can compare our results with those obtained by Kimura et al. [8] in humans. In this paper, the authors evidenced that only a selected population of trypsin-resistant hMECs were able to create a tight monolayer. It will certainly needs to be clarified if this is a species-specific difference, or is related to our method of selection that directly collected only pMECs with an intrinsic ability to form a barrier.
Another fundamental aspect is the reciprocal validation of the models obtained by integrating in vitro with the in vivo results within ConcePTION planned future activities. This will enable us to demonstrate if this intrinsic facility to form a strong barrier depends on a species-specific difference.
Finally, we have to consider that the activity of a functional barrier depends on the expression of specific transporters molecules [35]. Indeed, detailed reviews on the expression of transporters in the epithelial cells of mammary glands have been published [2,36,37,38,39]. Therefore, we characterized the drug transporter gene expression profile of the three cell lines by an array approach that includes 84 genes, belonging to the different categories of transporter families. Our results indicated that all the three lines expressed the studied genes, except the genes ABCC12 that code for multidrug resistance-associated protein 9, and the two genes SLC22A3 and SLC22A8 coding for ions transporter proteins. The most expressed genes are ABCE1, belonging to the ABC cassette family, which codes for the ATP-binding cassette sub-family E member 1, and SLC38A2, belonging to the solute carrier transporters family, which codes for sodium-dependent amino acid transporter protein.
The gene ABCC2, which codes for multidrug resistance-associated protein 2 (MRP2), was the only one with a high variation of expression among animals, showing a statistically significant higher expression in pMECs MG8, possibly in relation to sub-population complexity shown by flow cytometric investigation.
5. Conclusions
In conclusion, in the present paper we have reported an efficient method to obtain, from slaughtered pigs, primary mammary epithelial cells cultures as a model to study epithelial barrier function. pMECs could be utilized in pre-screening studies leading to the reduction of animals utilized for research. Overall, we believe that the results presented in this article are an excellent basis for future studies for lactational drug transfer.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11072012/s1, Figure S1: Representative images of “lumen like structure” in MG8 pMECs.
Author Contributions
C.B., M.F. conception and design of the work; C.B., D.L.M., R.S., A.Z., N.N., N.D., D.V., M.L.B., G.S.: experiments, acquisition analysis of data. C.B., M.F., G.S., M.B.-L., P.A.: interpretation of data. C.B. and M.F. drafted the work and substantively revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The ConcePTION project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 821520. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA. This work was supported also by the Research Foundation—Flanders (1S50721N).
Institutional Review Board Statement
All the biological materials used in this study were obtained from animals slaughtered for food purposes and no ethical approval was necessary.
Data Availability Statement
Data are available on AMSActa Institutional Research Repository by AlmaDL University of Bologna Digital Library: 10.6092/unibo/amsacta/6660.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ConcePTION Homepage. Available online: https://www.imi-conception.eu/ (accessed on 25 February 2021).
- Ventrella, D.; Forni, M.; Bacci, M.L.; Annaert, P. Non-clinical Models to Determine Drug Passage into Human Breast Milk. Curr. Pharm. Des. 2019, 25, 534–548. [Google Scholar] [CrossRef]
- ConcePTION, D3.2 Report on Lactation Characteristics of Animal Species; Selection of the Animal Species to be Used in In Vivo Studies. Available online: https://www.imi-conception.eu/wp-content/uploads/2020/04/ConcePTION-D3.2-Report-on-lactation-characteristics-of-animal-species-Selection-of-the-animal-species-to-be-used-in-in-vivo-studies.pdf (accessed on 25 February 2021).
- Ventrella, D.; Ashkenazi, N.; Elmi, A.; Allegaert, K.; Aniballi, C.; DeLise, A.; Devine, P.; Smits, A.; Steiner, L.; Forni, M.; et al. Animal Models for In Vivo Lactation Studies: Anatomy, Physiology and Milk Compositions in the Most Used Non-Clinical Species: A Contribution from the ConcePTION Project. Animals 2021, 11, 714. [Google Scholar] [CrossRef]
- McManaman, J.L.; Neville, M.C. Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 2003, 55, 629–641. [Google Scholar] [CrossRef]
- Hurley, W.L. Review: Mammary gland development in swine: Embryo to early lactation. Animal 2019, 13, s11–s19. [Google Scholar] [CrossRef] [PubMed]
- Toddywalla, V.S.; Kari, F.W.; Neville, M.C. Active transport of nitrofurantoin across a mouse mammary epithelial monolayer. J. Pharmacol. Exp. Ther. 1997, 280, 669–676. [Google Scholar]
- Kimura, S.; Morimoto, K.; Okamoto, H.; Ueda, H.; Kobayashi, D.; Kobayashi, J.; Morimoto, Y. Development of a human mammary epithelial cell culture model for evaluation of drug transfer into milk. Arch. Pharmacal Res. 2006, 29, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.M.; Pai, V.P.; Sartor, M.A.; Horseman, N.D. In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res. 2009, 335, 383–395. [Google Scholar] [CrossRef]
- Andersson, M.; Ersson, L.; Brandt, I.; Bergström, U. Potential transfer of neurotoxic amino acid β- N -methylamino-alanine (BMAA) from mother to infant during breast-feeding: Predictions from human cell lines. Toxicol. Appl. Pharmacol. 2017, 320, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Nauwelaerts, N.; Deferm, N.; Smits, A.; Bernardini, C.; Lammens, B.; Gandia, P.; Panchaud, A.; Nordeng, H.; Bacci, M.L.; Forni, M.; et al. A comprehensive review on non-clinical methods to study transfer of medication into breast milk – A contribution from the ConcePTION project. Biomed. Pharmacother. 2021, 136, 111038. [Google Scholar] [CrossRef]
- Kumura, H.; Tanaka, A.; Abo, Y.; Yui, S.; Shimazaki, K.-I.; Kobayashi, E.; Sayama, K. Primary Culture of Porcine Mammary Epithelial Cells as a Model System for Evaluation of Milk Protein Expression. Biosci. Biotechnol. Biochem. 2001, 65, 2098–2101. [Google Scholar] [CrossRef]
- Zheng, Y.-M.; He, X.-Y. Characteristics and EGFP expression of porcine mammary gland epithelial cells. Res. Vet. Sci. 2010, 89, 383–390. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, C.; Chou, Y. Establishment and characterization of a spontaneously immortalized porcine mammary epithelial cell line. Cell Biol. Int. 2006, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Dahanayaka, S.; Rezaei, R.; Porter, W.W.; Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Hou, Y.Q.; Wu, Z.L.; Wu, G. Technical note: Isolation and characterization of porcine mammary epithelial cells. J. Anim. Sci. 2015, 93, 5186–5193. [Google Scholar] [CrossRef]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific PurposesText with EEA Relevance. 47. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Murtagh, J.; McArdle, E.; Gilligan, E.; Thornton, L.; Furlong, F.; Martin, F. Organization of mammary epithelial cells into 3D acinar structures requires glucocorticoid and JNK signaling. J. Cell Biol. 2004, 166, 133–143. [Google Scholar] [CrossRef]
- Servais, H.; Van Der Smissen, P.; Thirion, G.; Van Der Essen, G.; Van Bambeke, F.; Tulkens, P.M.; Mingeot-Leclercq, M.-P. Gentamicin-induced apoptosis in LLC-PK1 cells: Involvement of lysosomes and mitochondria. Toxicol. Appl. Pharmacol. 2005, 206, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Dong, Q.; Sun, L.-Z. Stem/Progenitor Cells in Murine Mammary Gland: Isolation and Functional Characterization. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 879, pp. 179–193. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, D.; Bandyopadhyay, A.; Gao, H.; Gorena, K.M.; Hildreth, K.; Rebel, V.I.; Walter, C.A.; Huang, C.; Sun, L.-Z. Mammospheres from murine mammary stem cell-enriched basal cells: Clonal characteristics and repopulating potential. Stem Cell Res. 2013, 10, 396–404. [Google Scholar] [CrossRef]
- Ogorevc, J.; Zorc, M.; Dovč, P. Development of an In Vitro Goat Mammary Gland Model: Establishment, Characterization, and Applications of Primary Goat Mammary Cell Cultures. In Goat Science; IntechOpen: London, UK, 2018. [Google Scholar]
- Zaniboni, A.; Bernardini, C.; Alessandri, M.; Mangano, C.; Zannoni, A.; Bianchi, F.; Sarli, G.; Calzà, L.; Bacci, M.L.; Forni, M. Cells derived from porcine aorta tunica media show mesenchymal stromal-like cell properties in in vitro culture. Am. J. Physiol. Cell Physiol. 2014, 306, C322–C333. [Google Scholar] [CrossRef][Green Version]
- Levi, M.; Salaroli, R.; Parenti, F.; De Maria, R.; Zannoni, A.; Bernardini, C.; Gola, C.; Brocco, A.; Marangio, A.; Benazzi, C.; et al. Doxorubicin treatment modulates chemoresistance and affects the cell cycle in two canine mammary tumour cell lines. BMC Vet. Res. 2021, 17, 1–15. [Google Scholar] [CrossRef]
- Bernardini, C.; Algieri, C.; Mantia, L.D.; Zannoni, A.; Salaroli, R.; Trombetti, F.; Forni, M.; Pagliarani, A.; Nesci, S. Relationship between serum concentration, functional parameters and cell bioenergetics in IPEC-J2 cell line. Histochem. Cell Biol. 2021, 1–9. [Google Scholar] [CrossRef]
- Tubon, I.; Zannoni, A.; Bernardini, C.; Salaroli, R.; Bertocchi, M.; Mandrioli, R.; Vinueza, D.; Antognoni, F.; Forni, M. In Vitro Anti-Inflammatory Effect of Salvia sagittata Ethanolic Extract on Primary Cultures of Porcine Aortic Endothelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6829173. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Ingels, F.; Deferme, S.; Delbar, N.; Oth, M.; Augustijns, P. Implementation of the caco-2 cell culture model as a predictive tool for the oral absorption of drugs. In-house evaluation procedures. J. Pharm. Belg. 2003, 57, 153–158. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co Ltd.: London, UK, 1959. [Google Scholar]
- Sternlicht, M.D. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2005, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Oakes, S.; Hilton, H.N.; Ormandy, C.J. Key stages in mammary gland development - The alveolar switch: Coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res. 2006, 8, 207. [Google Scholar] [CrossRef]
- Moll, R.; Franke, W.W.; Schiller, D.L.; Geiger, B.; Krepler, R. The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982, 31, 11–24. [Google Scholar] [CrossRef]
- Diego, C.; Eugenio, M.; Silvia, M.; Elisabetta, M.; Paolo, A.; Mario, B. Bovine mammary epithelial cells retain stem-like phenotype in long-term cultures. Res. Vet. Sci. 2014, 97, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Molecular Structure of Tight Junctions and Their Role in Epithelial Transport. Physiology 2001, 16, 126–130. [Google Scholar] [CrossRef]
- Stelwagen, K.; Singh, K. The Role of Tight Junctions in Mammary Gland Function. J. Mammary Gland. Biol. Neoplasia 2013, 19, 131–138. [Google Scholar] [CrossRef]
- Kari, F.W.; Weaver, R.; Neville, M.C. Active transport of nitrofurantoin across the mammary epithelium in vivo. J. Pharmacol. Exp. Ther. 1997, 280, 664–668. [Google Scholar] [PubMed]
- Ito, S.; Alcorn, J. Xenobiotic transporter expression and function in the human mammary gland. Adv. Drug Deliv. Rev. 2003, 55, 653–665. [Google Scholar] [CrossRef]
- Dean, M. ABC Transporters, Drug Resistance, and Cancer Stem Cells. J. Mammary Gland. Biol. Neoplasia 2009, 14, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Vlaming, M.L.; Lagas, J.S.; Schinkel, A.H. Physiological and pharmacological roles of ABCG2 (BCRP): Recent findings in Abcg2 knockout mice. Adv. Drug Deliv. Rev. 2009, 61, 14–25. [Google Scholar] [CrossRef] [PubMed]
- García-Lino, A.M.; Fernández, I. Álvarez; Blanco-Paniagua, E.; Merino, G.; Álvarez, A.I. Transporters in the Mammary Gland—Contribution to Presence of Nutrients and Drugs into Milk. Nutrients 2019, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).