Application of Creep Feed and Phytase Super-Dosing as Tools to Support Digestive Adaption and Feed Efficiency in Piglets at Weaning
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets and Treatments
2.2. Animals and Housing
2.3. Capsule Administration
2.4. Blood Sampling
2.5. Statistical Analysis
3. Results
3.1. Performance
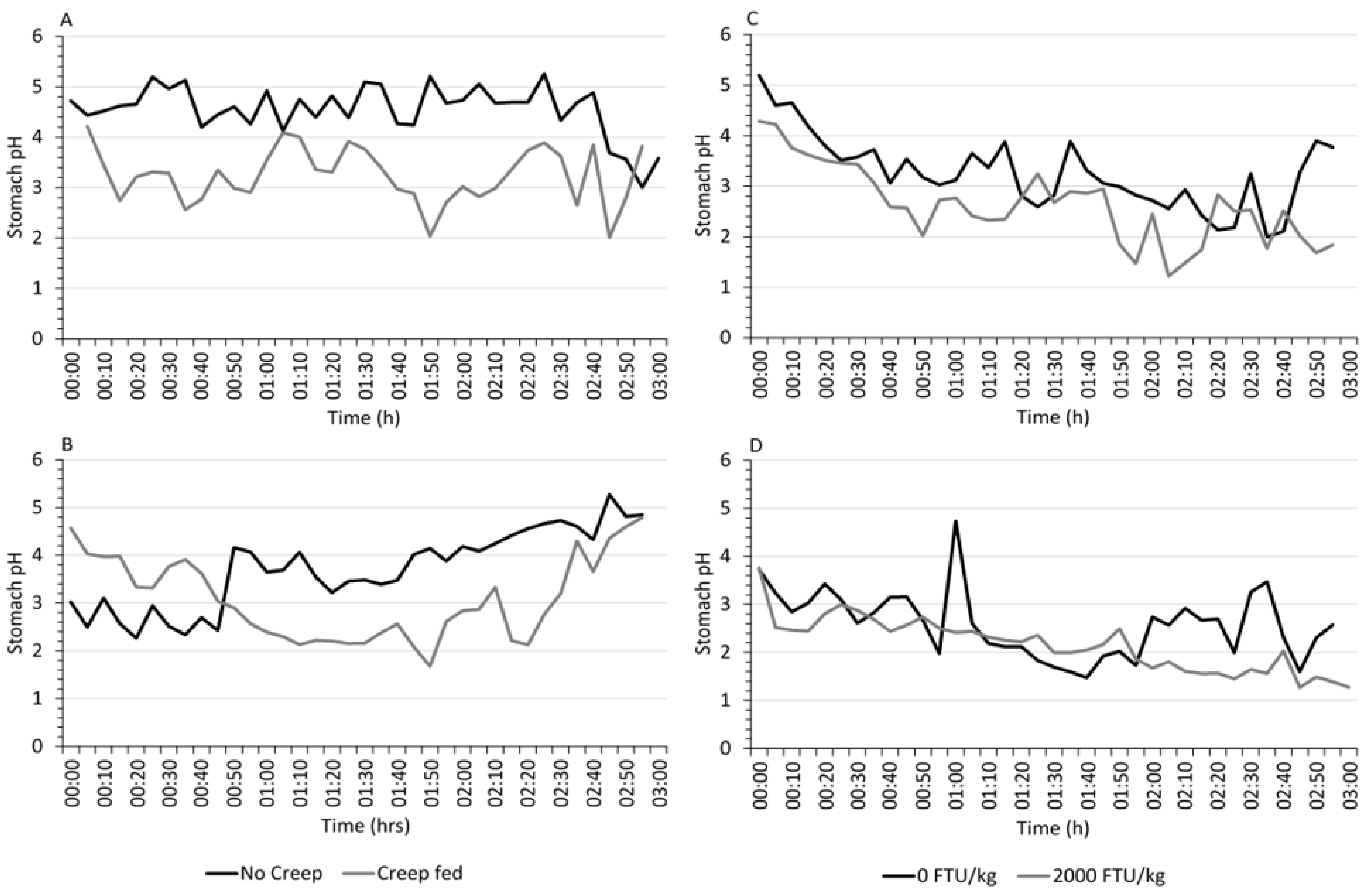
3.2. Real-Time Gastric pH
3.3. Plasma myo-Inositol and Cortisol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, G.; Pluske, J. The low feed intake in newly-weaned pigs: Problems and possible solutions. Asian Australas J. Anim. Sci. 2007, 20, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Lallès, J.-P.; Boudry, G.; Favier, C.; Le Floc’h, N.; Luron, I.; Montagne, L.; Oswald, I.P.; Pié, S.; Piel, C.; Sève, B. Gut function and dysfunction in young pigs: Physiology. Anim. Res. 2004, 53, 301–316. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Guidance for Industry# 213: New Animal Drugs and New Animal Drug Combination Products Administered in or on Medicated Feed or Drinking Water of Food Producing Animals: Recommendations for Drug Sponsors for Voluntarily Aligning Product Use Conditions with GFI# 209; Center for Veterinary Medicine; US Department of Health and Human Services: Washington, DC, USA, 2013. [Google Scholar]
- European Food Safety Authority and European Medicines Agency. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 2017, 15, e04666. [Google Scholar]
- Heo, J.; Opapeju, F.; Pluske, J.; Kim, J.; Hampson, D.; Nyachoti, C. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Lalles, J.-P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Schwarz, C.; Schedle, K. Phytate in pig and poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2015, 99, 605–625. [Google Scholar] [CrossRef]
- Selle, P.H.; Cowieson, A.J.; Cowieson, N.P.; Ravindran, V. Protein–phytate interactions in pig and poultry nutrition: A reappraisal. Nutr. Res. Rev. 2012, 25, 1–17. [Google Scholar] [CrossRef]
- Bedford, M.R. Exogenous enzymes in monogastric nutrition—Their current value and future benefits. Anim. Feed Sci. Technol. 2000, 86, 1–13. [Google Scholar] [CrossRef]
- Woyengo, T.; Weihrauch, D.; Nyachoti, C. Effect of dietary phytic acid on performance and nutrient uptake in the small intestine of piglets. J. Anim. Sci. 2012, 90, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Selle, P.H.; Ravindran, V. Phytate-degrading enzymes in pig nutrition. Livest. Sci. 2008, 113, 99–122. [Google Scholar] [CrossRef]
- Wilcock, P.; Walk, C.L. Low phytate nutrition—What is the pig and poultry industry doing to counter dietary phytate as an antinutrient and how is it being applied. In Phytate Destruction-Consequences for Precision Animal Nutrition; Walk, C.L., Kühn, I., Stein, H.H., Kidd, M.T., Rodehutscord, M., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 87–106. [Google Scholar]
- Santos, T.T.; Walk, C.L.; Wilcock, P.; Cordero, G.; Chewning, J. Performance and bone characteristics of growing pigs fed diets marginally deficient in available phosphorus and a novel microbial phytase. Can. J. Anim. Sci. 2014, 94, 493–497. [Google Scholar] [CrossRef]
- Laird, S.; Kühn, I.; Miller, H.M. Super-dosing phytase improves the growth performance of weaner pigs fed a low iron diet. Anim. Feed Sci. Technol. 2018, 242, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Kühn, I.; Bedford, M.R.; Whitfield, H.; Brearley, C.; Adeola, O.; Ajuwon, K.M. Effect of phytase on intestinal phytate breakdown, plasma inositol concentrations, and glucose transporter type 4 abundance in muscle membranes of weanling pigs. J. Anim. Sci. 2019, 97, 3907–3919. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Nagalakshmi, D.; Raju, M.V.L.N.; Rama Rao, S.V.; Bedford, M.R. Effect of phytase superdosing, myo-inositol and available phosphorus concentrations on performance and bone mineralisation in broilers. Anim. Nutr. 2017, 3, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-inositol: Its metabolism and potential implications for poultry nutrition—A review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef]
- Lee, S.A.; Bedford, M.R. Inositol—An Effective Growth Promotor? Worlds Poult. Sci. J. 2016, 72, 743–760. [Google Scholar] [CrossRef]
- Moran, K.; Wilcock, P.; Elsbernd, A.; Zier-Rush, C.; Boyd, R.D.; van Heugten, E. Effects of super-dosing phytase and inositol on growth performance and blood metabolites of weaned pigs housed under commercial conditions1. J. Anim. Sci. 2019, 97, 3007–3015. [Google Scholar] [CrossRef]
- Cranwell, P.D.; Noakes, D.E.; Hill, K.J. Gastric secretion and fermentation in the suckling pig. Br. J. Nutr. 1976, 36, 71–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cranwell, P.D. The development of acid and pepsin (EC 3. 4. 23. 1) secretory capacity in the pig; the effects of age and weaning: 1. Studies in anaesthetized pigs. Br. J. Nutr. 1985, 54, 305–320. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; Kim, I.H. Creep feeding improves growth performance of suckling piglets. Rev. Bras. Zootec. 2018, 47. [Google Scholar] [CrossRef] [Green Version]
- Sulabo, R.C.; Jacela, J.Y.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; DeRouchey, J.M.; Nelssen, J.L. Effects of lactation feed intake and creep feeding on sow and piglet performance. J. Anim. Sci. 2010, 88, 3145–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruininx, E.M.A.M.; Schellingerhout, A.B.; Binnendijk, G.P.; Peet-Schwering, C.M.C.v.d.; Schrama, J.W.; den Hartog, L.A.; Everts, H.; Beynen, A.C. Individually assessed creep food consumption by suckled piglets: Influence on post-weaning food intake characteristics and indicators of gut structure and hind-gut fermentation. Anim. Sci. 2004, 78, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Park, B.-C.; Ha, D.-M.; Park, M.-J.; Lee, C.Y. Effects of milk replacer and starter diet provided as creep feed for suckling pigs on pre- and post-weaning growth. Anim. Sci. J. 2014, 85, 872–878. [Google Scholar] [CrossRef]
- Lee, S.A.; Dunne, J.; Febery, E.; Wilcock, P.; Mottram, T.; Bedford, M.R. Superdosing phytase reduces real-time gastric pH in broilers and weaned piglets. Br. Poult. Sci. 2018, 59, 330–339. [Google Scholar] [CrossRef]
- Lee, S.A.; Dunne, J.; Febery, E.; Brearley, C.A.; Mottram, T.; Bedford, M.R. Exogenous phytase and xylanase exhibit opposing effects on real-time gizzard pH in broiler chickens. Br. Poult. Sci. 2018, 59, 568–578. [Google Scholar] [CrossRef] [Green Version]
- Mahan, D.C. Effect of weight, split-weaning, and nursery feeding programs on performance responses of pigs to 105 kilograms body weight and subsequent effects on sow rebreeding interval. J. Anim. Sci. 1993, 71, 1991–1995. [Google Scholar] [CrossRef]
- Sulabo, R.C.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; DeRouchey, J.M.; Nelssen, J.L. Effects of varying creep feeding duration on the proportion of pigs consuming creep feed and neonatal pig performance. J. Anim. Sci. 2010, 88, 3154–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Jang, H.D.; Kim, I.H. Effects of Varying Creep Feed Duration on Pre-weaning and Post-weaning Performance and Behavior of Piglet and Sow. Asian Australas J. Anim. Sci. 2011, 24, 1601–1606. [Google Scholar] [CrossRef]
- Yan, L.; Jang, H.D.; Kim, I.H. Effects of Creep Feed with Varied Energy Density Diets on Litter Performance. Asian Australas J. Anim. Sci. 2011, 24, 1435–1439. [Google Scholar] [CrossRef]
- Pajor, E.A.; Fraser, D.; Kramer, D.L. Consumption of solid food by suckling pigs: Individual variation and relation to weight gain. Appl. Anim. Behav. Sci. 1991, 32, 139–155. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.S.; Ijssennagger, N.; Kies, A.K.; Mil, S.W.C.v. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G159–G170. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Berrocoso, J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kuller, W.; van Beers-Schreurs, H.; Soede, N.; Langendijk, P.; Taverne, M.; Kemp, B.; Verheijden, J. Creep feed intake during lactation enhances net absorption in the small intestine after weaning. Livest. Sci. 2007, 108, 99–101. [Google Scholar] [CrossRef]
- Muns, R.; Magowan, E. The effect of creep feed intake and starter diet allowance on piglets’ gut structure and growth performance after weaning. J. Anim. Sci. 2018, 96, 3815–3823. [Google Scholar] [CrossRef]
- Brooks, P.; Russell, S.; Carpenter, J. Water intake of weaned piglets from three to seven weeks old. Vet. Rec. 1984, 115, 513–515. [Google Scholar] [CrossRef]
- Foltmann, B.; Lønblad, P.; Axelsen, N.H. Demonstration of chymosin (EC 3.4.23.4) in the stomach of newborn pig. Biochem. J. 1978, 169, 425–427. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, R.; Middelkoop, A.; de Souza, J.G.; van Veen, L.A.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E.; Kleerebezem, M. Impact of early-life feeding on local intestinal microbiota and digestive system development in piglets. Sci. Rep. 2021, 11, 4213. [Google Scholar] [CrossRef]
- Morgan, N.K.; Walk, C.L.; BEDFORD, M.R.; Burton, E.J. The effect of dietary calcium inclusion on broiler gastrointestinal pH: Quantification and method optimization. Poult. Sci. 2014, 93, 354–363. [Google Scholar] [CrossRef]
- Foltmann, B.; Jensen, A.L.; Lønblad, P.; Smidt, E.; Axelsen, N.H. A developmental analysis of the production of chymosin and pepsin in pigs. Comp. Biochem. Physiol. B Comp. Biochem. 1981, 68, 9–13. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic–pituitary–adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef]
- Ruis, M.A.W.; Te Brake, J.H.A.; Engel, B.; Ekkel, E.D.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. The Circadian Rhythm of Salivary Cortisol in Growing Pigs: Effects of Age, Gender, and Stress. Physiol. Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- van der Meulen, J.; Koopmans, S.J.; Dekker, R.A.; Hoogendoorn, A. Increasing weaning age of piglets from 4 to 7 weeks reduces stress, increases post-weaning feed intake but does not improve intestinal functionality. Animals 2010, 4, 1653–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeser, A.J.; Klok, C.V.; Ryan, K.A.; Wooten, J.G.; Little, D.; Cook, V.L.; Blikslager, A.T. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G173–G181. [Google Scholar] [CrossRef]
- Veum, T.L.; Bollinger, D.W.; Buff, C.E.; Bedford, M.R. A genetically engineered Escherichia coli phytase improves nutrient utilization, growth performance, and bone strength of young swine fed diets deficient in available phosphorus. J. Anim. Sci. 2006, 84, 1147–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jendza, J.A.; Dilger, R.N.; Adedokun, S.A.; Sands, J.S.; Adeola, O. Escherichia coli phytase improves growth performance of starter, grower, and finisher pigs fed phosphorus-deficient diets1. J. Anim. Sci. 2005, 83, 1882–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornegay, E.T.; Qian, H. Replacement of inorganic phosphorus by microbial phytase for young pigs fed on a maiz-soyabean-meal diet. Br. J. Nutr. 1996, 76, 563–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeola, O.; Olukosi, O.A.; Jendza, J.A.; Dilger, R.N.; Bedford, M.R. Response of growing pigs to Peniophora lycii- and Escherichia coli- derived phytases or varying ratios of calcium to total phosphorus. Anim. Sci. 2006, 82, 637–644. [Google Scholar] [CrossRef]
- Walk, C.L.; Bedford, M.R.; Santos, T.S.; Paiva, D.; Bradley, J.R.; Wladecki, H.; Honaker, C.; McElroy, A.P. Extra-phosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 2013, 92, 719–725. [Google Scholar] [CrossRef]
- Guggenbuhl, P.; Calvo, E.P.; Fru, F. Effect of a bacterial 6-phytase on plasma myo-inositol concentrations and P and Ca utilization in swine. J. Anim. Sci. 2016, 94, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Sommerfeld, V.; Kunzel, S.; Schollenberger, M.; Kuhn, I.; Rodehutscord, M. Influence of phytase or myo-inositol supplements on performance and phytate degradation products in the crop, ileum, and blood of broiler chickens. Poult. Sci. 2018, 97, 920–929. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ptak, A.; Mackowiak, P.; Sassek, M.; Pruszynska-Oszmalek, E.; Zyla, K.; Swiatkiewicz, S.; Kaczmarek, S.; Józefiak, D. The effect of microbial phytase and myo-inositol on performance and blood biochemistry of broiler chickens fed wheat/corn-based diets. Poult. Sci. 2013, 92, 2124–2134. [Google Scholar] [CrossRef]
- Rosenfelder-Kuon, P.; Klein, N.; Zegowitz, B.; Schollenberger, M.; Kühn, I.; Thuringer, L.; Seifert, J.; Rodehutscord, M. Phytate degradation cascade in pigs as affected by phytase supplementation and rapeseed cake inclusion in corn-soybean meal-based diets. J. Anim. Sci. 2020, 98, skaa053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Shin, S.; Kuehn, I.; Bedford, M.; Rodehutscord, M.; Adeola, O.; Ajuwon, K.M. Effect of phytase on nutrient digestibility and expression of intestinal tight junction and nutrient transporter genes in pigs. J. Anim. Sci. 2020, 98, skaa206. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Roos, F.F.; Ruckebusch, J.-P.; Wilson, J.W.; Guggenbuhl, P.; Lu, H.; Ajuwon, K.M.; Adeola, O. Time-series responses of swine plasma metabolites to ingestion of diets containing myo-inositol or phytase. Br. J. Nutr. 2017, 118, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Byun, S.M.; Jenness, R. Estimation of free myo-inositol in milks of various species and its source in milk of rats (Rattus norvegicus). J. Dairy Sci. 1982, 65, 531–536. [Google Scholar] [CrossRef]
- Boge, G.; Brækkan, O. Inositol i norsk fisk og fiskeprodukter (Inositol in Norwegian fish and fish products). Tidsskr. Hermetikkind. 1974, 60, 240–243. [Google Scholar]
- González-Ortiza, G.; Lee, S.A.; Vienola, K.; Raatikainen, K.; Jurgens, G.; Apajalahti, J.; Bedford, M.R. Interaction between xylanase and a proton pump inhibitor on broiler chicken performance and gut function. Anim. Nutr. 2021, in press. [Google Scholar]
- Tennant, S.M.; Hartland, E.L.; Phumoonna, T.; Lyras, D.; Rood, J.I.; Robins-Browne, R.M.; van Driel, I.R. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect. Immun. 2008, 76, 639–645. [Google Scholar] [CrossRef] [Green Version]

| Ingredient, g/kg | |
|---|---|
| Barley | 100 |
| Wheat whole meal | 346.2 |
| Wheat meal | 50 |
| Oats | 50 |
| Hypro soya | 173.7 |
| Full fat soybean | 30 |
| Whey powder | 138.9 |
| L-lysine HCl | 3.6 |
| DL-Methionine | 1.9 |
| L-Threonine | 2.0 |
| L-Tryptophan | 0.2 |
| L-Valine | 0.9 |
| Limestone | 0.9 |
| Dicalcium phosphate | 1.7 |
| Sodium chloride | 1.2 |
| Soya oil | 18.8 |
| Fish meal | 75 |
| Vitamin and mineral premix 2 | 5 |
| Analysed nutrient composition, % | |
| Digestible energy, MJ/Kg | 15.4 |
| Crude Protein | 22.25 |
| Dry Matter | 90.0 |
| Calcium | 0.87 |
| Phosphorus | 0.60 |
| Phytate phosphorus | 0.11 |
| Ash | 5.1 |
| Fat | 5.2 |
| Neutral detergent fibre | 8.8 |
| BW, kg | ADG, kg/d | ADFI, kg/d | FCR, kg:kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creep Feed | Phytase, FTU/kg | d0 | d7 | d21 | d0–7 | d7–21 | d0-21 | d0–7 | d7–21 | d0–21 | d0–7 | d7–21 | d0–21 |
| No | 6.6 | 7.6 | 14.1 | 0.14 b | 0.47 | 0.36 | 0.18 | 0.53 | 0.41 | 1.35 a | 1.13 | 1.15 | |
| Yes | 7.1 | 8.7 | 15.5 | 0.23 a | 0.49 | 0.40 | 0.22 | 0.56 | 0.45 | 0.99 b | 1.17 | 1.13 | |
| 0 | 6.8 | 8.1 | 14.5 | 0.18 | 0.46 | 0.36 | 0.20 | 0.54 | 0.43 | 1.23 | 1.19 | 1.18 a | |
| 2000 | 6.8 | 8.2 | 15.1 | 0.19 | 0.49 | 0.39 | 0.20 | 0.55 | 0.43 | 1.11 | 1.11 | 1.10 b | |
| SEM | 0.29 | 0.37 | 0.58 | 0.020 | 0.023 | 0.020 | 0.020 | 0.026 | 0.022 | 0.074 | 0.027 | 0.025 | |
| p-value | |||||||||||||
| Creep feed | 0.255 | 0.057 | 0.127 | 0.011 | 0.611 | 0.171 | 0.164 | 0.341 | 0.248 | 0.005 | 0.323 | 0.640 | |
| Phytase | 0.822 | 0.713 | 0.406 | 0.620 | 0.290 | 0.316 | 0.963 | 0.845 | 0.868 | 0.317 | 0.082 | 0.053 | |
| Creep feed × phytase | 0.797 | 0.853 | 0.996 | 0.962 | 0.839 | 0.860 | 0.727 | 0.594 | 0.755 | 0.859 | 0.560 | 0.681 | |
| 7 Days Pre-Weaning | Day of Weaning | |||||
|---|---|---|---|---|---|---|
| Creep | Min | Max | Average 1 | Min | Max | Average 2 |
| No | 3.00 | 5.25 | 4.55 a | 2.26 | 5.27 | 3.70 a |
| Yes | 2.01 | 4.21 | 3.24 b | 1.68 | 4.79 | 3.08 b |
| SEM | 0.087 | 0.124 | ||||
| p-value | <0.001 | 0.003 | ||||
| Day 7 Post-Weaning | Day 21 Post-Weaning | |||||
|---|---|---|---|---|---|---|
| Phytase, FTU/kg | Min | Max | Average 1 | Min | Max | Average 2 |
| 0 | 1.99 | 5.20 | 3.27 a | 1.47 | 4.73 | 2.58 a |
| 2000 | 1.22 | 4.29 | 2.62 b | 1.27 | 3.76 | 2.15 b |
| SEM | 0.111 | 0.082 | ||||
| p-value | <0.001 | 0.005 | ||||
| Myo-inositol (µM) | Cortisol (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Creep | Phytase, FTU/kg | d0 | d7 | d21 | d0 | d7 | d21 |
| No | 94.0 b | 44.3 | 31.3 | 87.1 | 32.0 | 10.3 | |
| Yes | 112.8 a | 39.9 | 33.2 | 91.9 | 20.3 | 10.4 | |
| 0 | - | 39.3 | 21.7 b | - | 32.3 | 10.7 | |
| 2000 | - | 44.9 | 42.7 a | - | 20.0 | 10.0 | |
| SEM | 6.07 | 3.28 | 3.07 | 16.24 | 6.95 | 1.45 | |
| p-value | |||||||
| Creep feed | 0.037 | 0.350 | 0.657 | 0.835 | 0.245 | 0.951 | |
| Phytase | - | 0.243 | <0.001 | - | 0.224 | 0.719 | |
| Creep feed × phytase | - | 0.245 | 0.271 | - | 0.730 | 0.810 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.A.; Febery, E.; Wilcock, P.; Bedford, M.R. Application of Creep Feed and Phytase Super-Dosing as Tools to Support Digestive Adaption and Feed Efficiency in Piglets at Weaning. Animals 2021, 11, 2080. https://doi.org/10.3390/ani11072080
Lee SA, Febery E, Wilcock P, Bedford MR. Application of Creep Feed and Phytase Super-Dosing as Tools to Support Digestive Adaption and Feed Efficiency in Piglets at Weaning. Animals. 2021; 11(7):2080. https://doi.org/10.3390/ani11072080
Chicago/Turabian StyleLee, Sophie A., Erica Febery, Pete Wilcock, and Michael R. Bedford. 2021. "Application of Creep Feed and Phytase Super-Dosing as Tools to Support Digestive Adaption and Feed Efficiency in Piglets at Weaning" Animals 11, no. 7: 2080. https://doi.org/10.3390/ani11072080
APA StyleLee, S. A., Febery, E., Wilcock, P., & Bedford, M. R. (2021). Application of Creep Feed and Phytase Super-Dosing as Tools to Support Digestive Adaption and Feed Efficiency in Piglets at Weaning. Animals, 11(7), 2080. https://doi.org/10.3390/ani11072080





