Potential Distribution of Amphibians with Different Habitat Characteristics in Response to Climate Change in South Korea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Species Occurrence Data
2.3. Environmental Variables
2.4. Habitat Characteristics Related to Breeding Behavior
2.5. Model Development, Evaluation, and Validation
2.6. Suitable Habitats and Species Richness
3. Results
3.1. Model Evaluation and Validation
3.2. Variable Contributions in the Model
3.3. Climate Change Impacts on Amphibian Species in Each Group
3.4. Habitat Suitability
3.4.1. Group 1: Wetland Amphibians
3.4.2. Group 2: Migrating Amphibians
3.4.3. Group 3: Forest-Dwelling Amphibians
3.5. Species Richness
3.5.1. Group 1: Wetland Amphibians
3.5.2. Group 2: Migrating Amphibians
3.5.3. Group 3: Forest-Dwelling Amphibians
3.6. Overall Species Richness
4. Discussion
4.1. Current and Future Potential Habitat Suitability
4.2. Potential Species Richness under Current Conditions
4.3. Potential Species Richness under Future Conditions
4.4. Limitations and Possible Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
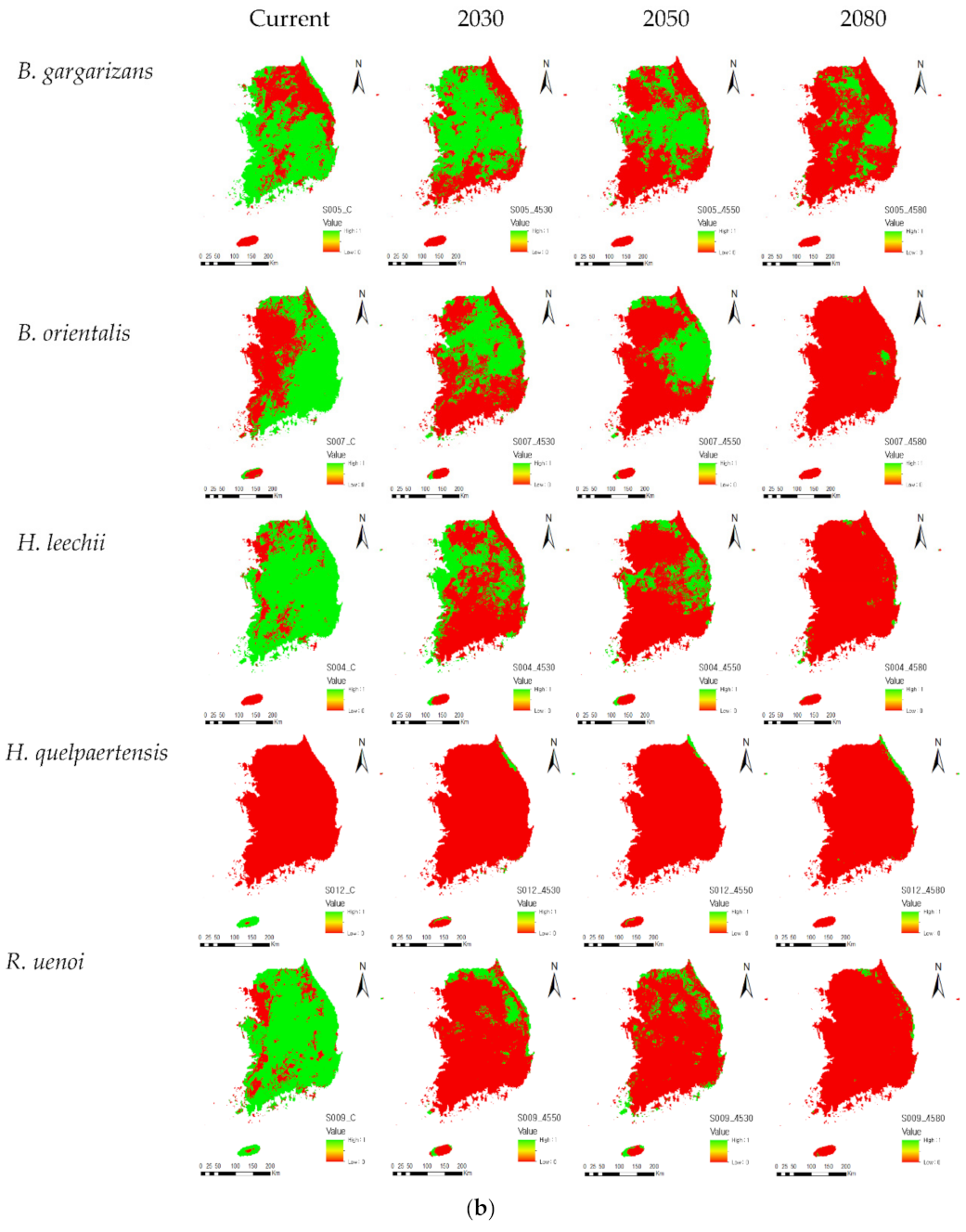
Appendix A. Preliminary Results of Species Distribution Analysis Using EcoBank Analysis Module for 16 Amphibians in Korea

Appendix B. Potential Spatial Distribution of Individual Amphibian Species under RCP4.5


References
- Wilby, R.L.; Perry, G.L. Climate change, biodiversity and the urban environment: A critical review based on London, UK. Prog. Phys. Geogr. 2006, 30, 73–98. [Google Scholar] [CrossRef]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, J.M.; Pineda, E.; López-Barrera, F.; Moreno, C.E. Amphibian species and functional diversity as indicators of restoration success in tropical montane forest. Biodivers. Conserv. 2017, 26, 2569–2589. [Google Scholar] [CrossRef]
- Ochoa-Ochoa, L.M.; Rodríguez, P.; Mora, F.; Flores-Villela, O.; Whittaker, R.J. Climate change and amphibian diversity patterns in Mexico. Biol. Conserv. 2012, 150, 94–102. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and Trends of Amphibian Declines and Extinctions Worldwide. Science 2004, 306, 1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pough, H.F. Amphibian biology and husbandry. ILAR J. 2007, 48, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2010; p. 1148. [Google Scholar]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Young, B.E.; Lips, K.R.; Reaser, J.K.; Ibáñez, R.; Salas, A.W.; Cedeño, J.R.; Coloma, L.A.; Ron, S.; La Marca, E.; Meyer, J.R. Population declines and priorities for amphibian conservation in Latin America. Conserv. Biol. 2001, 15, 1213–1223. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Agostini, M.G.; Roesler, I.; Bonetto, C.; Ronco, A.E.; Bilenca, D. Pesticides in the real world: The consequences of GMO-based intensive agriculture on native amphibians. Biol. Conserv. 2020, 241, 108355. [Google Scholar] [CrossRef]
- Knapp, R.A. Effects of nonnative fish and habitat characteristics on lentic herpetofauna in Yosemite National Park, USA. Biol. Conserv. 2005, 121, 265–279. [Google Scholar] [CrossRef]
- Miró, A.; Ventura, M. Introduced fish in Pyrenean high mountain lakes: Impact on amphibians and other organisms, and conservation implications. Limnetica 2020, 39, 283–297. [Google Scholar]
- Pilliod, D.S.; Hossack, B.R.; Bahls, P.F.; Bull, E.L.; Corn, P.S.; Hokit, G.; Maxell, B.A.; Munger, J.C.; Wyrick, A. Non-native salmonids affect amphibian occupancy at multiple spatial scales. Divers. Distrib. 2010, 16, 959–974. [Google Scholar] [CrossRef] [Green Version]
- Ficetola, G.F.; Rondinini, C.; Bonardi, A.; Baisero, D.; Padoa-Schioppa, E. Habitat availability for amphibians and extinction threat: A global analysis. Divers. Distrib. 2015, 21, 302–311. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Global Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Do, M.S.; Lee, J.-W.; Jang, H.-J.; Kim, D.-I.; Park, J.; Yoo, J.-C. Spatial distribution patterns and prediction of hotspot area for endangerd herpetofauna species in Korea. Korean J. Environ. Ecol. 2017, 31, 381–396. (In Korean) [Google Scholar] [CrossRef]
- Jeong, S.; Seo, C.; Yoon, J.; Lee, D.K.; Park, J. A sutudy on riparian habitats for amphibians using habitat suitability model. J. Environ. Impact Assess. 2015, 24, 175–189. (In Korean) [Google Scholar] [CrossRef]
- Jung, J.-H.; Lee, E.-J.; Lee, W.-S.; Park, C.-D. Habitat suitability models of Korean crevice salamander (Karsenia koreana) at forested area in Daejeon metropolitan city, Republic of Korea. J. For. Res. 2019, 24, 349–355. [Google Scholar] [CrossRef]
- Borzée, A.; Andersen, D.; Groffen, J.; Kim, H.-T.; Bae, Y.; Jang, Y. Climate change-based models predict range shifts in the distribution of the only Asian plethodontid salamander: Karsenia koreana. Sci. Rep. 2019, 9, 11838. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.Y.; Son, S.; Suh, G.U.; Herrando-Moraira, S.; Lee, C.H.; López-Pujol, J.; Chung, M.G. The Korean Baekdudaegan Mountains: A glacial refugium and a biodiversity hotspot that needs to be conserved. Front. Genet. 2018, 9, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-D.; Miller-Rushing, A.J. Degradation, urbanization, and restoration: A review of the challenges and future of conservation on the Korean Peninsula. Biol. Conserv. 2014, 176, 262–276. [Google Scholar] [CrossRef]
- Kim, J.-U.; Boo, K.-O.; Choi, J.; Byun, Y.-H. One hundred years of climate change on the Korean Peninsula. Annu. Rep. Natl. Inst. Meteorol. Sci. 2018. [Google Scholar]
- Borzée, A.; Min, M.-S. Disentangling the Impacts of speciation, sympatry and the island effect on the morphology of seven Hynobius sp. salamanders. Animals 2021, 11, 187. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. The Second and Third National Ecosystem Survey: 1997–2012; National Institute of Environmental Research: Incheon, Korea, 2013. (In Korean) [Google Scholar]
- Kim, J.-B.; Song, J.-Y. Amphibians and Reptiles of Korea; World Science Publisher: Seoul, Korea, 2010; p. 146. (In Korean) [Google Scholar]
- Kim, H.W.; Yoon, S.; Kim, M.; Shin, M.; Yoon, H.; Kim, K. EcoBank: A flexible database platform for sharing ecological data. Biodivers. Data J. 2021, 9, e61866. [Google Scholar] [CrossRef]
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Shin, M.-S.; Adhikari, P.; Seo, C. Potential impact of climate change on plant invasion in the Republic of Korea. J. Ecol. Environ. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Adhikari, P.; Shin, M.-S.; Jeon, J.-Y.; Kim, H.W.; Hong, S.; Seo, C. Potential impact of climate change on the species richness of subalpine plant species in the mountain national parks of South Korea. J. Ecol. Environ. 2018, 42, 36. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Adhikari, P.; Seo, C. Impact of climate change on potential dispersal of Paeonia obovata (paeoniaceae), a critically endangered medicinal plant of South Korea. Ecol. Environ. Conserv. 2020, 26, S145–S155. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Package ‘dismo’. Circles 2017, 9, 1–68. [Google Scholar]
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Oh, H.-S.; Adhikari, P.; Seo, C. Northward Range Expansion of Southern Butterflies According to Climate Change in South Korea. J. Clim. Chang. Res. 2020, 11, 643–656. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, D. The Encyclopedia of Korean Amphibians; The Nature and Ecology: Seoul, Korea, 2016. (In Korean) [Google Scholar]
- Hirai, T.; Matsui, M. Feeding Relationships between Hyla japonica and Rana nigromaculata in Rice Fields of Japan. J. Herpetol. 2002, 36, 662–667. [Google Scholar] [CrossRef]
- Muñoz-Carpena, R.; Parsons, J.E.; Gilliam, J.W. Numerical Approach to the Overland Flow Process in Vegetative Filter Strips. Trans. ASABE 1993, 36, 761–770. [Google Scholar] [CrossRef]
- Yoon, S.; Min, J.; Hwang, S. The climatic change during the historical age inferred from vegetation environment in alpine moors in the Korean Peninsula. Korean J. Geomorphol. Assoc. 2014, 21, 69–83. (In Korean) [Google Scholar]
- Todd, B.D.; Winne, C.T. Ontogenetic and interspecific variation in timing of movement and responses to climatic factors during migrations by pond-breeding amphibians. Can. J. Zool. 2006, 84, 715–722. [Google Scholar] [CrossRef]
- Choi, W.-J.; Park, D.; Kim, J.-K.; Lee, J.-H.; Kim, D.-I.; Kim, I.-H. Changes in the Reproductive Population Size of the Huanren Brown Frog (Rana huanrenensis) and Wonsan Salamander (Hynobius leechii), which Breeding in Mountain Valleys, According to Climate Change. Korean J. Environ. Ecol. 2018, 32, 582–590. (In Korean) [Google Scholar] [CrossRef]
- Park, D.; Park, S.-R. Multiple Insemination and Reproductive Biology of Hynobius leechii. J. Herpetol. 2000, 34, 594–598. [Google Scholar] [CrossRef]
- Kafash, A.; Ashrafi, S.; Ohler, A.; Yousefi, M.; Malakoutikhah, S.; Koehler, G.; Schmidt, B.R. Climate change produces winners and losers: Differential responses of amphibians in mountain forests of the Near East. Glob. Ecol. Conserv. 2018, 16, e00471. [Google Scholar] [CrossRef]
- Min, M.S.; Yang, S.Y.; Bonett, R.M.; Vieites, D.R.; Brandon, R.A.; Wake, D.B. Discovery of the first Asian plethodontid salamander. Nature 2005, 435, 87–90. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Préau, C.; Trochet, A.; Bertrand, R.; Isselin-Nondedeu, F. Modeling potential distributions of three european amphibian species comparing enfa and Maxent Préau et al.-MaxEnt and ENFA modeling on three amphibian species. Herpetol. Conserv. Biol. 2018, 13, 91–104. [Google Scholar]
- Swets, J. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.N.; Seo, C.; Thorne, J.; Nelson, J.K.; Erwin, S.; O’Brien, J.M.; Schwartz, M.W. Using species distribution models to predict new occurrences for rare plants. Divers. Distrib. 2009, 15, 565–576. [Google Scholar] [CrossRef]
- Yoo, C.; Kim, S.; Kim, T.-W. Assessment of drought vulnerability based on the soil moisture PDF. Stoch. Environ. Res. Risk Assess. 2006, 21, 131–141. [Google Scholar] [CrossRef]
- Hamer, A.J.; McDonnell, M.J. Amphibian ecology and conservation in the urbanising world: A review. Biol. Conserv. 2008, 141, 2432–2449. [Google Scholar] [CrossRef]
- Manzoor, S.A.; Griffiths, G.; Iizuka, K.; Lukac, M. Land Cover and Climate Change May Limit Invasiveness of Rhododendron ponticum in Wales. Front. Plant Sci. 2018, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Oh, H.-S.; Hong, C.-E. Current conditions of habitat for Rana catesbeiana and Trachemys scripta elegans imported to Jeju-do, including proposed management plans. Korean J. Environ. Ecol. 2007, 21, 311–317. (In Korean) [Google Scholar]
- Kim, H.-M.; Kim, M.-J.; Baek, H.-J.; Kim, S.-H.; An, K.-G.; Lee, J.-H. Predicting demographical change and potential habitat of the American bullfrog introduced to Korean peninsula. In Proceedings of the 11th Conference, Korean Society of Herpetologists, National Institute of Ecology, Seocheon-gun, Korea, 13 July 2018. [Google Scholar]
- Baek, H.-J.; Lee, J.; Lee, H.-S.; Hyun, Y.-K.; Cho, C.; Kwon, W.-T.; Marzin, C.; Gan, S.-Y.; Kim, M.-J.; Choi, D.-H.J. Climate change in the 21st century simulated by HadGEM2-AO under representative concentration pathways. Asia Pac. J. Atmos. Sci. 2013, 49, 603–618. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef] [Green Version]
- Gascon, C.; Collins, J.P.; Moore, R.D.; Church, D.R.; McKay, J.E.; Mendelson, J.R., III (Eds.) Amphibian Conservation Action Plan; International Union for Conservation of Nature-Species Survival Commission (IUCN-SSC) Amphibian Specialist Group: Gland, Switzerland; Cambridge, UK, 2007; p. 64. [Google Scholar]
- Zippel, K.; Johnson, K.; Gagliardo, R.; Gibson, R.; McFadden, M.; Browne, R.; Martinez, C.; Townsend, E. The amphibian ark: A global community for ex situ conservation of amphibians. Herpetol. Conserv. Biol. 2011, 6, 340–352. [Google Scholar]
- Ortega-Andrade, H.M.; Rodes Blanco, M.; Cisneros-Heredia, D.F.; Guerra Arévalo, N.; López de vargas-Marchuca, K.G.; Sánchez-Nivicela, J.C.; Armijos-Ojeda, D.; Cáceres Andrade, J.F.; Reyes-Puig, C.; Quezada Riera, A.B. Red list assessment of amphibian species of Ecuador: A multidimensional approach for their conservation. PLoS ONE 2021, 16, e0251027. [Google Scholar] [CrossRef]
- Gonçalves, J.; Honrado, J.P.; Vicente, J.R.; Civantos, E. A model-based framework for assessing the vulnerability of low dispersal vertebrates to landscape fragmentation under environmental change. Ecol. Complex. 2016, 28, 174–186. [Google Scholar] [CrossRef]
- Subba, B.; Sen, S.; Ravikanth, G.; Nobis, M.P. Direct modelling of limited migration improves projected distributions of Himalayan amphibians under climate change. Biol. Conserv. 2018, 227, 352–360. [Google Scholar] [CrossRef]





| Family Name | Scientific Name | Common Name | Presence | AUC | TSS |
|---|---|---|---|---|---|
| Bufonidae | Bufo gargarizans | Asian toad | 1233 | 0.79 | 0.59 |
| Bufonidae | Bufo stejnegeri | Water toad | 233 | 0.92 | 0.72 |
| Discoglossidae | Bombina orientalis | Oriental fire-bellied toad | 3302 | 0.73 | 0.54 |
| Hylidae | Dryophytes japonicus | Japanes tree frog | 4976 | 0.66 | 0.53 |
| Hynobiidae | Hynobius leechii | Korean salamander | 3401 | 0.77 | 0.65 |
| Hynobiidae | Hynobius quelpaertensis | Jeju salamander | 74 | 0.99 | 0.98 |
| Hynobiidae | Onychodactylus koreanus | Korean clawed salamander | 240 | 0.89 | 0.66 |
| Microhylidae | Kaloula borealis | Narrow-mouthed toad | 64 | 0.87 | 0.65 |
| Plethodontidae | Karsenia koreana | Korean crevice salamander | 13 | 0.82 | 0.69 |
| Ranidae | Glandirana rugosa | Wrinkled frog | 1683 | 0.71 | 0.61 |
| Ranidae | Lithobates catesbeianus | American bullfrog | 2527 | 0.87 | 0.59 |
| Ranidae | Pelophylax chosenicus | Korean golden frog | 31 | 0.97 | 0.89 |
| Ranidae | Pelophylax nigromaculatus | black-spotted pond frog | 6314 | 0.78 | 0.65 |
| Ranidae | Rana coreana | Korean brown frog | 1562 | 0.76 | 0.57 |
| Ranidae | Rana uenoi | Korean large brown frog | 3708 | 0.68 | 0.55 |
| Ranidae | Rana huanrenensis | Huanren brown frog | 930 | 0.86 | 0.54 |
| Hylidae | Dryophytes suweonensis * | Suweon tree frog | Presenc epoints < 10 | ||
| Hynobiidae | Hynobius yangi * | Kori salamander | Presenc epoints < 10 | ||
| Hylidae | Dryophytes flaviventrisa | Yellow-bellied tree frog | www.krsh.co.kr (accessed on 20 July 2021) | ||
| Hynobiidae | Hynobius geojeensis a | Geoje salamander | Borzée and Min, 2021 | ||
| Hynobiidae | Hynobius notialis a | Southern Korean salamander | Borzée and Min, 2021 | ||
| Hynobiidae | Hynobius perplicatus a | Cryptic Uiryeong salamander | Borzée and Min, 2021 | ||
| Hynobiidae | Hynobius unisacculus a | Korean small salamander | www.krsh.co.kr (accessed on 20 July 2021) | ||
| Code | Description | Unit | Source |
|---|---|---|---|
| Bio1 | Annual mean temperature | °C | KMA |
| Bio2 | Mean diurnal temperature range | °C | KMA |
| Bio3 | Isothermality (BIO2/BIO7) (×100) | Percent | KMA |
| Bio4 | Temperature seasonality | °C | KMA |
| Bio5 | Max temperature of warmest month | °C | KMA |
| Bio6 | Min temperature of coldest month | °C | KMA |
| Bio7 | Temperature annual range | °C | KMA |
| Bio8 | Mean temperature of wettest quarter | °C | KMA |
| Bio9 | Mean temperature of driest quarter | °C | KMA |
| Bio10 | Mean temperature of warmest quarter | °C | KMA |
| Bio11 | Mean temperature of coldest quarter | °C | KMA |
| Bio12 | Annual precipitation | mm | KMA |
| Bio13 | Precipitation of wettest month | mm | KMA |
| Bio14 | Precipitation of driest month | mm | KMA |
| Bio15 | Precipitation seasonality | Fraction | KMA |
| Bio16 | Precipitation of wettest quarter | mm | KMA |
| Bio17 | Precipitation of driest quarter | mm | KMA |
| Bio18 | Precipitation of warmest quarter | mm | KMA |
| Bio19 | Precipitation of coldest quarter | mm | KMA |
| Dem | Elevation | mm | KMA |
| Groups | Species | Altitude | Bio01 | Bio02 | Bio03 | Bio12 | Bio13 | Bio14 |
|---|---|---|---|---|---|---|---|---|
| Group 1 | D. japonicus | 81.4 | 4.4 | 3.5 | 3.7 | 0.4 | 6.2 | 0.3 |
| K. borealis | 48.5 | 7.1 | 0.3 | 6.9 | 16.9 | 10.6 | 9.7 | |
| G. rugosa | 60.1 | 2.6 | 6.9 | 12.7 | 11.6 | 1.9 | 4.3 | |
| L. catesbeianus | 31.2 | 46.1 | 0.8 | 2.3 | 2.6 | 16.4 | 0.6 | |
| P. chosenicus | 78.6 | 10.4 | 1.4 | 8.1 | 0.4 | 0.3 | 0.8 | |
| P. nigromaculatus | 77.4 | 9.0 | 3.6 | 7.3 | 1.6 | 0.8 | 0.2 | |
| R. coreana | 43.5 | 25.5 | 4.1 | 2.7 | 13.5 | 5.3 | 5.3 | |
| Group 2 | B. orientalis | 34.9 | 8.4 | 1.6 | 4.5 | 2.7 | 34.1 | 13.9 |
| B. gargarizans | 56.0 | 9.4 | 2.0 | 6.7 | 10.1 | 15.5 | 0.3 | |
| H. leechii | 44.4 | 4.6 | 3.9 | 1.9 | 15.3 | 27.4 | 2.4 | |
| H. quelpaertensis | 0.0 | 6.7 | 0.3 | 0.3 | 60.1 | 0.3 | 32.3 | |
| R. uenoi | 74.6 | 2.9 | 4.8 | 3.2 | 1.5 | 7.8 | 5.2 | |
| Group 3 | B. stejnegeri | 16.0 | 59.2 | 2.7 | 7.9 | 1.1 | 4.1 | 8.9 |
| K. koreana | 22.2 | 0.0 | 18.6 | 29.4 | 0.0 | 29.6 | 0.2 | |
| O. koreanus | 42.8 | 41.0 | 4.1 | 0.9 | 0.9 | 2.3 | 8.0 | |
| R. huanrenensis | 57.2 | 21.9 | 3.3 | 2.1 | 3.4 | 7.2 | 5.0 |
| Group | Scientific Name | Current | RCP 4.5 | PHL * (%) | RCP 8.5 | PHL * (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2030 | 2050 | 2080 | 2030 | 2050 | 2080 | |||||
| Group 1 | H. japonica | 69,191 | 74,273 | 50,748 | 23,119 | 66.6 | 52,626 | 41,463 | 22,798 | 67.1 |
| K. borealis | 24,392 | 30,814 | 39,835 | 61,206 | −150.9 | 48,429 | 33,656 | 41,009 | −68.1 | |
| G. rugosa | 60,730 | 52,518 | 41,887 | 19,256 | 68.3 | 35,123 | 34,411 | 31,959 | 47.4 | |
| L. catesbeianus | 28,523 | 62,436 | 37,986 | 27,067 | 5.1 | 40,263 | 55,541 | 42,311 | −48.3 | |
| P. chosenicus | 10,901 | 4298 | 298 | 2000 | 81.7 | 3255 | 412 | 168 | 98.5 | |
| P. nigromaculatus | 65,551 | 71,358 | 49,658 | 28,648 | 56.3 | 57,881 | 44,831 | 19,902 | 69.6 | |
| R. coreana | 47,791 | 33,675 | 17,128 | 15,420 | 67.7 | 28,215 | 25,815 | 16,617 | 65.2 | |
| Group 2 | B. gargarizans | 65,144 | 62,370 | 41,381 | 21,368 | 67.2 | 46,998 | 48,268 | 24,268 | 62.7 |
| B. orientalis | 56,561 | 47,634 | 30,044 | 2529 | 95.5 | 25,427 | 30,774 | 3354 | 94.1 | |
| H. leechii | 68,673 | 38,858 | 22,711 | 11,747 | 82.9 | 24,299 | 31,160 | 15,452 | 77.5 | |
| H. quelpaertensis | 1585 | 1427 | 888 | 1510 | 4.7 | 1343 | 253 | 1220 | 23.0 | |
| R. uenoi | 66,419 | 13,369 | 9417 | 2261 | 96.6 | 13,130 | 7152 | 2356 | 96.5 | |
| Group 3 | B. stejnegeri | 17,160 | 16,158 | 4288 | 265 | 98.5 | 3916 | 1448 | 59 | 99.7 |
| O. koreanus | 21,351 | 9675 | 5193 | 330 | 98.5 | 4920 | 2953 | 118 | 99.4 | |
| K. koreana | 45,627 | 76,576 | 84,130 | 87,674 | −92.2 | 79,896 | 78,035 | 87,252 | −91.2 | |
| R. huanrenensis | 31,061 | 13,481 | 6600 | 670 | 97.8 | 6466 | 3026 | 1228 | 96.0 | |
| Total area | 100,411 a | |||||||||
| RCP | Year | All | Group 1 | Group 2 | Group 3 | ||||
| Avg | Max | Avg | Max | Avg | Max | Avg | Max | ||
| Current | 7.33 | 12.00 | 2.60 | 6.00 | 3.48 | 5.00 | 1.25 | 4.00 | |
| 4.5 | 2030 | 6.19 | 14.00 | 2.94 | 6.00 | 2.01 | 5.00 | 1.23 | 4.00 |
| 2050 | 4.71 | 14.00 | 2.28 | 6.00 | 1.39 | 5.00 | 1.04 | 4.00 | |
| 2080 | 3.71 | 12.00 | 2.35 | 6.00 | 0.45 | 5.00 | 0.92 | 4.00 | |
| 8.5 | 2030 | 5.01 | 13.00 | 2.69 | 6.00 | 1.31 | 5.00 | 1.02 | 4.00 |
| 2050 | 5.00 | 13.00 | 2.92 | 6.00 | 1.19 | 5.00 | 0.88 | 4.00 | |
| 2080 | 4.30 | 11.00 | 2.79 | 6.00 | 0.6 | 5.00 | 0.91 | 4.00 | |
| Habitat | RCP4.5 | RCP8.5 | ||||||
|---|---|---|---|---|---|---|---|---|
| Groups | SR * Classes | Current | 2030 | 2050 | 2080 | 2030 | 2050 | 2080 |
| Low (0–1) | 23,489 | 15,970 | 34,572 | 51,231 | 29,348 | 33,026 | 52,852 | |
| Group 1 | Mid (2–4) | 44,823 | 52,094 | 48,351 | 35,381 | 47,418 | 48,409 | 34,879 |
| High (5~7) | 27,802 | 28,220 | 13,361 | 9672 | 19,505 | 14,838 | 8542 | |
| Low (0–1) | 22,604 | 41,113 | 62,642 | 89,066 | 63,930 | 59,266 | 86,693 | |
| Group 2 | Mid (2–3) | 36,568 | 49,692 | 29,856 | 7098 | 27,614 | 33,604 | 9359 |
| High (4~5) | 36,942 | 5479 | 3786 | 120 | 4727 | 3403 | 221 | |
| Low (0–1) | 65,259 | 72,324 | 86,187 | 95,317 | 86,137 | 92,280 | 95,001 | |
| Group 3 | Mid (2–3) | 25,866 | 19,700 | 9125 | 909 | 9458 | 3625 | 1262 |
| High (4) | 4989 | 4260 | 972 | 58 | 676 | 368 | 10 | |
| All | Low (0–4) | 13,785 | 22,241 | 50,037 | 73,827 | 43,616 | 49,586 | 70,203 |
| Mid (5–8) | 68,190 | 67,069 | 44,224 | 21,996 | 50,803 | 45,506 | 25,945 | |
| High (9~14) | 14,139 | 6974 | 2023 | 461 | 1852 | 1181 | 125 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.W.; Adhikari, P.; Chang, M.H.; Seo, C. Potential Distribution of Amphibians with Different Habitat Characteristics in Response to Climate Change in South Korea. Animals 2021, 11, 2185. https://doi.org/10.3390/ani11082185
Kim HW, Adhikari P, Chang MH, Seo C. Potential Distribution of Amphibians with Different Habitat Characteristics in Response to Climate Change in South Korea. Animals. 2021; 11(8):2185. https://doi.org/10.3390/ani11082185
Chicago/Turabian StyleKim, Hyun Woo, Pradeep Adhikari, Min Ho Chang, and Changwan Seo. 2021. "Potential Distribution of Amphibians with Different Habitat Characteristics in Response to Climate Change in South Korea" Animals 11, no. 8: 2185. https://doi.org/10.3390/ani11082185







