Developing Recommendations for Cumulative Endpoints and Lifetime Use for Research Animals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Scoping Survey about Frequency of Use, Lifetime Use, and Cumulative Use Considerations for Research Animals
2.1. Survey Methods
2.2. Data Analysis
2.3. Results
2.3.1. Respondent Demographics
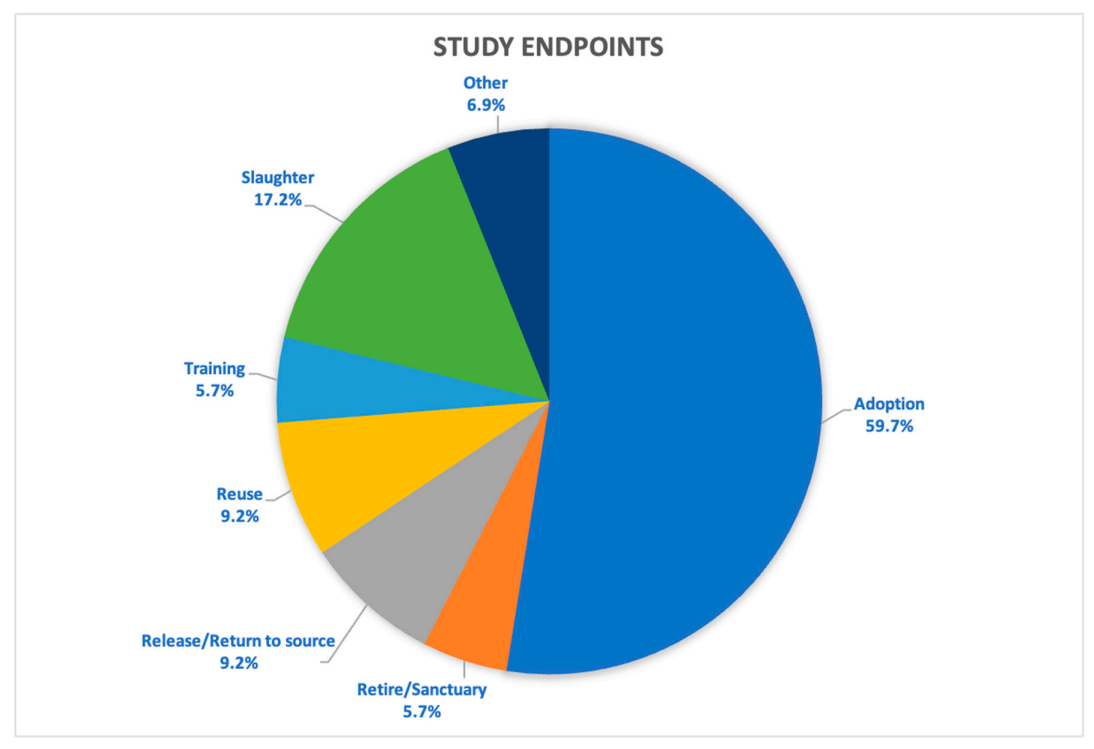
2.3.2. Institutional Endpoint Policy and Disposition of Animals at Study Conclusion
2.3.3. Quality of Life and Lifetime Use Decision Making
2.3.4. Consideration of Cumulative Use of Animals—Refinement vs. Reduction
2.3.5. Interest in Cumulative and Lifetime Use Guidelines at an Institutional Level
- “I think balance and good benefit/cost analysis is needed, we cannot create guidelines for a broad range of experiments and animal species. Each study needs a singular evaluation, discussion, and reflection from the Animal Welfare Body”.
- “I think blanket policies may be inappropriate, and instead there might need to be some general guidelines that can be applied on a case by case basis directly within protocols where all the details can be considered independently”.
- “I am always concerned that policies, while good as guidelines, may limit the ability for the veterinarian or IACUC to utilize professional judgment in deciding the fate of individual animals”.
- “The approach should be a whole animal assessment by a cross-disciplinary team”.
- “Principal investigators are strongly discouraged from advocating animal reuse as a reduction strategy, and reduction should not be a rationale for reusing animals or animals that have already undergone experimental procedures especially if the well-being of the animals would be compromised. My opinion is that you should strive for minimal suffering per individual animal even in the cost of using more animals”.
- “Committees should come up with objective criteria for the maximum number of lifetime procedures—similar to consideration for multiple major survival surgeries currently required by USDA—for a variety of procedures and apply those criteria to animals on a single study and then carefully weigh what should be allowable”.
2.4. Discussion
3. Relevant Cumulative Endpoint Guidance for Research Animals from Regulatory and Compliance Authorities
3.1. Ethical Review of Protocols by an AEC and/or Authority
3.2. Classifications of Major vs. Minor Surgical Procedures
3.3. Prospective Assessment of Severity/Invasiveness of Procedures and/or Protocol
3.4. Retrospective Assessment of Actual Severity of Procedures and/or Protocol
3.5. Establishment of Humane Endpoints for Research
4. Tools for Assessment of Animal Welfare
4.1. Animal Welfare Assessment Technique
4.1.1. The Five Domains
4.1.2. Extended Animal Welfare Assessment Grid
4.1.3. Biomarker Analysis
4.1.4. Quality of Life Assessments
4.1.5. Applying Technology to Animal Welfare Assessments
5. Recommendations for Cumulative and Lifetime Use of Animals Maintained for Teaching, Testing, and Research
5.1. Institutional Policy to Assess Cumulative Endpoints
5.2. Determination of Threshold Criteria
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK; UFAW: Potters Bar, UK; Herts, UK, 1959; p. 239. [Google Scholar]
- Turner, P.V. Moving Beyond the Absence of Pain and Distress: Focusing on Positive Animal Welfare. ILAR J. 2020, 1–7. [Google Scholar] [CrossRef]
- Canadian Council on Animal Care (CCAC). Guidelines on Choosing an Appropriate Endpoint in Experiments Using Animals for Research, Teaching and Testing; Canadian Council on Animal Care (CCAC): Ottawa, ON, Canada, 1998. [Google Scholar]
- Institute for Laboratory Animal Research (ILAR). Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- National Research Council (NRC) Committee on Recognition and Alleviation of Distress in Laboratory Animals. Recognition and Alleviation of Distress in Laboratory Animals; National Academies Press (US): Washington, DC, USA, 2008. [Google Scholar]
- Hendriksen, C.F.M.; Morton, D.B. (Eds.) Humane Endpoints in Animal Experiments for Biomedical Research. In Proceedings of the International Conference, Zeist, The Netherlands, 22–25 November 1998; Royal Society of Medicine Press Limited: London, UK, 1999. [Google Scholar]
- Fenwick, N.; Griffin, G.; Gauthier, C. The welfare of animals used in science: How the “Three Rs” ethic guides improvements. Can. Vet. J. 2009, 50, 523–530. [Google Scholar] [PubMed]
- Honess, P.; Wolfensohn, S. The extended welfare assessment grid: A matrix for the assessment of welfare and cumulative suffering in experimental animals. Altern. Lab. Anim. 2010, 38, 205–212. [Google Scholar] [CrossRef]
- Pound, P.; Nicol, C.J. Retrospective harm benefit analysis of pre-clinical animal research for six treatment interventions. PLoS ONE 2018, 13, e0193758. [Google Scholar] [CrossRef]
- Animals Procedures Committee APS. Review of Cost-Benefit Assessment in the Use of Animals in Research. 2003. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/119027/cost-benefit-assessment.pdf (accessed on 18 April 2021).
- National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Working Document on Project Evaluation and Retrospective Assessment. 2013. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/Endorsed_PE-RA.pdf (accessed on 18 April 2021).
- OIE. Use of animals in research and education. In Terrestrial Animal Health Code; World Organization for Animal Health: Paris, France, 2019; Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_aw_research_education.pdf (accessed on 18 April 2021).
- Brønstad, A.; Newcomer, C.E.; Decelle, T.; Everitt, J.I.; Guillen, J.; Laber, K. Current concepts of harm–benefit analysis of animal experiments–report from the AALAS–FELASA working group on harm–benefit analysis—Part 1. Lab. Anim. 2016, 50, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Laber, K.; Newcomer, C.E.; Decelle, T.; Everitt, J.I.; Guillen, J.; Brønstad, A. Recommendations for Addressing Harm-Benefit Analysis and Implementation in Ethical Evaluation—Report from the AALAS-FELASA Working Group on Harm-Benefit Analysis—Part 2. Lab. Anim. 2016, 50, 21–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiss Academies of Arts and Sciences. Weighing of Interests for Proposed Animal Experiments. Guidance for Applicants. Swiss Academies Communications. 2017. Available online: https://swiss-academies.ch/en/publications/ (accessed on 25 February 2021).
- Mohan, S.; Huneke, R. The Role of IACUCs in Responsible Animal Research. ILAR J. 2019, 60, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Foley, P.L. Everything You Need to Know About Satisfying IACUC Protocol Requirements. ILAR J. 2019, 60, 50–57. [Google Scholar] [CrossRef] [Green Version]
- AWA Section 2143(a)(3)(A,B,C,D,E) 9 CFR, Part 2, Section 2.31 (d)(1)(i,ii,iv,viii,ix,x). Available online: https://www.nal.usda.gov/sites/default/files/Policy14.pdf (accessed on 5 March 2021).
- Canadian Council on Animal Care (CCAC). Guide to the Care and Use of Experimental Animals Volume 1, 2nd ed.; Canadian Council on Animal Care (CCAC): Ottawa, ON, Canada, 1993; Available online: https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf (accessed on 5 March 2021).
- Carbone, L.; Austin, J. Pain and Laboratory Animals: Publication Practices for Better Data Reproducibility and Better Animal Welfare. PLoS ONE 2016, 11, e0155001. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, K.; Flecknell, P. Retrospective review of anesthetic and analgesic regimens used in animal research proposals. Altex 2019, 36, 65–80. [Google Scholar] [CrossRef]
- Yates, B.; Toth, L. Is it time to redefine “major operative procedures?”. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 8. [Google Scholar]
- National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes Working Document on a Severity Assessment Framework Brussels, 11–12 July 2012. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/guidance/severity/en.pdf (accessed on 3 March 2021).
- Smith, D.; Anderson, D.; Degryse, A.-D.; Bol, C.; Criado, A.; Ferrara, A.; Franco, N.H.; Gyertyan, I.; Orellana, J.M.; Ostergaard, G.; et al. Classification and reporting of severity experienced by animals used in scientific procedures: FELASA/ECLAM/ESLAV Working Group report. Lab. Anim. 2018, 52, 5–57. [Google Scholar] [CrossRef] [Green Version]
- Canadian Council on Animal Care (CCAC). CCAC Guidelines on: Animal Use Protocol Review 1997. Available online: https://www.ccac.ca/Documents/Standards/Guidelines/Protocol_Review.pdf (accessed on 3 March 2021).
- Olsson, I.A.S.; Nicol, C.J.; Niemi, S.M.; Sandøe, P. From unpleasant to unbearable—Why and how to implement an upper limit to pain and other forms of suffering in research with animals. ILAR J. 2020, 1–11. [Google Scholar] [CrossRef]
- Zintzsch, A.; Noe, E.; Grimm, H. Navigating Uncertainties: How to Assess Welfare and Harm in Genetically Altered Animals Responsibly-A Practical Guideline. Animals 2020, 10, 857. [Google Scholar] [CrossRef]
- Guidance on the Operation of the Animals (Scientific Procedures) Act 1986. UK Home Office. 2013. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/662364/Guidance_on_the_Operation_of_ASPA.pdf (accessed on 4 March 2021).
- Canadian Council on Animal Care (CCAC). Categories of Invasiveness in Animal Experiments. 1991. Available online: https://www.ccac.ca/Documents/Standards/Policies/Categories_of_invasiveness.pdf (accessed on 14 April 2021).
- Mai, S.H.C.; Sharma, N.; Kwong, A.C.; Dwivedi, D.J.; Khan, M.; Grin, P.M.; Fox-Robichaud, A.E.; Liaw, P.C. Body temperature and mouse scoring systems as surrogate markers of death in cecal ligation and puncture sepsis. Intens. Care Med. Exp. 2018, 6, 20. [Google Scholar] [CrossRef] [Green Version]
- McGinn, R.; Fergusson, D.A.; Stewart, D.J.; Kristof, A.S.; Barron, C.C.; Thebaud, B.; McIntyre, L.; Stacey, D.; Liepmann, M.; Dodelet-Devillers, A.; et al. Surrogate Humane Endpoints in Small Animal Models of Acute Lung Injury: A Modified Delphi Consensus Study of Researchers and Laboratory Animal Veterinarians. Crit. Care Med. 2021, 49, 311–323. [Google Scholar] [CrossRef]
- Canadian Council on Animal Care (CCAC) Guidelines: Animal Welfare Assessment. 2021. Available online: https://ccac.ca/Documents/Standards/Guidelines/CCAC_guidelines-Animal_welfare_assessment.pdf (accessed on 18 April 2021).
- Mellor, D.J.; Patterson-Kane, E.; Stafford, K.J. The Sciences of Animal Welfare; Wiley-Blackwell: Ames, IA, USA, 2009; ISBN 978-1405134958. [Google Scholar]
- Yeates, J. Animal Welfare in Veterinary Practice; Wiley-Blackwell: Ames, IA, USA, 2013; ISBN 978-1444334876. [Google Scholar]
- Canadian Council on Animal Care (CCAC) Guidelines: Mice Appendix 6—Indicators that May Be Used to Assess the Welfare of Mice. 2019. Available online: https://ccac.ca/Documents/Standards/Guidelines/CCAC_Guidelines_Mice.pdf (accessed on 18 April 2021).
- Canadian Council on Animal Care (CCAC) Guidelines: Rats. Appendix 4—Indicators that May Be Used to Assess the Welfare of raTs. 2020. Available online: https://ccac.ca/Documents/Standards/Guidelines/CCAC_Guidelines_Rats.pdf (accessed on 18 April 2021).
- Welfare Quality Network. Available online: http://www.welfarequalitynetwork.net/en-us/reports/assessment-protocols/ (accessed on 1 April 2021).
- Wemelsfelder, F.; Hunter, T.E.A.; Mendl, M.T.; Lawrence, A.B. Assessing the ‘Whole Animal’: A free choice profiling approach. Anim. Behav. 2001, 62, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, D.B.; Metzdorff, S.B.; Jensen, L.K.; Andersen, K.H.; Teilmann, A.C.; Jensen, H.E.; Frøkiær, H. Time-dependent pathologic and inflammatory consequences of various blood sampling techniques in mice. J. Am. Assoc. Lab. Anim. Sci. 2019, 58, 362–372. [Google Scholar] [CrossRef]
- Davis, J.N.; Courtney, C.L.; Superak, H.; Taylor, D.K. Behavioral, clinical and pathological effects of multiple daily intraperitoneal injections on female mice. Lab. Anim. 2014, 43, 131. [Google Scholar] [CrossRef]
- Broom, D.M. Animal welfare: The concept of the issues. In Attitudes to Animals: Views in Animal Welfare; Dolins, F.L., Ed.; Cambridge University Press: Cambridge, UK, 1999; pp. 129–143. [Google Scholar]
- Mellor, D.J.; Beausoleil, N.J. Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 2015, 24, 241. [Google Scholar] [CrossRef]
- Mellor, D.J. Updating animal welfare thinking: Moving beyond the “Five Freedoms” towards “a Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Mellor, D.J. Operational details of the five domains model and its key applications to the assessment and management of animal welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- McGreevy, P.; Berger, J.; De Brauwere, N.; Doherty, O.; Harrison, A.; Fiedler, J.; Jones, C.; McDonnell, S.; McLean, A.; Nakonechny, L.; et al. Using the five domains model to assess the adverse impacts of husbandry, veterinary, and equitation interventions on horse welfare. Animals 2018, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Mellor, D.J.; Burns, M. Using the Five Domains Model to develop welfare assessment guidelines for Thoroughbred horses in New Zealand. N. Z. Vet. J. 2020, 68, 150–156. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Honess, P. Laboratory animal, pet animal, farm animal, wild animal: Which gets the best deal? Anim. Welf. 2007, 16, 117–123. [Google Scholar]
- Wolfensohn, S.; Sharpe, S.; Hall, I.; Lawrence, S.; Kitchen, S.; Dennis, M. Refinement of welfare through development of a quantitative system for assessment of lifetime experience. Anim. Welf. 2015, 24, 139–149. [Google Scholar] [CrossRef]
- Wolfensohn, S. Too Cute to Kill? The Need for Objective Measurements of Quality of Life. Animals 2020, 10, 1054. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [Green Version]
- Dunn, R.A. The sooner the better: The arguments for the use of extended welfare assessment grids in animal welfare cases. Liverpool Law Rev. 2020, 41, 107–127. [Google Scholar] [CrossRef]
- Bateson, M. Cumulative stress in research animals: Telomere attrition as a biomarker in a welfare context? BioEssays 2016, 38, 201–212. [Google Scholar] [CrossRef]
- Chatelain, M.; Drobniak, S.M.; Szulkin, M. The association between stressors and telomeres in non-human vertebrates: A meta-analysis. Ecol. Lett. 2020, 23, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Debes, P.V.; Visse, M.; Panda, B.; Ilmonen, P.; Vasemägi, A. Is telomere length a molecular marker of past thermal stress in wild fish? Mol. Ecol. 2016, 25, 5412–5424. [Google Scholar] [CrossRef] [Green Version]
- Cai, N.; Chang, S.; Li, Y.; Li, Q.; Hu, J.; Liang, J.; Song, L.; Kretzschmar, W.; Gan, X.; Nicod, J.; et al. Molecular signatures of major depression. Curr. Biol. 2015, 25, 1146–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotrschal, A.; Ilmonen, P.; Penn, D.J. Stress impacts telomere dynamics. Biol. Lett. 2007, 3, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Beloor, J.; Kang, H.K.; Kim, Y.J.; Subramani, V.K.; Jang, I.S.; Sohn, S.H.; Moon, Y.S. The effect of stocking density on stress related genes and telomeric length in broiler chickens. Asian-Aust. J. Anim. Sci. 2010, 23, 437–443. [Google Scholar] [CrossRef]
- Nettle, D.; Monaghan, P.; Boner, W.; Gillespie, R.; Bateson, M. Bottom of the heap: Having heavier competitors accelerates early-life telomere loss in the European starling, Sturnus vulgaris. PLoS ONE 2013, 8, e83617. [Google Scholar] [CrossRef] [Green Version]
- Nettle, D.; Monaghan, P.; Gillespie, R.; Brilot, B.; Bedford, T.; Bateson, M. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 2015, 282, 20141610. [Google Scholar] [CrossRef]
- Ilmonen, P.; Kotrschal, A.; Penn, D.J. Telomere attrition due to infection. PLoS ONE 2008, 3, e2143. [Google Scholar] [CrossRef] [Green Version]
- Aydinonat, D.; Penn, D.J.; Smith, S.; Moodley, Y.; Hoelzl, F.; Knauer, F.; Schwarzenberger, F. Social isolation shortens telomeres in African Grey parrots (Psittacus erithacus erithacus). PLoS ONE 2014, 9, e93839. [Google Scholar] [CrossRef] [Green Version]
- Grosbellet, E.; Zahn, S.; Arrivé, M.; Dumont, S.; Gourmelen, S.; Pévet, P.; Challet, E.; Criscuolo, F. Circadian desynchronization triggers premature cellular aging in a diurnal rodent. FASEB J. 2015, 29, 4794–4803. [Google Scholar] [CrossRef]
- Sohn, S.H.; Subramani, V.K.; Moon, Y.S.; Jang, I.S. Telomeric DNA quantity, DNA damage, and heat shock protein gene expression as physiological stress markers in chickens. Poult. Sci. J. 2012, 91, 829–836. [Google Scholar] [CrossRef]
- Lambeth, S.P.; Schapiro, S.J.; Bernacky, B.J.; Wilkerson, G.K. Establishing ‘quality of life’parameters using behavioural guidelines for humane euthanasia of captive non-human primates. Anim. Welf. 2013, 22, 429. [Google Scholar] [CrossRef] [Green Version]
- Yeates, J. Whose Life is it Anyway? Vet. J. 2015, 206, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.; Morton, D.B.; Burman, O.; Dennison, N.; Honess, P.; Jennings, M.; Lane, S.; Middleton, V.; Roughan, J.V.; Wells, S.; et al. A guide to defining and implementing protocols for the welfare assessment of laboratory animals: Eleventh report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 2011, 45, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howerton, C.L.; Garner, J.P.; Mench, J.A. A system utilizing radio frequency identification (RFID) technology to monitor individual rodent behavior in complex social settings. J. Neurosci. Methods 2012, 209, 74–78. [Google Scholar] [CrossRef]
- Catarinucci, L.; Colella, R.; Mainetti, L.; Patrono, L.; Pieretti, S.; Secco, A.; Sergi, I. An animal tracking system for behavior analysis using radio frequency identification. Lab. Anim. 2014, 43, 321–327. [Google Scholar] [CrossRef]
- Redfern, W.S.; Tse, K.; Grant, C.; Keerie, A.; Simpson, D.J.; Pedersen, J.C.; Rimmer, V.; Leslie, L.; Klein, S.K.; Karp, N.A.; et al. Automated recording of home cage activity and temperature of individual rats housed in social groups: The Rodent Big Brother project. PLoS ONE 2017, 12, e0181068. [Google Scholar] [CrossRef] [PubMed]
- Tse, K.; Sillito, R.; Keerie, A.; Collier, R.; Grant, C.; Karp, N.A.; Vickers, C.; Chapman, K.; Armstrong, J.D.; Redfern, W.S. Pharmacological validation of individual animal locomotion, temperature and behavioural analysis in group-housed rats using a novel automated home cage analysis system: A comparison with the modified Irwin test. J. Pharmacol. Toxicol. Methods 2018, 94, 1–13. [Google Scholar] [CrossRef]
- Ahloy-Dallaire, J.; Klein, J.D.; Davis, J.K.; Garner, J.P. Automated monitoring of mouse feeding and body weight for continuous health assessment. Lab. Anim. 2019, 53, 342–351. [Google Scholar] [CrossRef]
- Yip, P.K.; Chapman, G.E.; Sillito, R.R.; Ip, T.R.; Akhigbe, G.; Becker, S.C.; Price, A.W.; Michael-Titus, A.T.; Armstrong, J.D.; Tremoleda, J.L. Studies on long term behavioural changes in group-housed rat models of brain and spinal cord injury using an automated home cage recording system. J. Neurosci. Methods 2019, 321, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, J.S.; Cook, N.J.; Schaefer, A.L. Recent applications of infrared thermography for animal welfare and veterinary research: Everything from chicks to elephants. In Proceedings of the InfraMation, Las Vegas, NV, USA, 19–22 October 2009; pp. 215–224. [Google Scholar]
- Barbosa Pereira, C.; Kunczik, J.; Zieglowski, L.; Tolba, R.; Abdelrahman, A.; Zechner, D.; Vollmar, B.; Janssen, H.; Thum, T.; Czaplik, M. Remote welfare monitoring of rodents using thermal imaging. Sensors 2018, 18, 3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Bermudez-Contreras, E.; Nazari, M.; Sutherland, R.J.; Mohajerani, M.H. Low-cost solution for rodent home-cage behaviour monitoring. PLoS ONE 2019, 14, e0220751. [Google Scholar] [CrossRef] [Green Version]
- Weary, D.M.; Fraser, D. Calling by domestic piglets: Reliable signals of need? Anim. Behav. 1995, 50, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Watts, J.M.; Stookey, J.M. Vocal behaviour in cattle: The animal’s commentary on its biological processes and welfare. Appl. Anim. Behav. Sci. 2000, 67, 15–33. [Google Scholar] [CrossRef]
- Manteuffel, G.; Puppe, B.; Schön, P.C. Vocalization of farm animals as a measure of welfare. Appl. Anim. Behav. Sci. 2004, 88, 163–182. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Mcloughlin, M.P.; Stewart, R.; McElligott, A.G. Automated bioacoustics: Methods in ecology and conservation and their potential for animal welfare monitoring. J. R. Soc. Interface 2019, 16, 20190225. [Google Scholar] [CrossRef]
- Ferrari, S.; Costa, A.; Guarino, M. Heat stress assessment by swine related vocalizations. Livest. Sci. 2013, 151, 29–34. [Google Scholar] [CrossRef]
- Schön, P.C.; Puppe, B.; Manteuffel, G. Automated recording of stress vocalisations as a tool to document impaired welfare in pigs. Anim. Welf. 2004, 13, 105–110. [Google Scholar]
- Zieske, L.R.; Dullen, D.; Mohanty, M. Comparing Veterinary Diagnosis and a Novel Non-Invasive Device (Paintrace) to Differentiate Location and Quantify Pain in Dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, A3660. [Google Scholar] [CrossRef]
- Paola, G.; Rachel, B.C.; Bernd, D. A pilot study on skin potential recordings as a measure of nociception in pain-free dogs and humans, and in dogs with persistent pain. Acta Vet. 2018, 68, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Ruíz-López, P.; Domínguez, J.M.; del Mar Granados, M. Intraoperative nociception-antinociception monitors: A review from the veterinary perspective. Vet. Anaesth. Analg. 2019, 47, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Aguado, D.; Bustamante, R.; García-Sanz, V.; González-Blanco, P.; de Segura, I.A.G. Efficacy of the Parasympathetic Tone Activity monitor to assess nociception in healthy dogs anaesthetized with propofol and sevoflurane. Vet. Anaesth. Analg. 2020, 47, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadavecchia, C.; Rohrbach, H.; Levionnois, O.; Leandri, M. The model of the Nociceptive Withdrawal Reflex in horses. Pferdeheilkunde 2016, 32, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Geers, R. Electronic monitoring of farm animals: A review of research and development requirements and expected benefits. Comput. Electron. Agric 1994, 10, 1–9. [Google Scholar] [CrossRef]
- De Mol, R.M.; André, G.; Bleumer, E.J.B.; Van der Werf, J.T.N.; De Haas, Y.; Van Reenen, C.G. Applicability of day-to-day variation in behavior for the automated detection of lameness in dairy cows. J. Dairy Sci. 2013, 96, 3703–3712. [Google Scholar] [CrossRef] [Green Version]
- Jukan, A.; Masip-Bruin, X.; Amla, N. Smart computing and sensing technologies for animal welfare: A systematic review. ACM Comput. Surv. 2017, 50, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Jukan, A.; Carpio, F.; Masip, X.; Ferrer, A.J.; Kemper, N.; Stetina, B.U. Fog-to-Cloud Computing for Farming: Low-Cost Technologies, Data Exchange, and Animal Welfare. Computer 2019, 52, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Buller, H.; Blokhuis, H.; Lokhorst, K.; Silberberg, M.; Veissier, I. Animal Welfare Management in a Digital World. Animals 2020, 10, 1779. [Google Scholar] [CrossRef]
- Munch, M. A Day’s Work on Facebook and Other Collaborative Trends in Animal Welfare. In Proceedings of the Conference Companion Publication of the Computer Supported Cooperative Work and Social Computing Conference, Austin, TX, USA, 9–13 November 2019; pp. 314–318. [Google Scholar]
- Junior, R.L. IoT applications for monitoring companion animals: A systematic literature review. In Proceedings of the 14th International Conference on Innovations in Information Technology (IIT), Al Ain, United Arab Emirates, 17–18 November 2020; pp. 239–246. [Google Scholar]
- Chen, Y.; Ding, S.; Xu, Z.; Zheng, H.; Yang, S. Blockchain-Based Medical Records Secure Storage and Medical Service Framework. J. Med. Syst. 2019, 43, 5. [Google Scholar] [CrossRef] [PubMed]
- Briney, K. Data Management for Researchers: Organize, Maintain and Share Your Data for Research Success; Pelagic Publishing Ltd.: Exeter, UK, 2015. [Google Scholar]




| Question | Response | % of Respondents |
|---|---|---|
| Geographic region | USA | 50.0 |
| E.U. | 24.0 | |
| Canada | 9.7 | |
| Asia | 3.9 | |
| Latin America | 0.7 | |
| Other | 22.7 | |
| Age range | 18–25 | 0 |
| 26–35 | 27.5 | |
| 36–45 | 27.5 | |
| 46–55 | 25.5 | |
| 56–65 | 16.9 | |
| Over 65 | 2.6 | |
| Gender | Male | 33.3 |
| Female | 66.7 | |
| Institution type | University/College | 60.5 |
| Industry | 5.3 | |
| Contract Research Organization | 3.9 | |
| Research Hospital | 7.2 | |
| Government | 9.8 | |
| NGO or Non-Profit | 4.6 | |
| Sanctuary | 0 | |
| Other | 8.6 | |
| Primary job function | Veterinarian | 58.2 |
| Veterinary Technician | 5.9 | |
| Animal Care | 5.9 | |
| Researcher | 3.3 | |
| Compliance | 4.6 | |
| Species worked with | Mouse | 77.7 |
| Rat | 68.8 | |
| Rabbit | 58.0 | |
| Pig | 57.1 | |
| Non-Human Primate | 44.6 | |
| Fish | 41.1 | |
| Dog | 36.6 | |
| Poultry | 26.8 | |
| Cat | 25.8 | |
| Other Rodent | 40.2 | |
| Livestock | 38.4 | |
| Teaching Animals | 34.8 | |
| Other Birds | 22.3 | |
| Other | 18.8 |
| Considerations | Responses (%) |
|---|---|
| My institutional AEC has a guidance document for determining animal disposition at study end that includes the use of objective metrics, such as number of studies conducted with animal, number of needle sticks, number of study days, protocol invasiveness, total blood volume provided over a lifetime, etc. | 36.3 |
| My institutional AEC has established adoption and/or retirement criteria for a species, regardless of study use (e.g., based on animal age, time in facility, etc.). | 25.0 |
| My facility has an assessment protocol for scoring animals exhibiting aversive behaviors to procedures (e.g., vocalization during blood collection, excessive trembling, struggling). | 31.8 |
| My institutional AEC periodically discusses quality of life of aging and long-term housed research and teaching animals. | 73.6 |
| Animal care and veterinary personnel at my facility regularly review quality of life and endpoint decision making for aging and long-term housed research and teaching animals. | 18.7 |
| Area of Protocol Review | Example | Possible Suggestions for Protocol Authors by AEC Reviewers |
|---|---|---|
| Multiplicity of procedures | Teaching animals in an animal science or veterinary medicine program | Consideration for inanimate models for skills development—use to replace some or all of procedures. When live animals are a required component of training programs, consideration of maintaining higher numbers to permit using animals at lower frequency (as well as inclusion of refinements to reduce welfare impact). |
| Colony or herd animals that are pooled for potential research use, e.g., ponies and horses maintained as a research herd | Define a maximum time that animals can remain in herd or colony. Retire or rehome animals once this timeline has been met. | |
| Scarce or special resource animals maintained as a colony and reused on studies | Clear treatment plans for maintenance of quality of life based on health conditions. Define a maximum number of studies an individual can be enrolled in. Define maximum number of minor surgeries. Define maximum number of anesthetic events. Define a lifetime maximum number of protocols in the moderate and severe welfare impact categories. | |
| Frequency of use | MRI imaging of animal with tumors under general anesthesia 2 x/week for multiple weeks | Try to limit imaging to most critical time points, provide extra food treats and high energy foods to offset weight loss from fasting. |
| Repeated studies of blood sampling for pharmacokinetic analyses | Habituate animals to bleeding, train for voluntary blood collection, when possible, counter condition animals with food treats, define a maximum period of time that animals are maintained in PK colony, rehome or adopt at study end. | |
| Intensity of use | Pharmacokinetic study with 12 time points in 24 h | Discuss expected PK results and test article half-life to determine most critical times. Ensure maximum blood volumes are not exceeded. Encourage microsampling and use of peripheral veins and replacement of fluids. Consider catheterization (temporary or permanent) for some/all of blood collections. Discuss effects of repeated or prolonged restraint stress. |
| Collecting body weights of rodent pups daily for first 2 weeks of life | Encourage surrogate forms of monitoring for pup wellness, such as presence of milk spot, and body color. | |
| Dosing animals with test article 2 or >times daily | Train animals to procedures and voluntary ingestion, if possible, counter condition with special resources, treats, and human interaction time, look for possible refinements, such as use of mini-pump, to avoid repeated handling/restraint stress. | |
| Duration of use | Multi-year protocols with fistulated cows | Establish clear goals for studies and determine duration of housing for animals that is consistent with current industry practices. |
| Blood donor animals kept to support research colonies or for teaching or clinical use at veterinary colleges | Attempt to establish donor animals living in local communities, determine clear duration periods if animals must live in colony/clinic setting, adopt or rehome at end of duration. Define maximum number of donations based on animal personality and response to handling and blood collections over time. | |
| Chronic toxicology studies | Have well defined humane endpoints, ensure personnel are trained to recognize, ensure robust behavioral management program is in place (e.g., exercise, food foraging and other resources, and positive human interaction time). Define a maximum number of studies an individual can be enrolled in. | |
| Aged rats used in multiple studies to assess longevity therapeutics | Establishment of a program that can evaluate ongoing welfare impacts so that these can be monitored and limited. A concurrent welfare assessment program should additionally be considered and incorporated into the intervention and endpoint determinations. |
| Consideration | Indicator |
|---|---|
| Is the environment appropriate for the individual animal: resource-based measures | Environment allows physical performance of important natural behaviors |
| Provision of appropriate housing and husbandry | |
| Presence of negative environmental features that might impair welfare | |
| General animal-based indicators of stress, illness, pain or discomfort | Altered food and water intake |
| Weight change | |
| Altered posture | |
| Altered grooming behavior | |
| Coat condition | |
| Chromodacryorrhea | |
| Damage to the fur or skin | |
| Abnormal repetitive behaviors | |
| Altered social behavior | |
| Altered activity levels | |
| Partially closed, sunken, or dull eyes | |
| Altered interactions with humans | |
| Altered physiological parameters | |
| 20 kHz vocalization | |
| Fecal corticosterone | |
| Animal-based indicators of neutral or positive welfare states | Exploratory behavior |
| Grooming | |
| Play | |
| 50 kHz vocalizations | |
| Nest building and time to integrate into nest test (TINT test) and nesting consolidation test scoring | |
| Indicators for assessing welfare in specific contexts | Facial grimace scale |
| Composite pain score | |
| Burrowing task | |
| Gait score | |
| Cornering behavior |
| Date | Clinical Health | Procedural Severity | Environmental Quality | Behavioral Assessments | Score | Total Score |
|---|---|---|---|---|---|---|
| 1/1/20 | 0 | 0 | 4 | 4 | 8 | 8 |
| 4/1/20 | 0 | 6 | 2 | 4 | 12 | 20 |
| 7/1/20 | 4 | 2 | 8 | 4 | 18 | 38 |
| 10/1/20 | 2 | 8 | 8 | 6 | 24 | 62 |
| 1/1/21 | 2 | 0 | 2 | 8 | 12 | 74 |
| 4/1/21 | 2 | 0 | 2 | 4 | 8 | 82 |
| Category | Description |
|---|---|
| A good life | The balance of salient positive and negative experiences is strongly positive. Achieved by full compliance with best practices advice well above the minimum requirements of codes of practice or welfare. |
| A life worth living | The balance of salient positive and negative experiences is favorable, but less so. Achieved by full compliance with the minimum requirements of code of practice or welfare that include elements which promote some positive experiences. |
| Point of balance | The neutral point where salient positive and negative experiences are equally balanced. |
| A life worth avoiding | The balance of salient positive and negative experiences is unfavorable but can be remedied rapidly by veterinary treatment or a change in husbandry practices. |
| A life not worth living | The balance of salient positive and negative experiences is strongly negative and cannot be remedied rapidly so that euthanasia is the only humane alternative. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunamaker, E.A.; Davis, S.; O’Malley, C.I.; Turner, P.V. Developing Recommendations for Cumulative Endpoints and Lifetime Use for Research Animals. Animals 2021, 11, 2031. https://doi.org/10.3390/ani11072031
Nunamaker EA, Davis S, O’Malley CI, Turner PV. Developing Recommendations for Cumulative Endpoints and Lifetime Use for Research Animals. Animals. 2021; 11(7):2031. https://doi.org/10.3390/ani11072031
Chicago/Turabian StyleNunamaker, Elizabeth A., Shawn Davis, Carly I. O’Malley, and Patricia V. Turner. 2021. "Developing Recommendations for Cumulative Endpoints and Lifetime Use for Research Animals" Animals 11, no. 7: 2031. https://doi.org/10.3390/ani11072031
APA StyleNunamaker, E. A., Davis, S., O’Malley, C. I., & Turner, P. V. (2021). Developing Recommendations for Cumulative Endpoints and Lifetime Use for Research Animals. Animals, 11(7), 2031. https://doi.org/10.3390/ani11072031






