Toxicity of Zinc Oxide Nanoparticles on the Embryo of Javanese Medaka (Oryzias javanicus Bleeker, 1854): A Comparative Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of ZnO NPs and Test Organisms
2.2. Physicochemical Characterization of ZnO NPs
2.3. Measured Exposure Concentrations and Zinc Ion Release
2.4. Toxicity Tests
2.5. Statistical Analysis
3. Results
3.1. Characterization of ZnO NPS
3.2. Dissolution of ZnO NPs
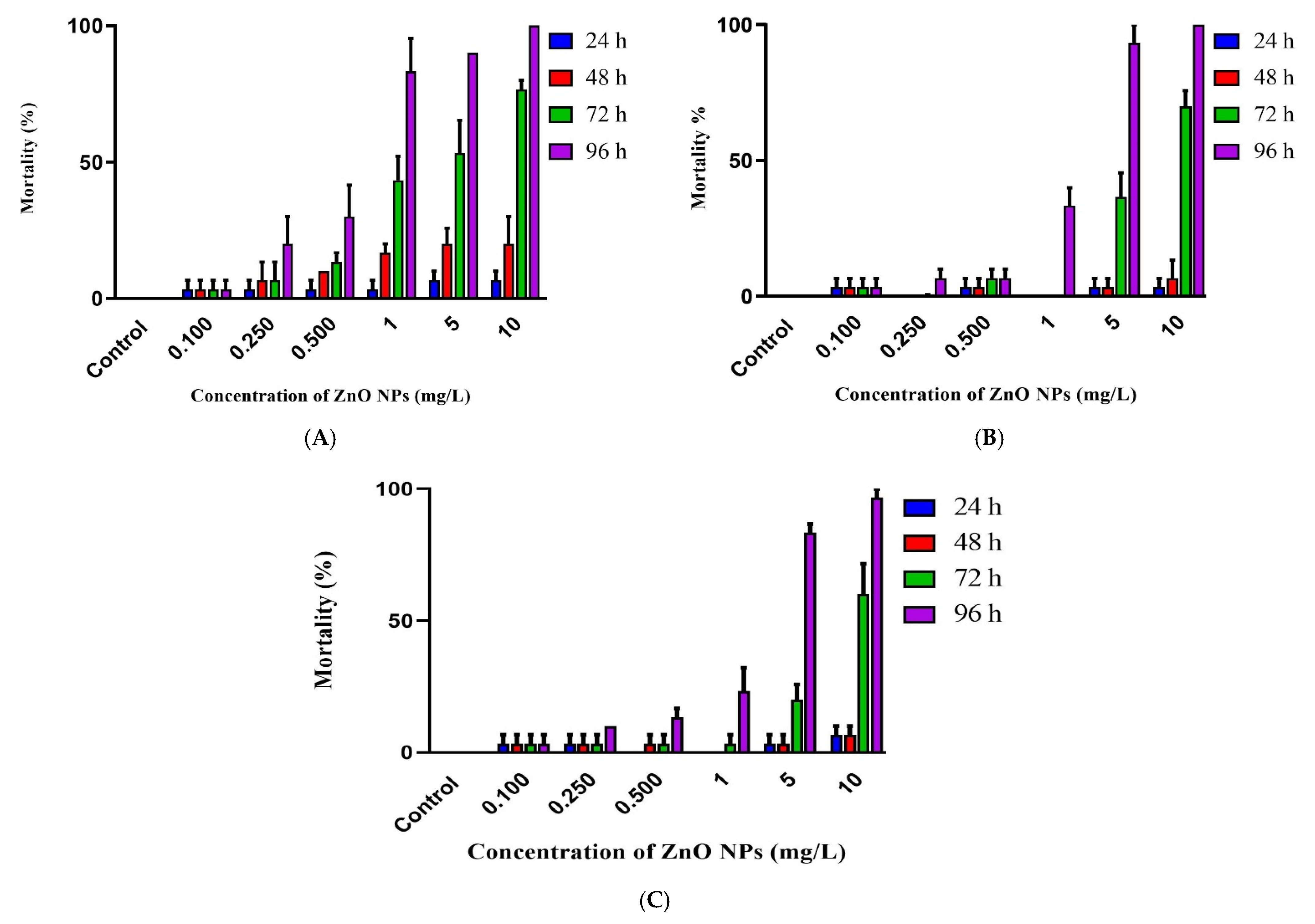
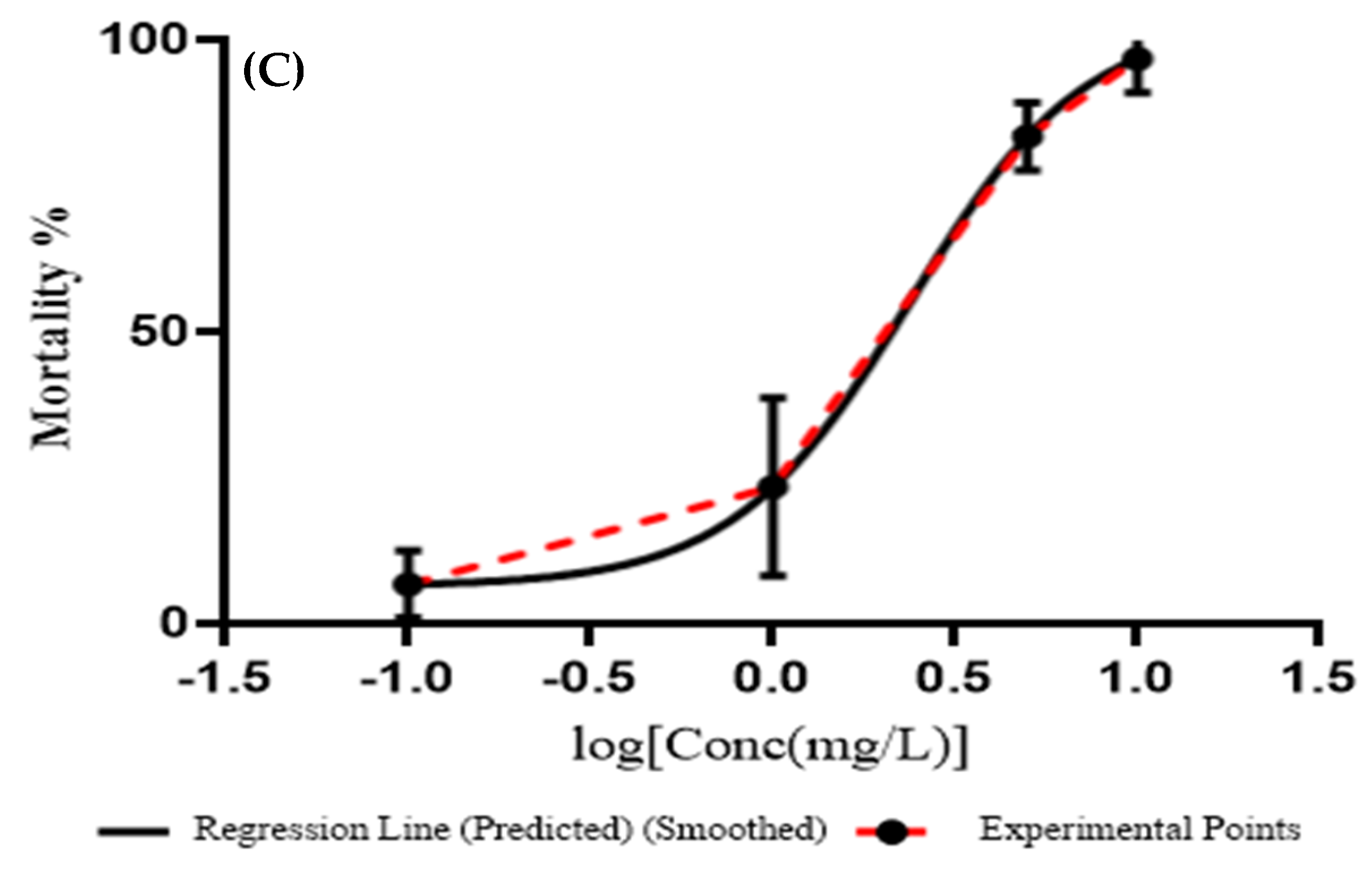
3.3. Embryotoxicity of ZnO NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide-engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef] [PubMed]
- Patibandla, S.; Zhang, Y.; Tohari, A.M.; Gu, P.; Reilly, J.; Chen, Y.; Shu, X. Comparative analysis of the toxicity of gold nanoparticles in zebrafish. J. Appl. Toxicol. 2018, 38, 1153–1161. [Google Scholar] [CrossRef]
- Li, M.; Lin, D.; Zhu, L. Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ. Pollut. 2013, 173, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.C. From ecotoxicology to nanoecotoxicology. Toxicol. 2010, 269, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A.; et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applications. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Chen, L.; Hao, J.; Zhong, N. Bioaccumulation and sub-acute toxicity of zinc oxide nanoparticles in juvenile carp (Cyprinus carpio): A comparative study with its bulk counterparts. Ecotoxicol. Environ. Saf. 2013, 91, 52–60. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Huang, R.; Miao, Z.; Cai, L.; Du, Q. Toxicity assessment and histopathological analysis of nano ZnO against marine fish (Mugilogobius chulae) embryos. J. Environ. Sci. 2018, 73, 78–88. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanoparticle Res. 2010, 12, 1645–1654. [Google Scholar] [CrossRef]
- Pereira, A.C.; Gomes, T.; Machado, M.R.F.; Rocha, T.L. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: From morphological to molecular approach. Environ. Pollut. 2019, 252, 1841–1853. [Google Scholar] [CrossRef]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total. Environ. 2014, 476–477, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.Y.; Leung, K.M.Y. Temperature-dependent toxicities of nano zinc oxide to marine diatom, amphipod and fish in relation to its aggregation size and ion dissolution. Nanotoxicology 2014, 8, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.; Mehennaoui, K.; Cambier, S.; Libralato, G.; Jomini, S.; Domingos, R.F. Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: A critical overview. Aquat. Toxicol. 2016, 170, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Poynton, H.C.; Chen, C.; Alexander, S.L.; Major, K.M.; Blalock, B.J.; Unrine, J.M. Enhanced toxicity of environmentally transformed ZnO nanoparticles relative to Zn ions in the epibenthic amphipod Hyalella Azteca. Environ. Sci. Nano 2019, 6, 325–340. [Google Scholar] [CrossRef]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.M.N.; Fougères, P.A.; Leung, Y.H.; Liu, F.; Djurišić, A.B.; Giesy, J.P.; Leung, K.M.Y. Physicochemical characteristics and toxicity of surface-modified zinc oxide nanoparticles to freshwater and marine microalgae. Sci. Rep. 2017, 7, 15909. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.P.; Fang, T.; Xiong, D.W.; Zhu, W.T.; Sima, X.F. Comparative toxicity of nano-ZnO and bulk ZnO suspensions to zebrafish and the effects of sedimentation, OH production and particle dissolution in distilled water. J. Environ. Monit. 2011, 13, 1975–1982. [Google Scholar] [CrossRef]
- Cong, Y.; Jin, F.; Wang, J.; Mu, J. The embryotoxicity of ZnO nanoparticles to marine medaka, Oryzias melastigma. Aquat. Toxicol. 2017, 185, 11–18. [Google Scholar] [CrossRef]
- Xiong, D.; Fang, T.; Yu, L.; Sima, X.; Zhu, W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: Acute toxicity, oxidative stress and oxidative damage. Sci. Total. Environ. 2011, 409, 1444–1452. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136–137, 49–59. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Heal. 2008, 43, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Sagar, B.; Doshi, S.; Chandrasekaran, N.; Mukherjee, A. Comparative study on toxicity of ZnO and TiO2 nanoparticles on Artemia salina: Effect of pre-UV-A and visible light irradiation. Environ. Sci. Pollut. Res. 2017, 24, 5633–5646. [Google Scholar] [CrossRef]
- Magtoon, W.; Termvidchakorn, A. A revised taxonomic account of Ricefish Oryzias (Beloniformes; Adrianichthyidae), in Thailand, Indonesia and Japan. Natur. Hist. J. Chula. Uni. 2009, 9, 35–68. Available online: https://li01.tci-thaijo.org/index.php/tnh/article/view/102961 (accessed on 4 February 2021).
- Inoue, K.; Takei, Y. Diverse adaptability in Oryzias species to high environmental salinity. Zool. Sci. 2002, 19, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Yusof, S. Effect of mercury and cadmium on early life stages of Java medaka (Oryzias javanicus): A potential tropical test fish. Mar. Pollut. Bull. 2011, 63, 347–349. [Google Scholar] [CrossRef]
- Yusof, S.; Ismail, A.; Koito, T.; Kinoshita, M.; Inoue, K. Occurrence of two closely related ricefishes, Javanese medaka (Oryzias javanicus) and Indian medaka (O. dancena) at sites with different salinity in Peninsular Malaysia. Environ. Biol. Fishes 2012, 93, 43–49. [Google Scholar] [CrossRef]
- Woo, S.; Won, H.; Lee, A.; Yum, S. Oxidative stress and gene expression in diverse tissues of Oryzias javanicus exposed to 17β-estradiol. Mol. Cell. Toxicol. 2012, 8, 263–269. [Google Scholar] [CrossRef]
- Salleh, A.F.M.; Amal, M.N.A.; Nasruddin, N.S.; Zulkifli, S.Z.; Yusuff, F.M.; Ibrahim, W.N.W.; Ismail, A. Water pH effects on survival, reproductive performances, and ultrastructure of gonads, gills, and skins of the Javanese medaka (Oryzias javanicus). Turk. J. Vet. Anim. Sci. 2017, 41, 471–481. [Google Scholar] [CrossRef]
- Yusuff, F.M.; Nabila, A.G.S.; Zulkifli, S.Z.; Azmai, M.N.A.; Ibrahim, W.N.W.; Yusof, S.; Ismail, A. Acute toxicity test of copper pyrithione on Javanese medaka and the behavioural stress symptoms. Mar. Pollut. Bull. 2018, 127, 150–153. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Zulkifli, S.Z.; Azmai, M.N.A.; Muhammad-Yusuff, F.M.; Ismail, A. Embryonic toxicity of 3,4-dichloroaniline (3,4-DCA) on Javanese medaka (Oryzias javanicus Bleeker, 1854). Toxicol. Rep. 2020, 7, 1039–1045. [Google Scholar] [CrossRef]
- Imai, S.; Koyama, J.; Fujii, K. Effects of estrone on full life cycle of Java medaka (Oryzias javanicus), a new marine test fish. Environ. Toxicol. Chem. 2007, 26, 726. [Google Scholar] [CrossRef]
- Kamarudin, N.A.; Zulkifli, S.Z.; Azmai, M.N.A.; Aziz, F.Z.A.; Ismail, A. Herbicide diuron as endocrine disrupting chemicals (EDCs) through histopathalogical analysis in gonads of Javanese Medaka (Oryzias javanicus, Bleeker 1854). Animals 2020, 10, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.M.; Fu, X.E.; Zeng, Y.; Liu, Y.D.; Kim, H.S.; Chon, T.S. The stepwise behavioral responses of medaka (Oryzias latipes) to organophosphorus pesticides in an online monitoring system. Procedia Environ. Sci. 2012, 13, 1122–1133. [Google Scholar] [CrossRef] [Green Version]
- OECD. Test. No. 210: Fish., Early-Life Stage Toxicity Test; OECD publishing: Paris, France, 1992. [Google Scholar]
- Chen, T.H.; Lin, C.C.; Meng, P.J. Zinc oxide nanoparticles alter hatching and larval locomotor activity in zebrafish (Danio rerio). J. Hazard. Mater. 2014, 277, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Fairbairn, E.A.; Keller, A.A.; Mädler, L.; Zhou, D.; Pokhrel, S.; Cherr, G.N. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571. [Google Scholar] [CrossRef]
- Yung, M.M.N.; Wong, S.W.Y.; Kwok, K.W.H.; Liu, F.Z.; Leung, Y.H.; Chan, W.T.; Li, X.Y.; Djurišić, A.B.; Leung, K.M.Y. Salinity-dependent toxicities of zinc oxide nanoparticles to the marine diatom Thalassiosira pseudonana. Aquat. Toxicol. 2015, 165, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, S.; Yoo, J.; Lee, J.S.; Park, J.W.; Jung, J. Effect of salinity on acute copper and zinc toxicity to Tigriopus japonicus: The difference between metal ions and nanoparticles. Mar. Pollut. Bull. 2014, 85, 526–531. [Google Scholar] [CrossRef]
- Yung, M.M.N.; Kwok, K.W.H.; Djurišić, A.B.; Giesy, J.P.; Leung, K.M.Y. Influences of temperature and salinity on physicochemical properties and toxicity of zinc oxide nanoparticles to the marine diatom Thalassiosira pseudonana. Sci. Rep. 2017, 7, 3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, M.M.N.; Mouneyrac, C.; Leung, K.M.Y. Ecotoxicity of zinc oxide nanoparticles in the marine environment. Encycl. Nanotechnol. 2016, 1–17. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Wiesner, M.R.; Bottero, J.Y. Chemical stability of metallic nanoparticles: A parameter controlling their potential cellular toxicity in vitro. Environ. Pollut. 2009, 157, 1127–1133. [Google Scholar] [CrossRef]
- Hogstrand, C.; Wilson, R.W.; Polgar, D.; Wood, C.M. Effects of zinc on the kinetics of branchial calcium uptake in freshwater rainbow trout during adaptation to waterborne zinc. J. Exp. Biol. 1994, 186, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.; McGeer, J.C. Development of a biotic ligand model for the acute toxicity of zinc to Daphnia pulex in soft waters. Aquat. Toxicol. 2009, 91, 26–32. [Google Scholar] [CrossRef]
- Heijerick, D.G.; Schamphelaere, K.A.C.D.; Janssen, C.R. Biotic ligand model development predicting Zn toxicity to the alga Pseudokirchneriella subcapitata: Possibilities and limitations. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 207–218. [Google Scholar] [CrossRef]
- Saddick, S.; Afifi, M.; Zinada, O.A.A. Effect of zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi J. Biol. Sci. 2017, 24, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cai, J.; Wang, S.; You, H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health. 2017, 30, 213–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehmas, L.C.; Anders, C.; Chess, J.; Punnoose, A.; Pereira, C.B.; Greenwood, J.A.; Tanguay, R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015, 2, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Morgalev, Y.N.; Gosteva, I.A.; Morgaleva, T.G.; Morgalev, S.Y.; Kostenko, E.V.; Kudryavtsev, B.A. Parameters of embryogenesis in zebrafish Danio rerio as indicators of the ecological toxicity of zinc oxide nanoparticles. Nanotechnologies Russ. 2018, 13, 311–316. [Google Scholar] [CrossRef]
- Taherian, S.M.R.; Hosseini, S.A.; Jafari, A.; Etminan, A.; Birjandi, M. Acute toxicity of zinc oxide nanoparticles from satureja hortensis on rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 2020, 20, 481–489. [Google Scholar] [CrossRef]
- Aziz, S.; Abdullah, S.; Abbas, K.; Zia, M.A. Effects of engineered zinc oxide nanoparticles on freshwater fish, Labeo rohita: Characterization of ZnO nanoparticles, acute toxicity and oxidative stress. Pak. Vet. J. 2020, 40, 479–483. [Google Scholar] [CrossRef]



| Medium | Ultra-Pure Water | Deionized Water | Dechlorinated Tap Water |
|---|---|---|---|
| Zeta potential (mV) | −6.43 | 3.04 | 2.01 |
| Size distribution (nm) | 1079 | 3209 | 3652 |
| Species | Life Stage | Duration of Exposure (Hours) | Medium | Median Lethal Concentration (LC50) (mg/L)/(ppm) | Reference |
|---|---|---|---|---|---|
| Oryzias Javanicus (Javanese medaka) | Embryo | 96 | Ultra-pure water | 0.6438 | Present study |
| Deionized water | 1.333 | ||||
| Dechlorinated tap water | 2.370 | ||||
| Danio rerio (Zebrafish) | Embryo | 96 | Zebrafish culture medium | 60 | [49] |
| Danio rerio (Zebrafish) | Embryo | 96 | Drinking water | 30.51 | [51] |
| Danio rerio (Zebrafish) | Embryo | 96 | Distilled water | 4.92 | [20] |
| Danio rerio (Zebrafish) | Embryo | 96 | Milli-Q® water | 1.793 | [22] |
| Oncorhynchus mykiss (Rainbow trout) | Adult | 96 | Dechlorinated tap water | 25.50 | [52] |
| Oreochromis niloticu (Nile tilapia) | Adult | 96 | Deionized water | 5.5 | [48] |
| Coptodon zilli (Red belly tilapia) | 5.6 | ||||
| Labeo rohita | Adult | 96 | Deionized water | 31.15 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, N.; Zulkifli, S.Z.; Azmai, M.N.A.; Ismail, A. Toxicity of Zinc Oxide Nanoparticles on the Embryo of Javanese Medaka (Oryzias javanicus Bleeker, 1854): A Comparative Study. Animals 2021, 11, 2170. https://doi.org/10.3390/ani11082170
Amin N, Zulkifli SZ, Azmai MNA, Ismail A. Toxicity of Zinc Oxide Nanoparticles on the Embryo of Javanese Medaka (Oryzias javanicus Bleeker, 1854): A Comparative Study. Animals. 2021; 11(8):2170. https://doi.org/10.3390/ani11082170
Chicago/Turabian StyleAmin, Naweedullah, Syaizwan Zahmir Zulkifli, Mohammad Noor Amal Azmai, and Ahmad Ismail. 2021. "Toxicity of Zinc Oxide Nanoparticles on the Embryo of Javanese Medaka (Oryzias javanicus Bleeker, 1854): A Comparative Study" Animals 11, no. 8: 2170. https://doi.org/10.3390/ani11082170
APA StyleAmin, N., Zulkifli, S. Z., Azmai, M. N. A., & Ismail, A. (2021). Toxicity of Zinc Oxide Nanoparticles on the Embryo of Javanese Medaka (Oryzias javanicus Bleeker, 1854): A Comparative Study. Animals, 11(8), 2170. https://doi.org/10.3390/ani11082170








