Straw as an Alternative to Grass Forage in Horses—Effects on Post-Prandial Metabolic Profile, Energy Intake, Behaviour and Gastric Ulceration
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
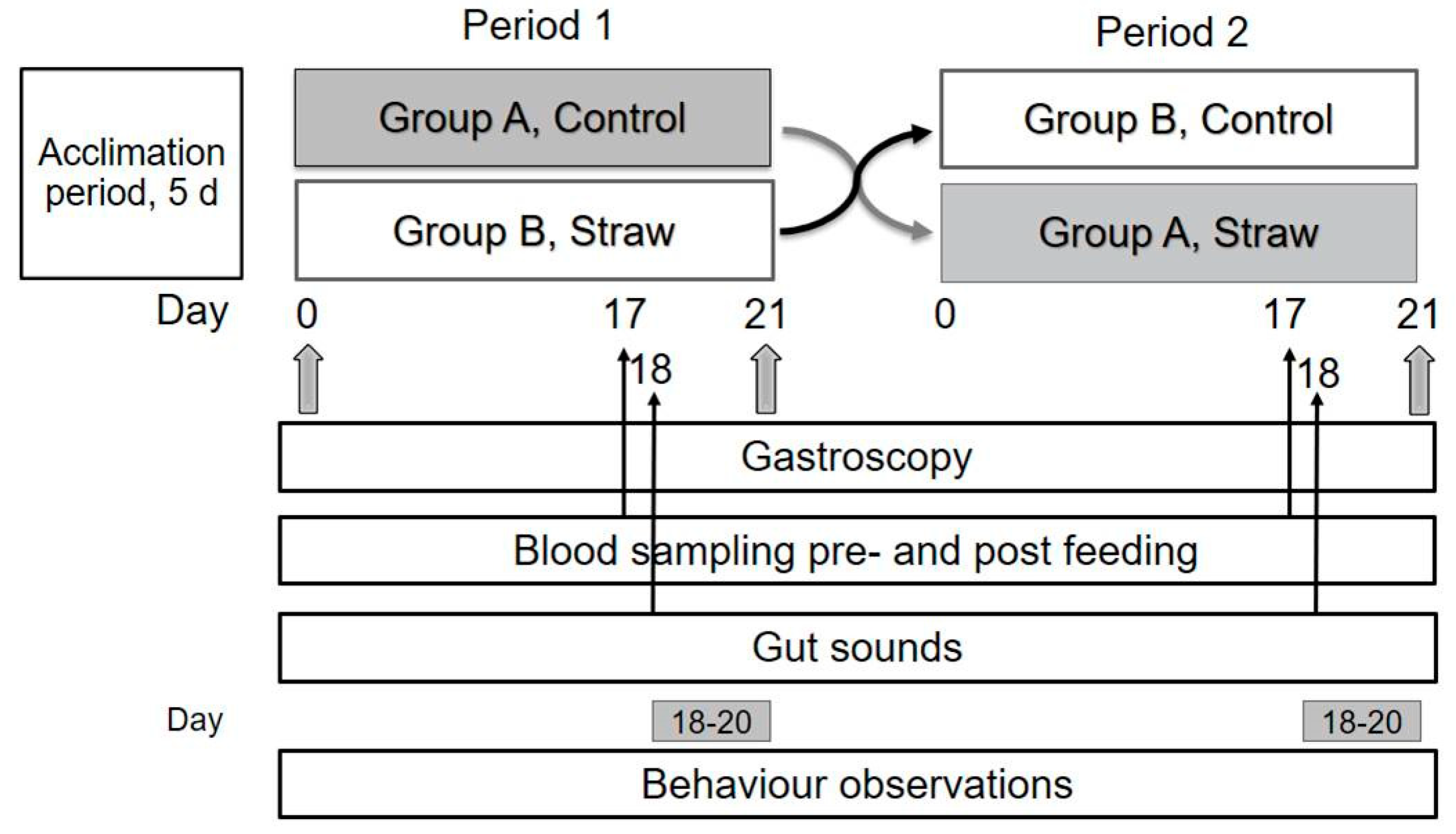
2.2. Study Design and Diets
2.3. Management
2.4. Examination of Gastric Mucosa
2.5. Blood Sampling and Plasma Analyses
2.6. Intestinal Sounds and Faecal Sampling
2.7. Feeding Time, Prefeeding Behaviour and Heart Rate
2.8. Statistical Analyses
3. Results
3.1. Nutrient Intake and Body Condition
3.2. Gastric Ulcers
3.3. Intestinal Sounds and Faecal Dry Matter
3.4. Plasma Insulin, TPP and Metabolite Concentration
3.5. Feeding Time, Prefeeding Behaviour, Heart Rate, Cortisol and Serotonin Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsson, N.; Kihlén, G.; Cagell, W. Smältbarhetsförsök med hästar (Digestibility experiments on horses). Lantbrukshögskolan Husdjursförsöksanstalten 1949, Meddelande nr 36, 1–51. [Google Scholar]
- Slagsvold, P.; Hintz, H.F.; Schryver, H.F. Digestibility by ponies of oat straw treated with anhydrous ammonia. Anim. Sci. 1979, 28, 347–352. [Google Scholar] [CrossRef]
- Hansen, D.K.; Webb, G.W.; Webb, S.P. Digestibility of wheat straw or ammoniated wheat straw in equine diets. J. Equine Vet. Sci. 1992, 12, 223–226. [Google Scholar] [CrossRef]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Rendle, D.; Argo, P.C.M.; Bowen, P.M.; Carslake, H.; German, P.A.; Harris, P.; Knowles, E.; Menzies-Gow, D.N.; Morgan, R. Equine obesity: Current perspectives. UK-Vet Equine 2018, 2, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Ellis, A.D.; Fradinho, M.J.; Jansson, A.; Julliand, V.; Luthersson, N.; Santos, A.S.; Vervuert, I. Review: Feeding conserved forage to horses: Recent advances and recommendations. Animal 2017, 11, 958–967. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Ahiswedeu, L.; Reinhardt, H.J. Studies on the duration of feeding, masticatory frequency and mincing of feed in horses. DTW Deutsche Tierarztliche Wochenschrift 1975, 82, 54–58. [Google Scholar]
- McGreevy, P.D.; Cripps, P.J.; French, N.P.; Green, L.E.; Nicol, C.J. Management factors associated with stereotypic and redirected behaviour in the thoroughbred horse. Equine Vet. J. 1995, 27, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Redbo, I.; Redbo-Torstensson, P.; Ödberg, F.O.; Hedendahl, A.; Holm, J. Factors affecting behavioural disturbances in race-horses. Anim. Sci. 1998, 66, 475–481. [Google Scholar] [CrossRef]
- Hockenhull, J.; Creighton, E. Pre-feeding behaviour in UK leisure horses and associated feeding routine risk factors. Anim. Welf. 2014, 23, 297–308. [Google Scholar] [CrossRef]
- Luthersson, N.; Nielsen, K.H.; Harris, P.; Parkin, T.D. Risk factors associated with equine gastric ulceration syndrome (EGUS) in 201 horses in Denmark. Equine Vet. J. 2009, 41, 625–630. [Google Scholar] [CrossRef]
- Airaksinen, S.; Heinonen-Tanski, H.; Heiskanen, M.L. Quality of different bedding materials and their influence on the compostability of horse manure. J. Equine Vet. Sci. 2001, 21, 125–130. [Google Scholar] [CrossRef]
- Wichert, B.; Nater, S.; Wittenbrink, M.M.; Wolf, P.; Meyer, K.; Wanner, M. Judgement of hygienic quality of roughage in horse stables in Switzerland. J. Anim. Physiol. Anim. Nutr. 2008, 92, 432–437. [Google Scholar] [CrossRef] [Green Version]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body-fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- Nadeau, J.A.; Andrews, F.M.; Mathew, A.G.; Argenzio, R.A.; Blackford, J.T.; Sohtell, M.; Saxton, A.M. Evaluation of diet as a cause of gastric ulcers in horses. Am. J. Vet. Res. 2000, 61, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, B.W.; Hewetson, M.; Hepburn, R.J.; Luthersson, N.; Tamzali, Y. European College of Equine Internal Medicine Consensus Statement-Equine Gastric Ulcer Syndrome in Adult Horses. J. Vet. Intern. Med. 2015, 29, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Sykes, B.W.; Kathawala, K.; Song, Y.; Garg, S.; Page, S.W.; Underwood, C.; Mills, P.C. Preliminary investigations into a novel, long-acting, injectable, intramuscular formulation of omeprazole in the horse. Equine Vet. J. 2017, 49, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, A.; Harris, P.A. A bibliometric review on nutrition of the exercising horse from 1970 to 2010. Comp. Exerc. Physiol. 2013, 9, 169–180. [Google Scholar] [CrossRef]
- NRC. National Research Council Committee Nutrient Requirements of Horses; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Ringmark, S.; Roepstorff, L.; Essén-Gustavsson, B.; Revold, T.; Lindholm, A.; Hedenström, U.; Rundgren, M.; Ögren, G.; Jansson, A. Growth, training response and health in Standardbred yearlings fed a forage-only diet. Animal 2013, 7, 746–753. [Google Scholar] [CrossRef] [Green Version]
- Connysson, M.; Muhonen, S.; Jansson, A. Road transport and diet affect metabolic response to exercise in horses. J. Anim. Sci. 2017, 95, 4869–4879. [Google Scholar] [CrossRef] [Green Version]
- Connysson, M.; Rhodin, M.; Jansson, A. Effects of Horse Housing System on Energy Balance during Post-Exercise Recovery. Animals 2019, 9, 976. [Google Scholar] [CrossRef] [Green Version]
- Jansson, A.; Sandin, A.; Lindberg, J. Digestive and metabolic effects of altering feeding frequency in athletic horses. Equine Comp. Exerc. Physiol. 2006, 3, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Frank, N.; Tadros, E.M. Insulin dysregulation. Equine Vet. J. 2014, 46, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Dosi, M.C.M.; Kirton, R.; Hallsworth, S.; Keen, J.A.; Morgan, R.A. Inducing weight loss in native ponies: Is straw a viable alternative to hay? Vet. Rec. 2020, 187, e60. [Google Scholar] [CrossRef]
- Carter, R.A.; Treiber, K.H.; Geor, R.J.; Douglass, L.; Harris, P.A. Prediction of incipient pasture-associated laminitis from hyperinsulinaemia, hyperleptinaemia and generalised and localised obesity in a cohort of ponies. Equine Vet. J. 2009, 41, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Glunk, E.C.; Hathaway, M.R.; Weber, W.J.; Sheaffer, C.C.; Martinson, K.L. The Effect of Hay Net Design on Rate of Forage Consumption When Feeding Adult Horses. J. Equine Vet. Sci. 2014, 34, 986–991. [Google Scholar] [CrossRef]
- Jansson, A.; Saastamoinen, M.; Lindberg, J. Forage feeding systems. In Forages and Grazing in Horse Nutrition; Saastamoinen, M., Fradinho, M.J., Santos, A.S., Miraglia, N., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 289–303. [Google Scholar]
- Clarke, L.L.; Argenzio, R.A.; Roberts, M.C. Effect of meal feeding on plasma volume and urinary electrolyte clearance in ponies. Am. J. Vet. Res. 1990, 51, 571–576. [Google Scholar]
- Jansson, A.; Dahlborn, K. Effects of feeding frequency and voluntary salt intake on fluid and electrolyte regulation in athletic horses. J. Appl. Physiol. 1999, 86, 1610–1616. [Google Scholar] [CrossRef]
- Jansson, A.; Lindberg, J.; Rundgren, M.; Müller, C.; Connysson, M.; Kjellberg, L.; Lundberg, M. Utfodringsrekommendationer för häst; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2011; pp. 1–39. [Google Scholar]
- Jose-Cunilleras, E.; Hinchcliff, K.W.; Sams, R.A.; Devor, S.T.; Linderman, J.K. Glycemic index of a meal fed before exercise alters substrate use and glucose flux in exercising horses. J. Appl. Physiol. 2002, 92, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Van Weyenberg, S.; Buyse, J.; Janssens, G.P. Digestibility of a complete ration in horses fed once or three times a day and correlation with key blood parameters. Vet. J. 2007, 173, 311–316. [Google Scholar] [CrossRef]
- Lindedam, J.; Bruun, S.; Jørgensen, H.; Felby, C.; Magid, J. Cellulosic ethanol: Interactions between cultivar and enzyme loading in wheat straw processing. Biotechnol. Biofuels 2010, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Tishler, Y.; Samach, A.; Rogachev, I.; Elbaum, R.; Levy, A.A. Analysis of Wheat Straw Biodiversity for Use as a Feedstock for Biofuel Production. BioEnergy Res. 2015, 8, 1831–1839. [Google Scholar] [CrossRef] [Green Version]
- Alberghina, D.; Giannetto, C.; Visser, E.K.; Ellis, A.D. Effect of diet on plasma tryptophan and serotonin in trained mares and geldings. Vet. Rec. 2010, 166, 133–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banskota, S.; Ghia, J.E.; Khan, W.I. Serotonin in the gut: Blessing or a curse. Biochimie 2019, 161, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Tecott, L.H. Serotonin and the Orchestration of Energy Balance. Cell Metab. 2007, 6, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Simansky, K.J. Serotonergic control of the organization of feeding and satiety. Behav. Brain Res. 1996, 73, 37–42. [Google Scholar] [CrossRef]
- Miquel-Kergoat, S.; Azais-Braesco, V.; Burton-Freeman, B.; Hetherington, M.M. Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiol. Behav. 2015, 151, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, M.D. Review article: Serotonin receptors and transporters—Roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. S7), 3–14. [Google Scholar] [CrossRef]
- Sumara, G.; Sumara, O.; Kim, J.K.; Karsenty, G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012, 16, 588–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderBroek, A.R.; Reef, V.B.; Aitken, M.R.; Stefanovski, D.; Southwood, L.L. Assessing gastrointestinal motility in healthy horses comparing auscultation, ultrasonography and an acoustic gastrointestinal surveillance biosensor: A randomised, blinded, controlled crossover proof of principle study. Equine Vet. J. 2019, 51, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.M.; Cohen, N.D.; Gibbs, P.G.; Thompson, J.A. Feeding practices associated with colic in horses. J. Am. Vet. Med. Assoc. 2001, 219, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Connysson, M.; Muhonen, S.; Lindberg, J.E.; Essen-Gustavsson, B.; Nyman, G.; Nostell, K.; Jansson, A. Effects on exercise response, fluid and acid-base balance of protein intake from forage-only diets in Standardbred horses. Equine Vet. J. 2006, 38, 648–653. [Google Scholar] [CrossRef]
- Essen-Gustavsson, B.; Connysson, M.; Jansson, A. Effects of crude protein intake from forage-only diets on muscle amino acids and glycogen levels in horses in training. Equine Vet. J. 2010, 42, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Muhonen, S.; Lindberg, J.E.; Bertilsson, J.; Jansson, A. Effects on fluid balance, digestion and exercise response in Standardbred horses fed silage, haylage and hay. Comp. Exerc. Physiol. 2008, 5, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Muhonen, S.; Julliand, V.; Lindberg, J.E.; Bertilsson, J.; Jansson, A. Effects on the equine colon ecosystem of grass silage and haylage diets after an abrupt change from hay. J. Anim. Sci. 2009, 87, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, S.; Jansson, A. Comparison of grass haylage digestibility and metabolic plasma profile in Icelandic and Standardbred horses. J. Anim. Physiol. Anim. Nutr. 2011, 95, 273–279. [Google Scholar] [CrossRef]
- Ringmark, S.; Jansson, A. Insulin response to feeding forage with varying crude protein and amino acid content in horses at rest and after exercise. Comp. Exerc. Physiol. 2013, 9, 209–217. [Google Scholar] [CrossRef]
- Ringmark, S.; Revold, T.; Jansson, A. Effects of training distance on feed intake, growth, body condition and muscle glycogen content in young Standardbred horses fed a forage-only diet. Animal 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Muhonen, S.; Connysson, M.; Lindberg, J.E.; Julliand, V.; Bertilsson, J.; Jansson, A. Effects of crude protein intake from grass silage-only diets on the equine colon ecosystem after an abrupt feed change. J. Anim. Sci. 2008, 86. [Google Scholar] [CrossRef] [Green Version]


| Item | Grass Haylage 1 | Grass Haylage 2 | Wheat Straw |
|---|---|---|---|
| Dry matter (DM), % | 59 | 49 | 89 |
| Metabolisable energy (ME), MJ | 11.2 | 9.8 | 6.7 |
| Crude protein (CP), g | 180 | 79 | 22 |
| Digestible crude protein (dCP), g | 137 | 43 | 9 |
| Neutral detergent fibre (NDF), g | 483 | 608 | 802 |
| Water soluble carbohydrates (WSC), g | 77 | 49 | 8 |
| Ca, g | 3.9 | 6.2 | 2.0 |
| P, g | 2.6 | 1.7 | 0.3 |
| Mg, g | 1.7 | 1.2 | 0.5 |
| Item | Control Diet 1 | Straw Diet 1 | p |
|---|---|---|---|
| Dry matter (DM), kg | 1.22 ± 0.01 | 1.43 ± 0.01 | <0.05 |
| Metabolisable energy (ME), MJ | 12.9 ± 0.6 | 12.7 ± 0.6 | >0.05 |
| Crude protein (CP), g | 158 ± 7 | 141 ± 7 | >0.05 |
| Digestible crude protein (dCP), g | 111 ± 5 | 103 ± 5 | >0.05 |
| Neutral detergent fibre (NDF), g | 667 ± 36 | 938 ± 36 | <0.05 |
| Water soluble carbohydrates (WSC), g | 77 ± 3 | 60 ± 3 | <0.05 |
| Ca, g | 7.1 ± 0.3 | 5.2 ± 0.3 | <0.05 |
| P, g | 3.6 ± 0.2 | 3.1 ± 0.2 | <0.05 |
| Mg, g | 2.8 ± 0.1 | 2.7 ± 0.1 | >0.05 |
| Na, g | 3.9 ± 0.2 | 3.9 ± 0.2 | >0.5 |
| Item | Control Diet | Straw Diet | p |
|---|---|---|---|
| DM, kg | 1.2 ± 0.06 | 1.2 ± 0.06 | >0.05 |
| ME, MJ | 12.4 ± 0.5 | 10.7 ± 0.5 | <0.001 |
| CP, g | 155 ± 5 | 134 ± 5 | <0.0001 |
| dCP, g | 108 ± 3 | 99 ± 3 | <0.0001 |
| NDF, g | 633 ±41 | 696 ± 41 | >0.05 |
| WSC, g | 75 ± 2 | 60 ± 2 | <0.0001 |
| Item | Control Diet | Straw Diet | p |
|---|---|---|---|
| Oral behaviours prior to feeding | 11 ± 1 | 6 ± 2 | 0.07 |
| Motion-related behaviours prior to feeding | 6 ± 1 | 5 ± 2 | >0.05 |
| Feeding pause, min | 1 ± 2 | 16 ± 2 | <0.001 |
| Possible feeding time, min | 44 ± 3 | 54 ± 3 | <0.05 |
| Feed left overs, kg | 0.1 ± 0.0 | 0.2 ± 0.0 | <0.05 |
| Item | Control Diet | Straw Diet | p |
|---|---|---|---|
| Cortisol feeding 15.00, nmol/L | 210 ± 60 | 210 ± 60 | >0.05 |
| Cortisol feeding 22.00, nmol/L | 130 ± 60 | 176 ± 60 | <0.05 |
| P feeding occasion | <0.001 | 0.09 | |
| Serotonin feeding 15.00, nmol/L | 162 ± 55 | 261 ± 55 | 0.05 |
| Serotonin feeding 22.00, nmol/L | 211 ± 55 | 218 ± 55 | >0.05 |
| P feeding occasion | >0.05 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansson, A.; Harris, P.; Davey, S.L.; Luthersson, N.; Ragnarsson, S.; Ringmark, S. Straw as an Alternative to Grass Forage in Horses—Effects on Post-Prandial Metabolic Profile, Energy Intake, Behaviour and Gastric Ulceration. Animals 2021, 11, 2197. https://doi.org/10.3390/ani11082197
Jansson A, Harris P, Davey SL, Luthersson N, Ragnarsson S, Ringmark S. Straw as an Alternative to Grass Forage in Horses—Effects on Post-Prandial Metabolic Profile, Energy Intake, Behaviour and Gastric Ulceration. Animals. 2021; 11(8):2197. https://doi.org/10.3390/ani11082197
Chicago/Turabian StyleJansson, Anna, Patricia Harris, Sara Larsdotter Davey, Nanna Luthersson, Sveinn Ragnarsson, and Sara Ringmark. 2021. "Straw as an Alternative to Grass Forage in Horses—Effects on Post-Prandial Metabolic Profile, Energy Intake, Behaviour and Gastric Ulceration" Animals 11, no. 8: 2197. https://doi.org/10.3390/ani11082197
APA StyleJansson, A., Harris, P., Davey, S. L., Luthersson, N., Ragnarsson, S., & Ringmark, S. (2021). Straw as an Alternative to Grass Forage in Horses—Effects on Post-Prandial Metabolic Profile, Energy Intake, Behaviour and Gastric Ulceration. Animals, 11(8), 2197. https://doi.org/10.3390/ani11082197





