Age-Related Changes in the Primary Motor Cortex of Newborn to Adult Domestic Pig Sus scrofa domesticus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Localization of Motor Cortex and Tissue Preparation
2.3. Western Blot
2.4. Immunohistochemistry
2.5. Double Immunofluorescence
2.6. Morphometry and Statistical Analysis
3. Results
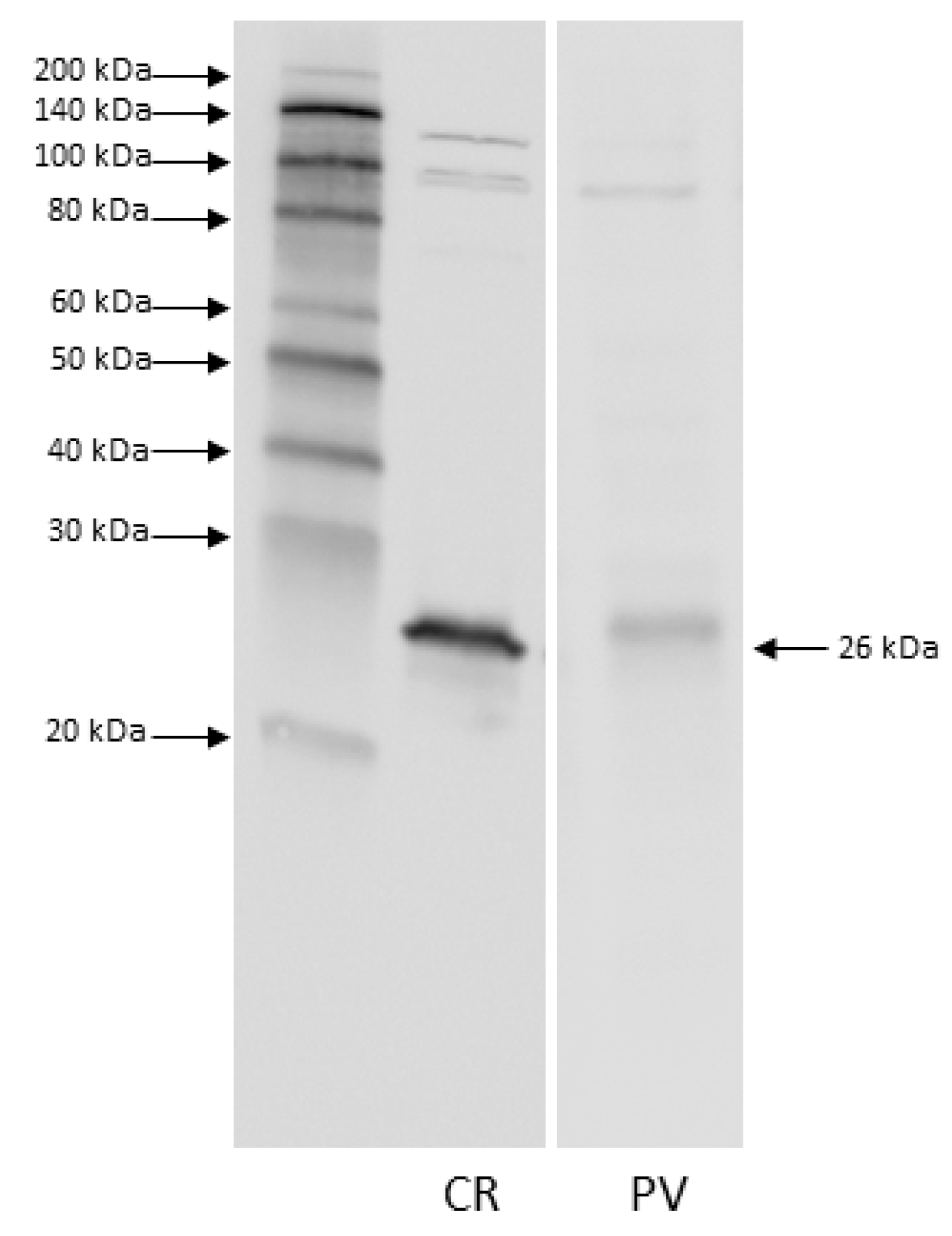
3.1. Western Blot
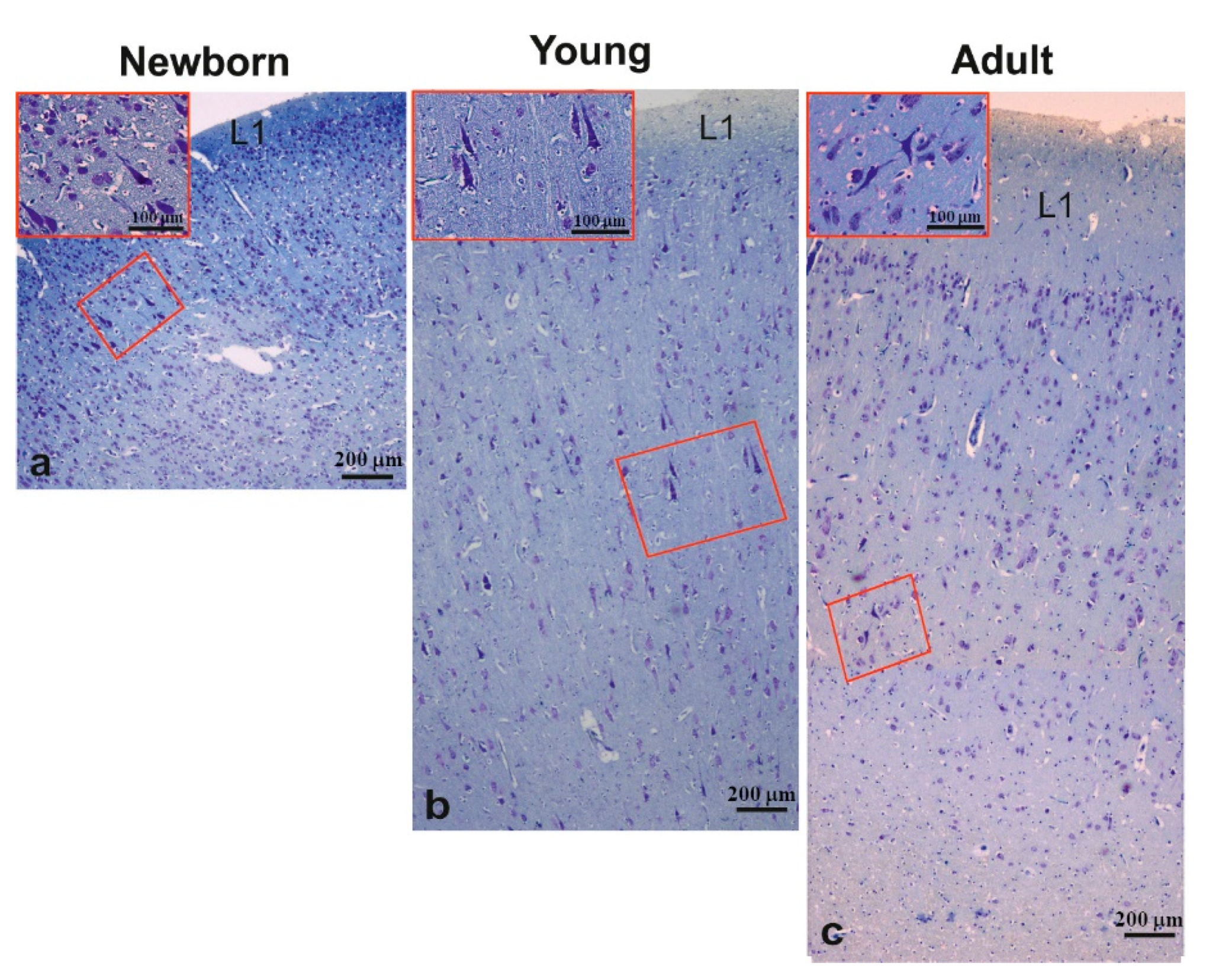
3.2. Morphology
3.3. Immunohistochemistry
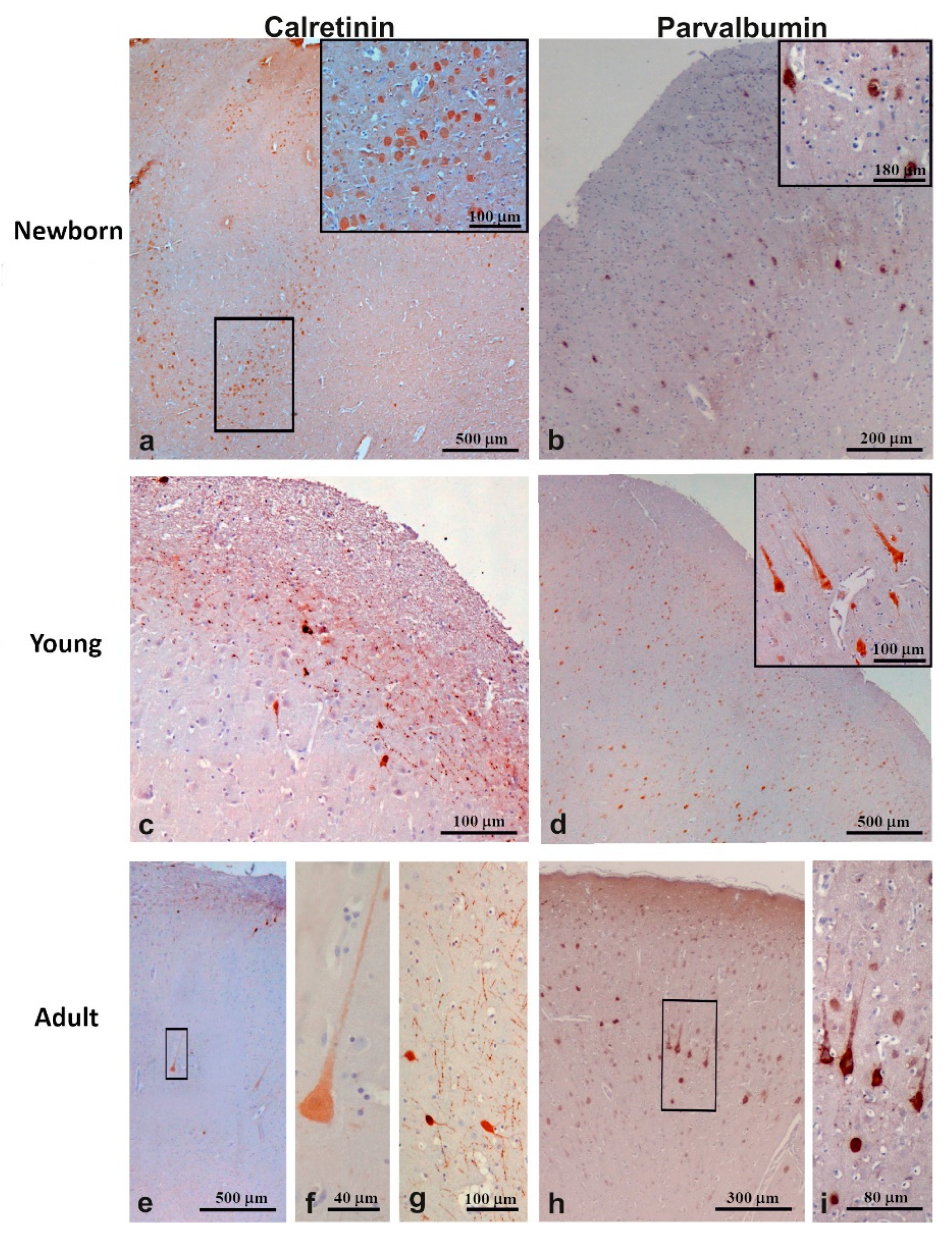
3.3.1. Calretinin
3.3.2. Parvalbumin
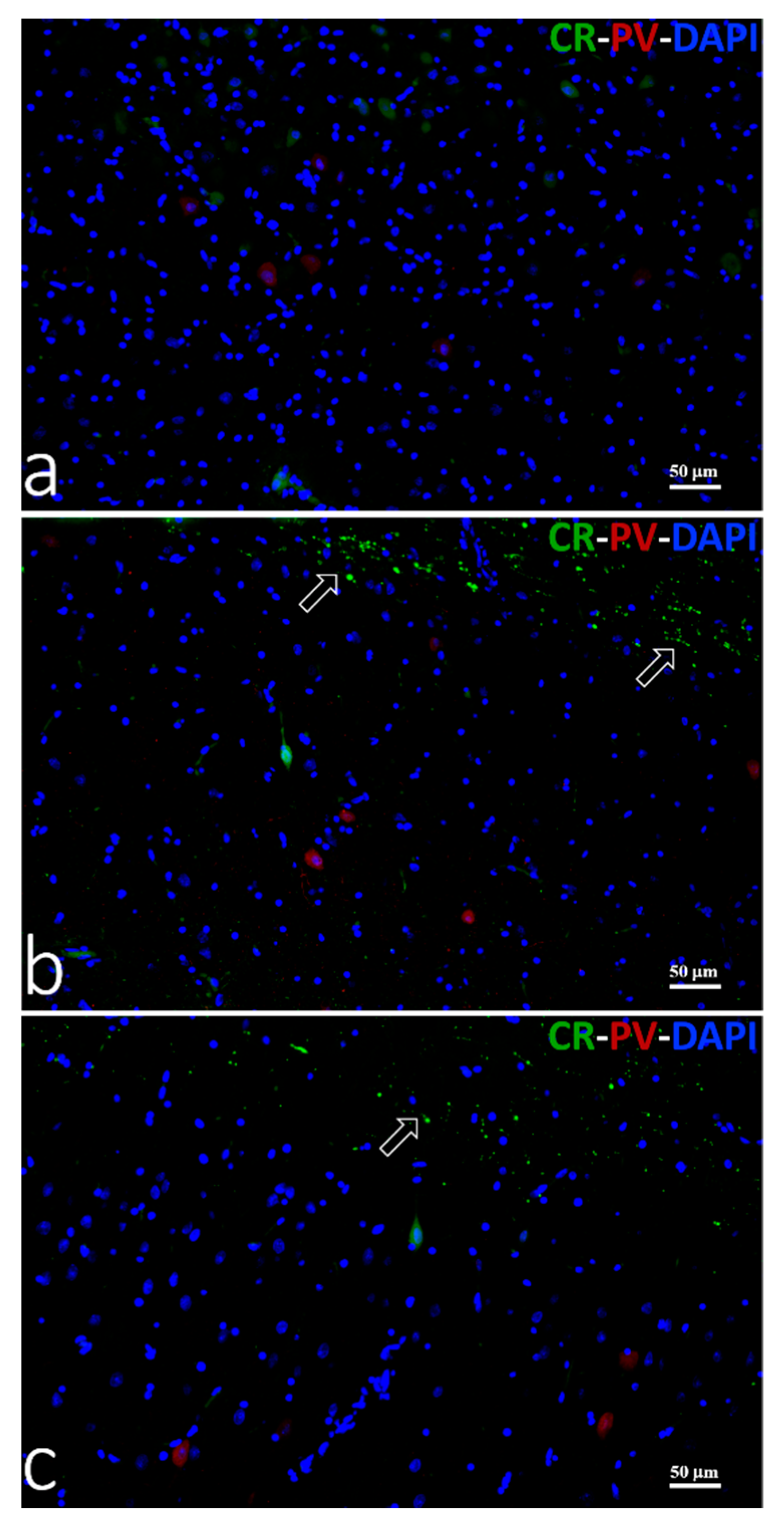
3.3.3. Double Immunofluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Manger, P.R. Establishing order at the systems level in mammalian brain evolution. Brain Res. Bull. 2005, 66, 282–289. [Google Scholar] [CrossRef]
- Raghanti, M.A.; Spocter, M.A.; Butti, C.; Hof, P.R.; Sherwood, C.C. A comparative perspective on minicolumns and inhibitory GABAergic interneurons in the neocortex. Front. Neuroanat. 2010, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Fritsch, G.; Hitzig, E. Uber die elektrische Erregbarkeitt des Grossshirns. Arch. Anat. Physiol. 1870, 87, 300–332. [Google Scholar]
- Campbell, A.W. Histological Studies on the Localization of Cerebral Function; University Press: Cambridge, UK, 1905. [Google Scholar]
- Breazile, J.E.; Swafford, B.C.; Thompson, W.D. Study of the motor cortex of the domestic pig. Am. J. Vet. Res. 1966, 27, 1369–1373. [Google Scholar]
- Palmieri, G.; Farina, V.; Panu, R.; Asole, A.; Sanna, L.; De Riu, P.L.; Gabbi, C. Course and termination of the pyramidal tract in the pig. Arch. Anat. Microsc. Morphol. Exp. 1987, 75, 167–176. [Google Scholar]
- Schmidt, V. Motor cortex. In Comparative Anatomy of the Pig Brain—An Integrative Magnetic Resonance Imaging (MRI) Study of the Porcine Brain with Special Emphasis on the External Morphology of the Cerebral Cortex, 1st ed.; Schmidt, V., Ed.; VVB Laufersweiler Verlag: Giessen, Germany, 2015; pp. 104–105. [Google Scholar]
- Bjarkam, C.R.; Glud, A.N.; Orlowski, D.; Sørensen, J.C.H.; Palomero-Gallagher, N. The telencephalon of the Göttingen minipig, cytoarchitecture and cortical surface anatomy. Brain. Struct. Funct. 2016, 222, 2093–2114. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, P.A. Cytoarchitectonic volumetric comparison of the area gigantopyramidalis in wild and domestic sheep. Anat. Embryol. 1975, 147, 167–175. [Google Scholar] [CrossRef]
- Kojima, T. On the brain of the sperm whale (Physeter catodon L.). Sci. Rep. Whales Res. Inst. 1951, 6, 49–72. [Google Scholar]
- Kesarev, V.S.; Malofeeva, L.I.; Trykova, O.V. Structural organization of the cerebral neocortex in cetaceans. Arkh. Anat. Gistol. Embriol. 1977, 73, 23–30. [Google Scholar] [PubMed]
- Hof, P.R.; Van der Gucht, E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat. Rec. 2007, 290, 1–31. [Google Scholar] [CrossRef]
- Badlangana, N.L.; Bhagwandin, A.; Fuxe, K.; Manger, P.R. Observations on the giraffe central nervous system related to the corticospinal tractmotor cortex and spinalcord: What difference does along neck make? Neuroscience 2007, 148, 522–534. [Google Scholar] [CrossRef] [PubMed]
- DeFelipe, J.; Fariñas, I. The pyramidal neuron of the cerebral cortex: Morphological and chemical characteristics of the synaptic inputs. Prog. Neurobiol. 1992, 39, 5632–607. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, Y.; Kubota, Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex. 1997, 7, 476–486. [Google Scholar] [CrossRef] [Green Version]
- Porter, L.L.; Matin, D.; Asaf, K. Characteristics of GABAergic neurons and their synaptic relationships with intrinsic axons in the cat motor cortex. Somatosens. Mot. Res. 2000, 17, 67–81. [Google Scholar]
- Barinka, F.; Druga, R. Calretinin expression in the mammalian neocortex: A review. Physiol. Res. 2010, 59, 665–677. [Google Scholar] [CrossRef]
- Baimbridge, K.G.; Celio, M.R.; Rogers, J.H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992, 15, 303–308. [Google Scholar] [CrossRef]
- Rogers, J.H. Calretinin: A gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 1987, 105, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polans, A.; Baehr, W.; Palczewski, K. Turned on by Ca2+! The physiology and pathology of Ca(2+)-binding proteins in the retina. Trends Neurosci. 1996, 19, 547–554. [Google Scholar] [CrossRef]
- Schäfer, B.W.; Heizmann, C.W. The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem. Sci. 1996, 21, 134–140. [Google Scholar] [CrossRef]
- Baldellon, C.; Alattia, J.R.; Strub, M.P.; Pauls, T.; Berchtold, M.W.; Cave, A.; Padilla, A. 15N NMR Relaxation studies of calcium-loaded parvalbumin show tight dynamics compared to those of other EF-hand proteins. Biochemistry 1998, 37, 9964–9975. [Google Scholar] [CrossRef] [PubMed]
- Clements, R.J.; Mcdonough, J.; Freeman, E.J. Distribution of parvalbumin and calretinin immunoreactive interneurons in motor cortex from multiple sclerosis post-mortem tissue. Exp. Brain. Res. 2008, 187, 459–465. [Google Scholar] [CrossRef]
- Celio, M.R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 1990, 35, 375–475. [Google Scholar] [CrossRef]
- Arai, R.; Winsky, L.; Arai, M.; Jacobowitz, D.M. Immunohistochemical localization of calretinin in the rat hindbrain. J. Comp. Neurol. 1991, 310, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Jacobowitz, D.M.; Winsky, L. Immunocytochemical localization of calretinin in the forebrain of the rat. J. Comp. Neurol. 1991, 304, 198–218. [Google Scholar] [CrossRef] [PubMed]
- Resibois, A.; Rogers, J.H. Calretinin in rat brain: An immunohistochemical study. Neuroscience 1991, 46, 101–134. [Google Scholar] [CrossRef]
- Van Brederode, J.F.; Helliesen, M.K.; Hendrickson, A.E. Distribution of the calcium-binding proteins parvalbumin and calbindin-D28k in the sensorimotor cortex of the rat. Neuroscience 1991, 44, 157–171. [Google Scholar] [CrossRef]
- Hof, P.R.; Glezer, I.I.; Condé, F.; Flagg, R.A.; Rubin, M.B.; Nimchinsky, E.A.; Vogt Weisenhorn, D.M. Cellular distribution of the calcium binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: Phylogenetic and developmental patterns. J. Chem. Neuroanat. 1999, 16, 77–116. [Google Scholar] [CrossRef]
- Bu, J.; Sathyendra, V.; Nagykery, N.; Geula, C. Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp. Neurol. 2003, 182, 220–231. [Google Scholar] [CrossRef]
- Litwinowicz, B.; Labuda, C.; Kowiański, P.; Spodnik, J.H.; Ludkiewicz, B.; Wójcik, S.; Moryś, J. Developmental pattern of calbindin D28k protein expression in the rat striatum and cerebral cortex. Folia Morphol. 2003, 62, 327–329. [Google Scholar]
- Abrahám, H.; Tóth, Z.; Bari, F.; Domoki, F.; Seress, L. Novel calretinin and reelin expressing neuronal population includes Cajal-Retzius-type cells in the neocortex of adult pigs. Neuroscience 2005, 136, 217–230. [Google Scholar] [CrossRef]
- Gonchar, Y.; Wang, Q.; Burkhalter, A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 2008, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.J.; Lee, J.C.; Kang, B.G.; An, J.; Song, H.S.; Son, O.; Nam, D.H.; Cha, C.I.; Joo, K.M. Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebral cortex and in the hippocampal region of nNOS knock-out(-/-) mice. Anat. Cell. Biol. 2011, 44, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirone, A.; Castagna, M.; Granato, A.; Peruffo, A.; Quilici, F.; Cavicchioli, L.; Piano, I.; Lenzi, C.; Cozzi, B. Expression of calcium-binding proteins and selected neuropeptides in the human, chimpanzee, and crab-eating macaque claustrum. Front. Syst. Neurosci. 2014, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirone, A.; Magliaro, C.; Giannessi, E.; Ahluwalia, A. Parvalbumin expression in the claustrum of the adult dog. An immunohistochemical and topographical study with comparative notes on the structure of the nucleus. J. Chem. Neuroanat. 2015, 64, 33–42. [Google Scholar] [CrossRef]
- Ahn, J.H.; Hong, S.; Park, J.H.; Kim, I.H.; Cho, J.H.; Lee, T.K.; Lee, J.C.; Chen, B.H.; Shin, B.N.; Bae, E.J.; et al. Immunoreactivities of calbindin-D28k, calretinin and parvalbumin in the somatosensory cortex of rodents during normal aging. Mol. Med. Rep. 2017, 16, 7191–7198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirone, A.; Miragliotta, V.; Cozzi, B.; Granato, A. The claustrum of the pig: An immunohistochemical and a quantitative Golgi study. Anat. Rec. 2019, 302, 1638–1646. [Google Scholar] [CrossRef]
- Pirone, A.; Graïc, J.M.; Grisan, E.; Cozzi, B. The claustrum of the sheep and its connections to the visual cortex. J. Anat. 2021, 238, 1–12. [Google Scholar] [CrossRef]
- Cozzi, B.; De Giorgio, A.; Peruffo, A.; Montelli, S.; Panin, M.; Bombardi, C.; Grandis, A.; Pirone, A.; Zambenedetti, P.; Corain, L.; et al. The laminar organization of the motor cortex in monodactylous mammals: A comparative assessment based on horse, chimpanzee, and macaque. Brain Struct. Funct. 2017, 222, 2743–2757. [Google Scholar] [CrossRef]
- Preuss, T.M.; Kaas, J.H. Parvalbumin-like immunoreactivity of layer V pyramidal cells in the motor and somatosensory cortex of adult primates. Brain Res. 1996, 712, 353–357. [Google Scholar] [CrossRef]
- Elston, G.N.; Gonzalez-Albo, M.C. Parvalbumin-, calbindin-, and calretinin-immunoreactive neurons in the prefrontal cortex of the owl monkey (Aotus tri-virgatus): A standardized quantitative comparison with sensory and motor areas. Brain. Behav. Evol. 2003, 62, 19–30. [Google Scholar] [CrossRef]
- Ernst, L.; Darschnik, S.; Roos, J.; González-Gómez, M.; Beemelmans, C.; Beemelmans, C.; Engelhardt, M.; Meyer, G.; Wahle, P. Fast prenatal development of the NPY neuron system in the neocortex of the European wild boar Sus scrofa. Brain Struct. Funct. 2018, 223, 3855–3873. [Google Scholar] [CrossRef]
- Minervini, S.; Accogli, G.; Pirone, A.; Graïc, J.M.; Cozzi, B.; Desantis, S. Brain mass and encephalization quotients in the domestic industrial pig (Sus scrofa). PLoS ONE 2016, 11, e0157378. [Google Scholar] [CrossRef] [Green Version]
- Reiland, S. Growth and skeletal development of the pig. Acta Radiol. Suppl. 1978, 358, 15–22. [Google Scholar]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef] [Green Version]
- Olkowicz, S.; Turlejski, K.; Bartkowska, K.; Wielkopolska, E.; Djavadian, R.L. Thalamic nuclei in the opossum Monodelphis domestica. J. Chem. Neuroanat. 2008, 36, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Wernera, T.E.R.; Bernsona, D.; Esbjörnera, E.K.; Rochaa, S.; Wittung-Stafshede, P. Amyloid formation of fish β-parvalbumin involvesprimar y nucleation triggered by disulfide-bridged protein dimers. Proc. Natl. Acad. Sci. USA 2020, 117, 27997–28004. [Google Scholar] [CrossRef]
- Das Dores, S.; Chopin, C.; Villaume, C.; Fleurence, J.; Guéant, J.-L. A new oligomeric parvalbumin allergen of Atlantic cod (Gad mI) encoded by a gene distinct from that of Gad cI. Allergy 2002, 57, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Bastianelli, E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum 2003, 2, 242–262. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Arendt, O.; Brown, E.B.; Schwaller, B.; Eilers, J. Parvalbumin is freely mobile in axons, somata and nuclei of cerebellar Purkinje neurones. J. Neurochem. 2007, 100, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peruffo, A.; Corain, L.; Bombardi, C.; Centelleghe, C.; Grisan, E.; Graïc, J.M.; Bontempi, P.; Grandis, A.; Cozzi, B. The motor cortex of the sheep: Laminar organization, projections and diffusion tensor imaging of the intracranial pyramidal and extrapyramidal tracts. Brain Struct. Funct. 2019, 224, 1933–1946. [Google Scholar] [CrossRef]
- Hof, P.R.; Chanis, R.; Marino, L. Cortical complexity in cetacean brains. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 287, 1142–1152. [Google Scholar] [CrossRef] [Green Version]
- Amunts, K.; Istomin, V.; Schleicher, A.; Ziles, K. Postnatal development of the human primary motor cortex: A quantitative cytoarchitectonic analysis. Anat. Embryol. 1995, 192, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Jelsing, J.; Nielsen, R.; Olsen, A.K.; Grand, N.; Hemmingsen, R.; Pakkenberg, B. The postnatal development of neocortical neurons and glial cells in the Gottingen minipig and the domestic pig brain. J. Exp. Biol. 2006, 209, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, W.T.; Dobbing, J. Prenatal and postnatal growth and development of the central nervous system of the pig. Proc. R. Soc. Lond. B Biol. Sci. 1967, 166, 384–395. [Google Scholar] [PubMed]
- Pond, W.G.; Boleman, S.L.; Fiorotto, M.L.; Ho, H.; Knabe, D.A.; Mersmann, H.J.; Savell, J.W.; Su, D.R. Perinatal ontogeny of brain growth in the domestic pig. Proc. Soc. Exp. Biol. Med. 2000, 223, 102–108. [Google Scholar] [CrossRef]
- Winter, J.D.; Dorner, S.; Lukovic, J.; Fisher, J.A.; StLawrence, K.S.; Kassner, A. Noninvasive MRI measures of microstructural and cerebrovascular changes during normal swine brain development. Pediatr. Res. 2011, 69, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Conrad, M.S.; Dilger, R.N.; Johnson, R.W. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: A longitudinal MRI study. Dev. Neurosci. 2012, 34, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health. Perspect. 2000, 108, 511–533. [Google Scholar]
- Marin-Padilla, M.; Marin-Padilla, T.M. Origin, prenatal development and structural organization of layer I of the human cerebral (motor) cortex. A Golgi study. Anat. Embryol. 1982, 164, 161–206. [Google Scholar] [CrossRef] [PubMed]
- Marin-Padilla, M. Prenatal and early postnatal ontogenesis of the human motor cortex: A Golgi study. I. The sequential development of the cortical layers. Brain Res. 1970, 23, 167–183. [Google Scholar] [CrossRef]
- Letzkus, J.J.; Wolff, S.B.E.; Meyer, E.M.M.; Tovote, P.; Courtin, J.; Herry, C.; Lüthi, A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 2011, 480, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Manning, T.F.; Ostry, D.J. Somatosensory cortex participates in the consolidation of human motor memory. PLoS Biol. 2019, 17, e3000469. [Google Scholar] [CrossRef] [Green Version]
- Lema Tomé, C.M.; Miller, R.; Bauer, C.; Smith, C.; Blackstone, K.; Leigh, A.; Busch, J.; Turner, C.P. Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: Somatosensory and motor cortex. Dev. Psychobiol. 2008, 50, 665–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFelipe, J.; Hendry, S.H.; Hashikawa, T.; Molinari, M.; Jones, E.G. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience 1990, 37, 655–673. [Google Scholar] [CrossRef]
- Hof, P.R.; Bogaert, Y.E.; Rosenthal, R.E.; Fiskum, G. Distribution of neuronal populations containing neurofilament protein and calcium-binding proteins in the canine neocortex: Regional analysis and cell typology. J. Chem. Neuroanat. 1996, 11, 81–98. [Google Scholar] [CrossRef]
- Pugliese, M.; Carrasco, J.; Geloso, M.C.; Mascort, J.; Michetti, F.; Mahy, N. γ-aminobutyric acidergic interneuron vulnerability to aging in canine prefrontal cortex. J. Neurosci. Res. 2004, 77, 913–920. [Google Scholar] [CrossRef]
- Ryan, M.C.; Sherman, P.; Rowland, L.M.; Wijtenburg, S.A.; Acheson, A.; Fieremans, E.; Veraart, J.; Novikov, D.S.; Hong, L.E.; Sladky, J.; et al. Miniature pig model of human adolescent brain white matter development. J. Neurosci. Methods. 2018, 296, 99–108. [Google Scholar] [CrossRef]

| Antibody | Immunogen | Manufacturing Details | Dilution |
|---|---|---|---|
| Anti-PV | PARV-19 hybridoma produced by the fusion of mouse myeloma cells and splenocytes from an immunized mouse. Purified frog muscle parvalbumin was used as the immunogen | Sigma-Aldrich, mouse monoclonal, Clone PARV-19, Product No. P 3088 RRID: AB477329 | 1:2000 |
| Anti-CR | Full length protein | abcam, rabbit polyclonal, ab702 RRID: AB305702 | 1:100 |
| Antibody | Type | Manufacturing Details | Dilution |
|---|---|---|---|
| Biotinylated | Anti-mouse IgG (H + L) | Vector Labs, Burlingame, horse, Cat.n. BA-2001, Lot.n. ZC1230 RRID: AB2336180 | 5 µg/mL |
| Biotinylated | Anti-rabbit IgG (H + L) | Vector Labs, Burlingame, horse, Cat.n. BA-1100, Lot.n. ZA0319 RRID: AB2336201 | 5 µg/mL |
| DyLight 549 | Anti-mouse IgG (H + L) | Vector Labs, Burlingame, horse, Cat.n. DI-22549, Lot.n. Z0416 | 10 µg/mL |
| DyLight 488 | Anti-rabbit IgG (H + L) | Vector Labs, Burlingame, horse, Cat.n. DI-1088, Lot.n. Z1005 | 10 µg/mL |
| HRP conjugate | Anti-rabbit IgG | Enzo life science, goat, Cat.n. ADI-SAB-300J | 1:10,000 |
| HRP conjugate | Anti-mouse IgG | Perkin Elmer, goat, Cat.n. NEF822 | 1:10,000 |
| Parameters | Age (Days) | |||
|---|---|---|---|---|
| 1 (n = 4) | 150–180 (n = 4) | >300 (n = 19) | p-Value | |
| Brain weight (g) | 33.97 ± 1.99 a | 126.21 ± 7.5 b | 142.30 ± 6.22 c | <0.001 |
| Cell density (n/mm2) | 425.71 ± 68.40 a | 270.19 ± 33.51 b | 251.33 ± 28.01 b | <0.001 |
| M1 thickness (µm) | 1250.39 ± 41.04 a | 1962.23 ± 48.12 b | 2058.12 ± 37.27 b | <0.001 |
| Layer 1 thickness (µm) | 135.14 ± 21.9 a | 264.73 ± 19.76 b | 438.33 ± 50.12 c | <0.001 |
| Large pyramidal neurons size in layer 5 (µm) | 33.21 ± 4.21 | 34.82 ± 3.04 | 34.68 ± 3.13 | 0.359 |
| CR-ir density (n/mm2) * | 25.04 ± 3.16 a | 15.78 ± 2.90 b | 16.51 ± 2.02 b | <0.001 |
| PV-ir density (n/mm2) * | 27.44 ± 2.98 a | 17.86 ± 4.22 b | 19.55 ± 4.67 b | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desantis, S.; Minervini, S.; Zallocco, L.; Cozzi, B.; Pirone, A. Age-Related Changes in the Primary Motor Cortex of Newborn to Adult Domestic Pig Sus scrofa domesticus. Animals 2021, 11, 2019. https://doi.org/10.3390/ani11072019
Desantis S, Minervini S, Zallocco L, Cozzi B, Pirone A. Age-Related Changes in the Primary Motor Cortex of Newborn to Adult Domestic Pig Sus scrofa domesticus. Animals. 2021; 11(7):2019. https://doi.org/10.3390/ani11072019
Chicago/Turabian StyleDesantis, Salvatore, Serena Minervini, Lorenzo Zallocco, Bruno Cozzi, and Andrea Pirone. 2021. "Age-Related Changes in the Primary Motor Cortex of Newborn to Adult Domestic Pig Sus scrofa domesticus" Animals 11, no. 7: 2019. https://doi.org/10.3390/ani11072019
APA StyleDesantis, S., Minervini, S., Zallocco, L., Cozzi, B., & Pirone, A. (2021). Age-Related Changes in the Primary Motor Cortex of Newborn to Adult Domestic Pig Sus scrofa domesticus. Animals, 11(7), 2019. https://doi.org/10.3390/ani11072019






