Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Anatomical Locations of Thermal Windows in Cattle and River Buffalo
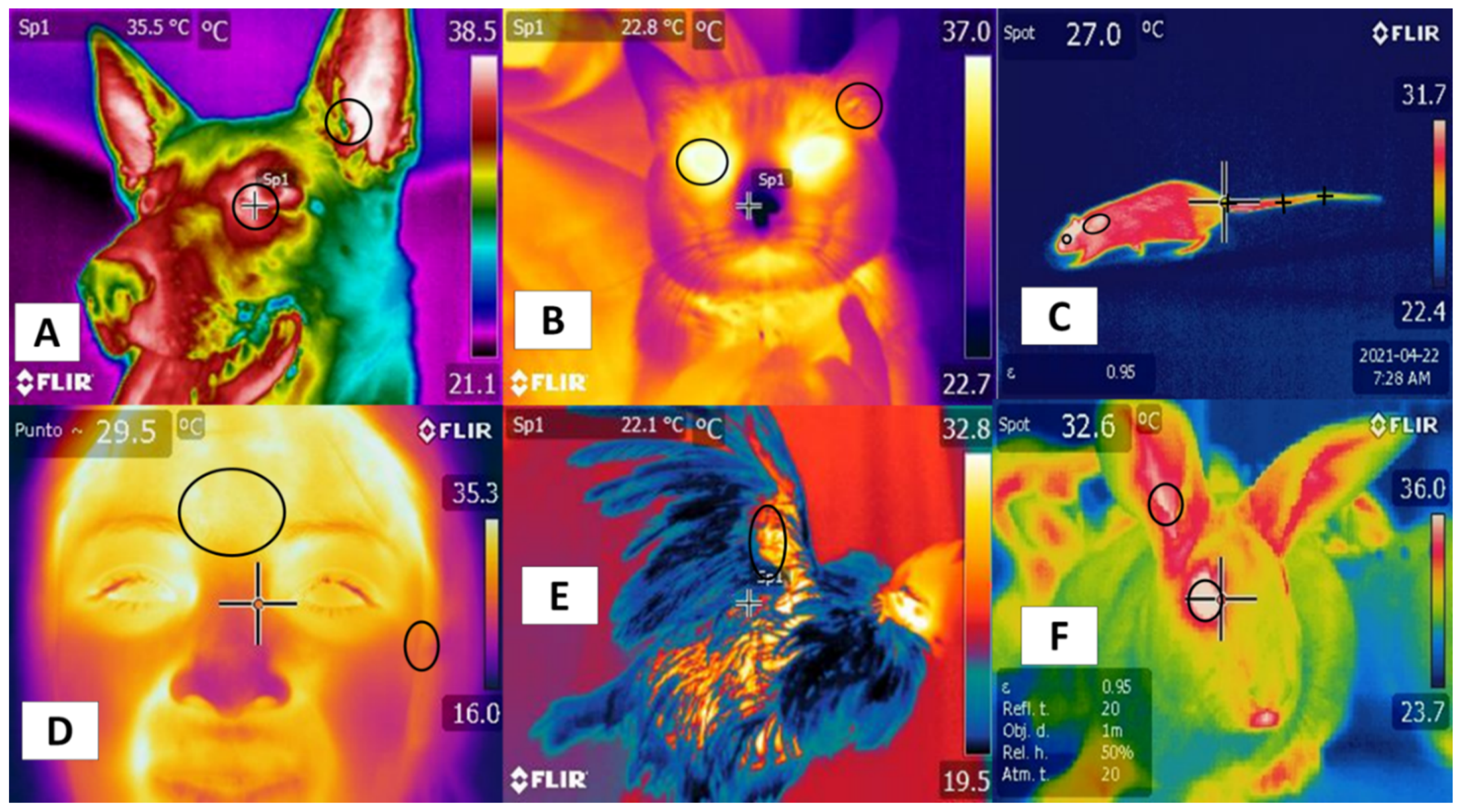
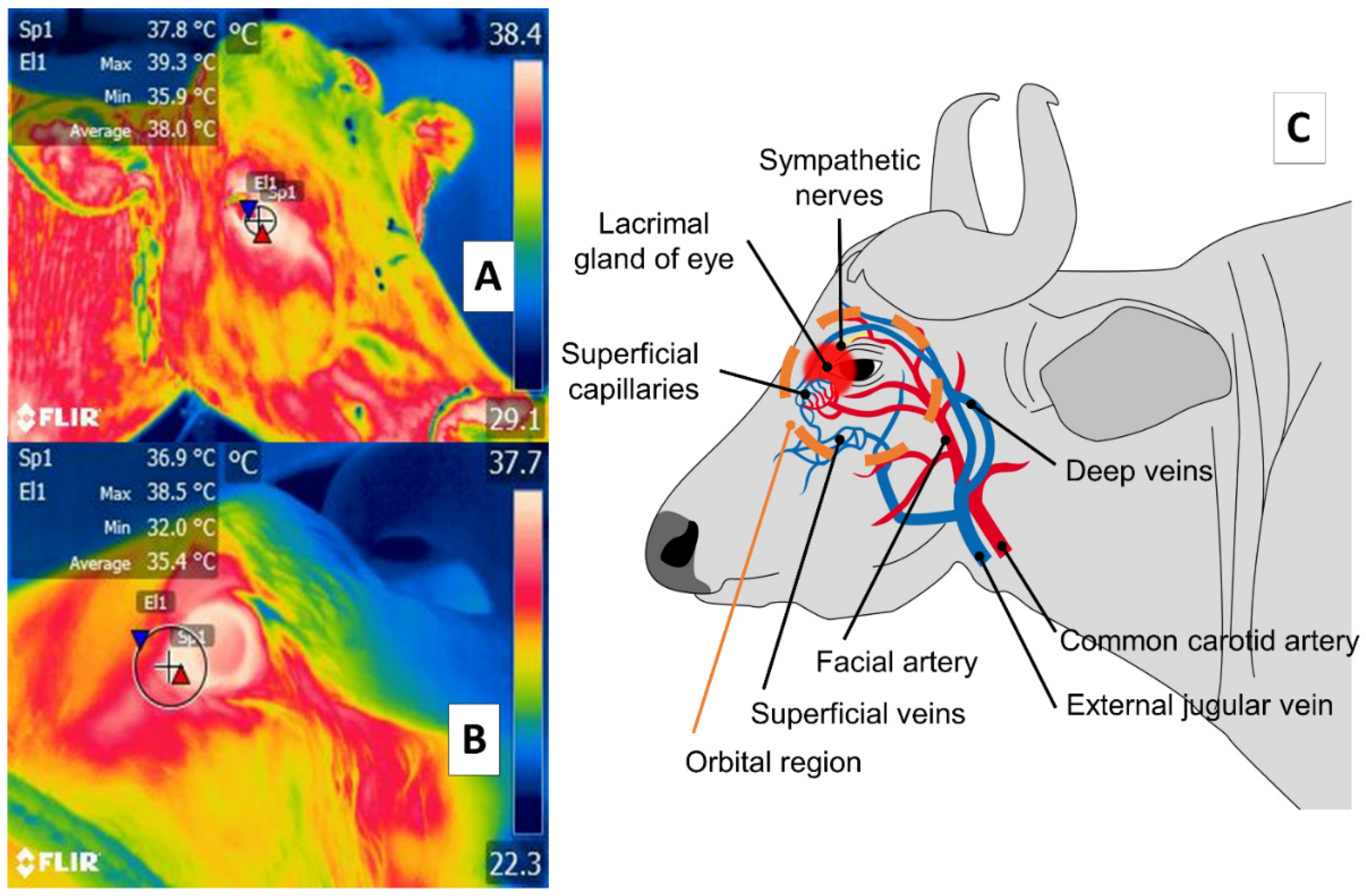
3. Orbital Region (Regio Orbitalis)
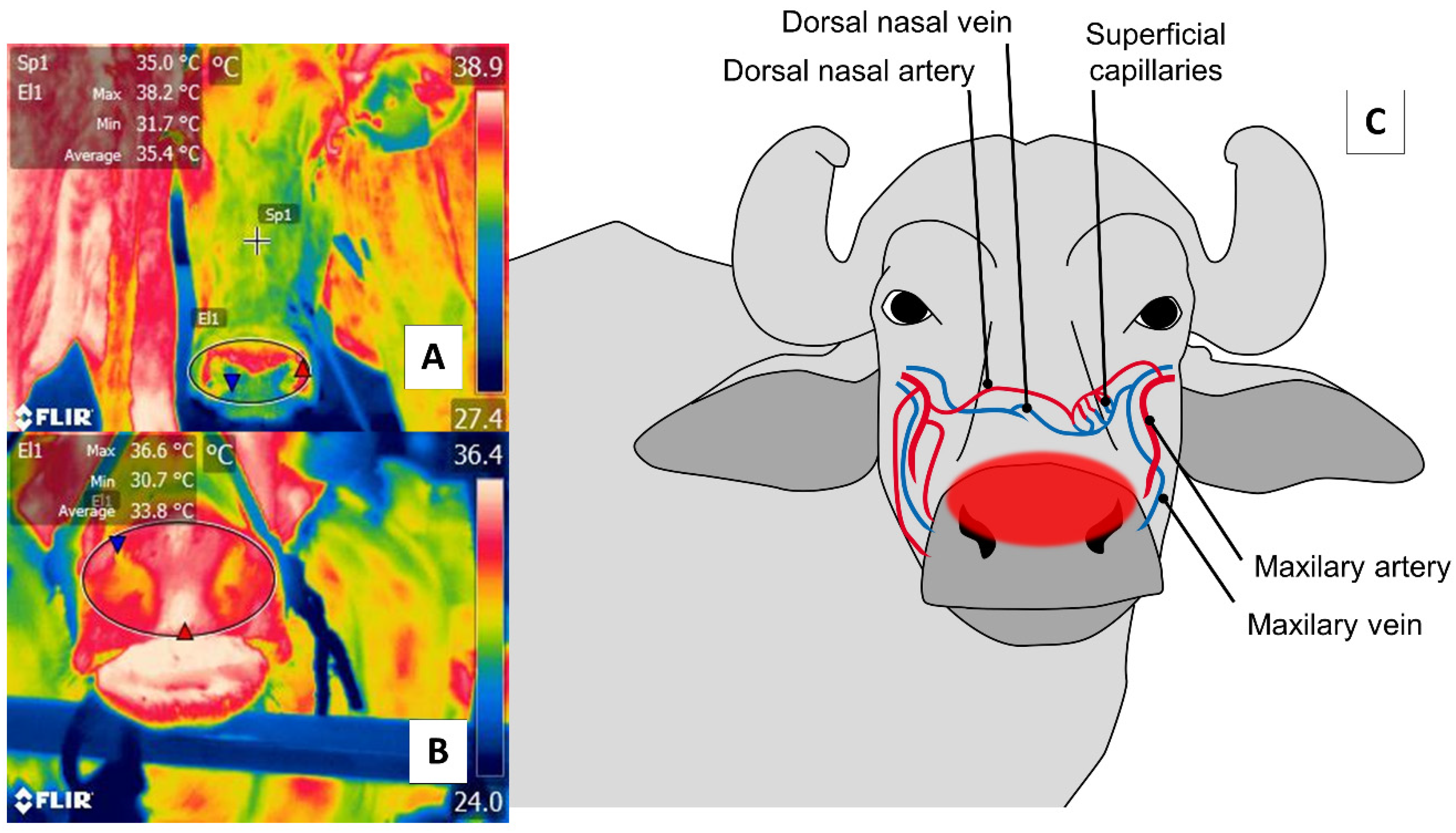
4. Nasal Region (Regio Nasalis)
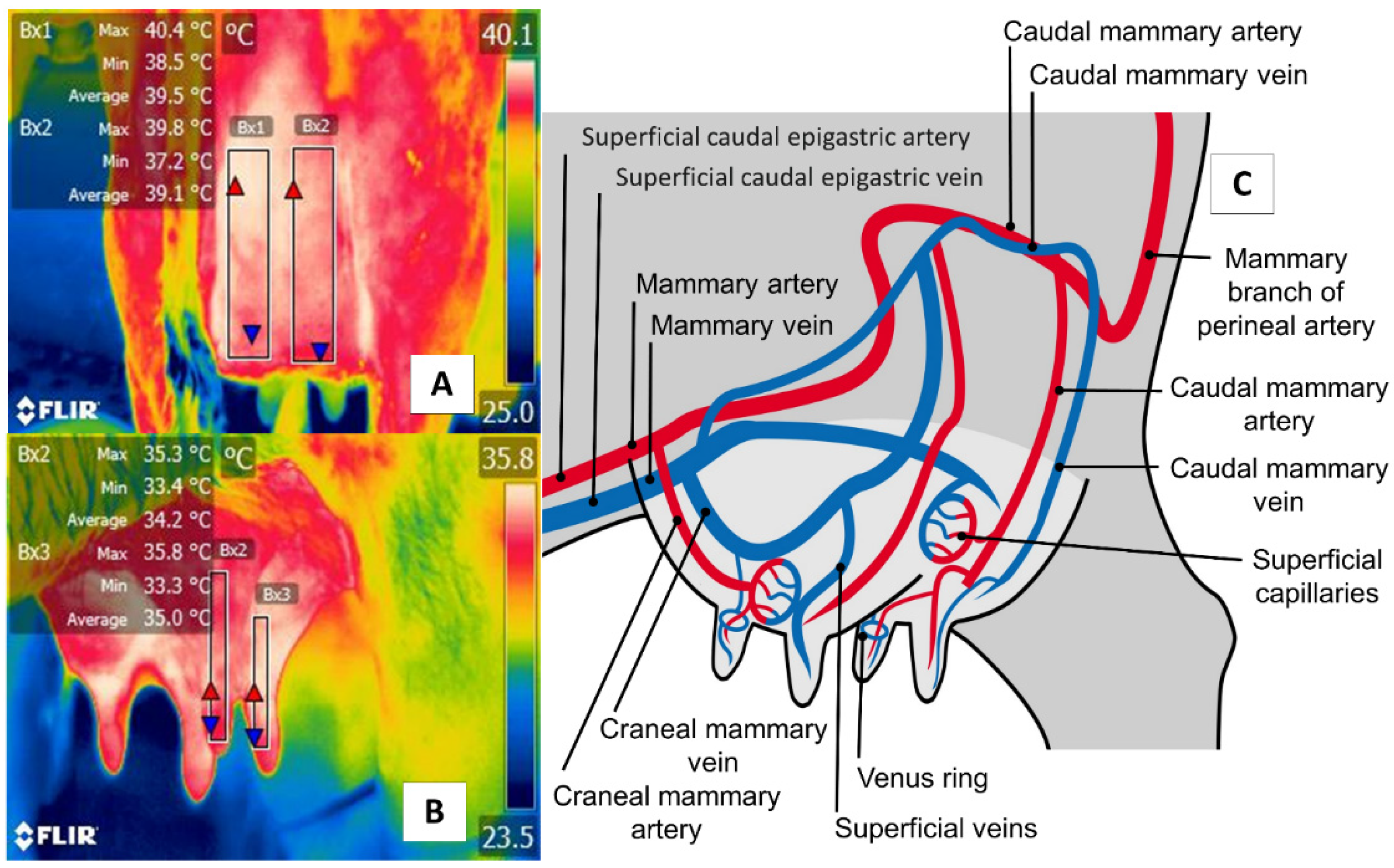
5. Udder Region (Regio Uberis) and Mammary Gland
6. Perineal Region (Regio Perinealis)
7. IRT and the Assessment of Pathological States
8. Environmental Influence on the Physiological Responses of Thermoregulation
9. Perspectives and Areas of Opportunity
10. Limitations of Infrared Thermography due to Environmental Conditions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Ethics Statement
References
- Petrc, K.; Kinizcova, I. The Use of Infrared Thermography in Livestock Production and Veterinary Field. In Infrared Thermography Recent Advances and Future Trends; Kunc, P., Knizkova, I., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 85–101. [Google Scholar]
- Stewart, M.; Wilson, M.T.; Schaefer, A.L.; Huddart, F.; Sutherland, M.A. The Use of Infrared Thermography and Accelerometers for Remote Monitoring of Dairy Cow Health and Welfare. J. Dairy Sci. 2017, 100, 3893–3901. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Velarde, A.; Maris-Huertas, S.; Cajiao, M.N. Animal Welfare, a Global Vision in Ibero-America; Elsevier Press: Barcelona, Spain, 2016; pp. 1–516. [Google Scholar]
- Mota-Rojas, D.; Orihuela, A.; Strappini-Asteggiano, A.; Nelly Cajiao-Pachón, M.; Agüera-Buendía, E.; Mora-Medina, P.; Ghezzi, M.; Alonso-Spilsbury, M. Teaching Animal Welfare in Veterinary Schools in Latin America. Int. J. Vet. Sci. Med. 2018, 6, 131–140. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, D.; Yang, Q.; Wen, Z.; Lv, L. Review: Application of Infrared Thermography in Livestock Monitoring. Trans. ASABE 2020, 63, 389–399. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I.; Napolitano, F.; Mota-Rojas, D.; Orihuela, A. The Water Buffalo in the Americas, Practical and Experimental Approaches, 2nd ed.; BM Editores: Mexico City, Mexico, 2018; pp. 1–881. [Google Scholar]
- Napolitano, F.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Orihuela, A. The Latin American River Buffalo, Recent Findings, 3rd ed.; BM Editores: Mexico City, Mexico, 2020; pp. 1–1545. [Google Scholar]
- Mota-Rojas, D.; Miranda-Córtes, A.; Casas-Alvarado, A.; Mora-Medina, P.; Boscato, L.; Hernández-Ávalos, I. Neurobiology and Modulation of Stress- Induced Hyperthermia and Fever in Animals. Abanico Vet. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-García, D.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Mora-Medina, P.; Salmerón, C.; Gómez, J.; Boscato, L.; Gutiérrez-Pérez, O.; Cruz, V.; et al. Hypothermia in Newly Born Piglets: Mechanisms of Thermoregulation and Pathophysiology of Death. J. Anim. Behav. Biometeorol. 2021, 9. [Google Scholar] [CrossRef]
- Reyes-Sotelo, B.; Mota-Rojas, D.; Martínez-Burnes, J.; Olmos-Hernández, A.; Hernández-Ávalos, I.; José, N.; Casas-Alvarado, A.; Gómez, J.; Mora-Medina, P. Thermal Homeostasis in the Newborn Puppy: Behavioral and Physiological Responses. J. Anim. Behav. Biometeorol. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Lowe, G.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Infrared Thermography—A Non-Invasive Method of Measuring Respiration Rate in Calves. Animals 2019, 9, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, D.V. Thermal Windows and Heat Exchange. Temperature 2015, 2, 451. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in Infrared Thermography: Surgical Aspects, Vascular Changes, and Pain Monitoring in Veterinary Medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected Contribution: Ambient Temperature for Experiments in Rats: A New Method for Determining the Zone of Thermal Neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef] [Green Version]
- Hankenson, F.C.; Marx, J.O.; Gordon, C.J.; David, J.M. Effects of Rodent Thermoregulation on Animal Models in the Research Environment. Comp. Med. 2018, 68, 425–438. [Google Scholar] [CrossRef]
- Flores-Peinado, S.; Mota-Rojas, D.; Guerrero-Legarreta, I.; Mora-Medina, P.; Cruz-Monterrosa, R.; Gómez-Prado, J.; Guadalupe Hernández, M.; Cruz-Playas, J.; Martínez-Burnes, J. Physiological Responses of Pigs to Preslaughter Handling: Infrared and Thermal Imaging Applications. Int. J. Vet. Sci. Med. 2020, 8, 71–84. [Google Scholar] [CrossRef]
- Lazaro, C.; Conte-Junior, C.A.; Medina-Vara, M.; Mota-Rojas, D.; Cruz-Monterrosa, R.; Guerrero-Legarreta, I. Effect of Pre-Slaughter Confinement Stress on Physicochemical Parameters of Chicken Meat. Ciênc. Anim. Bras. 2019, 20. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared Thermography: A Non-Invasive Window into Thermal Physiology. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Lecona-Butrón, H.; Martínez-Burnes, J.; Mora-Medina, P.; Gómez-Prado, J.; Orihuela, A. Infrared Thermal Imaging Associated with Pain in Laboratory Animals. Exp. Anim. 2020. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Napolitano, F.; Braghieri, A.; Guerrero-Legarreta, I.; Bertoni, A.; Martínez-Burnes, J.; Cruz-Monterrosa, R.; Gómez, J.; Ramírez-Bribiesca, E.; Barrios-García, H.; et al. Thermal Biology in River Buffalo in the Humid Tropics: Neurophysiological and Behavioral Responses Assessed by Infrared Thermography. J. Anim. Behav. Biometeorol. 2021, 9. [Google Scholar] [CrossRef]
- Martello, L.S.; da Luz e Silva, S.; da Costa Gomes, R.; da Silva Corte, R.R.P.; Leme, P.R. Infrared Thermography as a Tool to Evaluate Body Surface Temperature and Its Relationship with Feed Efficiency in Bos Indicus Cattle in Tropical Conditions. Int. J. Biometeorol. 2016, 60, 173–181. [Google Scholar] [CrossRef]
- Thompson, S.; Schaefer, A.L.; Crow, G.H.; Basarab, J.; Colyn, J.; Ominski, K. Relationship between Residual Feed Intake and Radiated Heat Loss Using Infrared Thermography in Young Beef Bulls. J. Therm. Biol. 2018, 78, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ootsuka, Y.; Tanaka, M. Control of Cutaneous Blood Flow by Central Nervous System. Temperature 2015, 2, 392–405. [Google Scholar] [CrossRef]
- Vainionpää, M. Thermographic Imaging in Cats and Dogs Usability as a Clinical Method. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2014. [Google Scholar]
- Lim, S.; Honek, J.; Xue, Y.; Seki, T.; Cao, Z.; Andersson, P.; Yang, X.; Hosaka, K.; Cao, Y. Cold-Induced Activation of Brown Adipose Tissue and Adipose Angiogenesis in Mice. Nat. Protoc. 2012, 7, 606–615. [Google Scholar] [CrossRef]
- Gordon, C.J.; Aydin, C.; Repasky, E.A.; Kokolus, K.M.; Dheyongera, G.; Johnstone, A.F.M. Behaviorally Mediated, Warm Adaptation: A Physiological Strategy When Mice Behaviorally Thermoregulate. J. Therm. Biol. 2014, 44, 41–46. [Google Scholar] [CrossRef]
- Barros, D.V.; Silva, L.K.X.; Kahwage, P.R.; Lourenço Júnior, J.B.; Sousa, J.S.; Silva, A.G.M.; Franco, I.M.; Martorano, L.G.; Garcia, A.R. Assessment of Surface Temperatures of Buffalo Bulls (Bubalus bubalis) Raised under Tropical Conditions Using Infrared Thermography. Arq. Bras. Med. Vet. Zootec. 2016, 68, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Talukder, S.; Thomson, P.C.; Kerrisk, K.L.; Clark, C.E.F.; Celi, P. Evaluation of Infrared Thermography Body Temperature and Collar-Mounted Accelerometer and Acoustic Technology for Predicting Time of Ovulation of Cows in a Pasture-Based System. Theriogenology 2015, 83, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, A.; Mota-Rojas, D.; Álvarez-Macias, A.; Mora-Medina, P.; Guerrero-Legarreta, I.; Morales-Canela, A.; Gómez-Prado, J.; José-Pérez, N.; Martínez-Burnes, J. Scientific Findings Related to Changes in Vascular Microcirculation Using Infrared Thermography in the River Buffalo. J. Anim. Behav. Biometeorol. 2020, 8, 288–297. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Habeeb, A.A.; Napolitano, F.; Sarubbi, J.; Ghezzi, M.; Ceriani, M.C.; Cuibus, A.; Martínez-Burnes, J.; Braghieri, A.; Lendez, P.A.; et al. River Buffalo, European Cattle and Indian Cattle Welfare: Environmental, Physiological and Behavioral Aspects in Response to Natural and Artificial Shade. In El búfalo de Agua en Latinoamérica, Hallazgos Recientes; Napolitano, F., Mota-Rojas, D., Guerrero-Legarreta, J., Orihuela, A., Eds.; BM Editores: Mexico City, Mexico, 2020; pp. 960–1016. Available online: https://www.lifescienceglobal.com/journals/journal-of-buffalo-science/97-abstract/jbs/4550-el-bufalo-de-agua-en-latinoamerica-hallazgos-recientes (accessed on 11 December 2020).
- Nääs, I.A.; Garcia, R.G.; Caldara, F.R. Infrared Thermal Image for Assessing Animal Health and Welfare. J. Anim. Behav. Biometeorol. 2014, 2, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Gigantesco, A.; Giuliani, M. Quality of Life in Mental Health Services with a Focus on Psychiatric Rehabilitation Practice. Ann. Ist. Super Sanità 2011, 47, 363–372. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria; World Association of Veterinary Anatomist: Oslo, Norway, 2017. [Google Scholar]
- Constantinescu, M.G. Illustrated Veterinary Anatomical Nomenclature, 4th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2018; pp. 1–576. [Google Scholar]
- Childs, C. Body temperature and clinical thermometry. In Thermoregulation: From Basic Neuroscience to Clinical Neurology, Part II; Romanovsky, A.A., Ed.; Elsevier Press: Amsterdam, The Netherlands, 2018; pp. 467–482. [Google Scholar]
- Romanovsky, A.A. Thermoregulation: Some Concepts Have Changed. Functional Architecture of the Thermoregulatory System. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R37–R46. [Google Scholar] [CrossRef]
- Romanovsky, A.A. Skin Temperature: Its Role in Thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Habeeb, A.A.; Ghezzi, M.D.; Kanth Reddy, R.; Napolitano, F.; Lendez, P.A.; Cuibus, A.; Ceriani, C.M.; Sarubbi, J.; Braghieri, A.; et al. Termorragulación del búfalo de agua: Mecanismos neurobiológicos, cambios microcirculatorios y aplicaciones prácticas de la termografía infrarroja. In El Búfalo de Agua en Latinoamérica, Hallazgos Recientes; Napolitano, F., Mota Rojas, D., Guerrero-Legarreta, I., Orihuela, A., Eds.; BM Editores: Mexico City, Mexico, 2020; pp. 922–934. [Google Scholar]
- Osaka, T. Hypoxia-Induced Hypothermia Mediated by GABA in the Rostral Parapyramidal Area of the Medulla Oblongata. Neuroscience 2014, 267, 46–56. [Google Scholar] [CrossRef]
- Cerri, M.; Zamboni, G.; Tupone, D.; Dentico, D.; Luppi, M.; Martelli, D.; Perez, E.; Amici, R. Cutaneous Vasodilation Elicited by Disinhibition of the Caudal Portion of the Rostral Ventromedial Medulla of the Free-Behaving Rat. Neuroscience 2010, 165, 984–995. [Google Scholar] [CrossRef]
- Labeur, L.; Villiers, G.; Small, A.H.; Hinch, G.N.; Schmoelzl, S. Infrared Thermal Imaging as a Method to Evaluate Heat Loss in Newborn Lambs. Res. Vet. Sci. 2017, 115, 517–522. [Google Scholar] [CrossRef]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Jia, G.; Li, W.; Meng, J.; Tan, H.; Feng, Y. Non-Contact Evaluation of Pigs’ Body Temperature Incorporating Environmental Factors. Sensors 2020, 20, 4282. [Google Scholar] [CrossRef]
- Vainionpää, M.; Tienhaara, E.-P.; Raekallio, M.; Junnila, J.; Snellman, M.; Vainio, O. Thermographic Imaging of the Superficial Temperature in Racing Greyhounds before and after the Race. Sci. World J. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Agbigbe, O.; Nigro, N.; Yakobi, G.; Shapiro, J.; Ginosar, Y. Use of High-Resolution Thermography as a Validation Measure to Confirm Epidural Anesthesia in Mice: A Cross-over Study. Int. J. Obstet. Anesth. 2021, 46, 102981. [Google Scholar] [CrossRef] [PubMed]
- Küls, N.; Blissitt, K.J.; Shaw, D.J.; Schöffmann, G.; Clutton, R.E. Thermography as an Early Predictive Measurement for Evaluating Epidural and Femoral–Sciatic Block Success in Dogs. Vet. Anaesth. Anal. 2017, 44, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Church, J.S.; Hegadoren, P.R.; Paetkau, M.J.; Miller, C.C.; Regev-Shoshani, G.; Schaefer, A.L.; Schwartzkopf-Genswein, K.S. Influence of Environmental Factors on Infrared Eye Temperature Measurements in Cattle. Res. Vet. Sci. 2014, 96, 220–226. [Google Scholar] [CrossRef]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive Physiological Indicators of Heat Stress in Cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Montanholi, Y.R.; Odongo, N.E.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Miller, S.P. Application of Infrared Thermography as an Indicator of Heat and Methane Production and Its Use in the Study of Skin Temperature in Response to Physiological Events in Dairy Cattle (Bos Taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Perez Marquez, H.J.; Ambrose, D.J.; Schaefer, A.L.; Cook, N.J.; Bench, C.J. Infrared Thermography and Behavioral Biometrics Associated with Estrus Indicators and Ovulation in Estrus-Synchronized Dairy Cows Housed in Tiestalls. J. Dairy Sci. 2019, 102, 4427–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huggins, J.; Rakobowchuk, M. Utility of Lacrimal Caruncle Infrared Thermography When Monitoring Alterations in Autonomic Activity in Healthy Humans. Eur. J. Appl. Physiol. 2019, 119, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Stookey, J.M.; Stafford, K.J.; Tucker, C.B.; Rogers, A.R.; Dowling, S.K.; Verkerk, G.A.; Schaefer, A.L.; Webster, J.R. Effects of Local Anesthetic and a Nonsteroidal Antiinflammatory Drug on Pain Responses of Dairy Calves to Hot-Iron Dehorning. J. Dairy Sci. 2009. [Google Scholar] [CrossRef]
- Stewart, M.; Verkerk, G.A.; Stafford, K.J.; Schaefer, A.L.; Webster, J.R. Noninvasive Assessment of Autonomic Activity for Evaluation of Pain in Calves, Using Surgical Castration as a Model. J. Dairy Sci. 2010, 93, 3602–3609. [Google Scholar] [CrossRef]
- Giro, A.; de Campos Bernardi, A.C.; Barioni Junior, W.; Lemes, A.P.; Botta, D.; Romanello, N.; do Nascimento Barreto, A.; Garcia, A.R. Application of Microchip and Infrared Thermography for Monitoring Body Temperature of Beef Cattle Kept on Pasture. J. Therm. Biol. 2019, 84, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Stafford, K.J.; Dowling, S.K.; Schaefer, A.L.; Webster, J.R. Eye Temperature and Heart Rate Variability of Calves Disbudded with or without Local Anaesthetic. Physiol. Behav. 2008, 93, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A Lameness Scoring System That Uses Posture and Gait to Predict Dairy Cattle Reproductive Performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Lowe, G.; McCane, B.; Sutherland, M.; Waas, J.; Schaefer, A.; Cox, N.; Stewart, M. Automated Collection and Analysis of Infrared Thermograms for Measuring Eye and Cheek Temperatures in Calves. Animals 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikkagoudara, K.P.; Singh, P.; Barman, D.; Potshangbam, C.; Bhatt, N.; Singh, S.V.; Lathwal, S.S. Eye Temperature, an Indicator for Stress Levels in Young Buffalo Bulls—A Case Study of Micro-Environment Modification. J. Agrometeorol. 2020, 22, 266–273. [Google Scholar]
- Scoley, G.E.; Gordon, A.W.; Morrison, S.J. Use of Thermal Imaging in Dairy Calves: Exploring the Repeatability and Accuracy of Measures Taken from Different Anatomical Regions1. Trans. Anim. Sci. 2019, 3, 564–576. [Google Scholar] [CrossRef]
- Athaíde, L.G.; Joset, W.C.; de Almeida, J.F.; Pantoja, M.H.; Noronha, R.D.; Bezerra, A.S.; Barbosa, A.V.; Martorano, L.G.; da Silva, J.A.; Lourenço Júnior, J.D. Thermoregulatory and Behavioral Responses of Buffaloes With and Without Direct Sun Exposure During Abnormal Environmental Condition in Marajó Island, Pará, Brazil. Front. Vet. Sci. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Brcko, C.C.; Silva, J.A.; Martorano, L.G.; Vilela, R.A.; Nahúm, B.D.; Silva, A.G.; Barbosa, A.V.; Bezerra, A.S.; Lourenço Júnior, J.D. Infrared Thermography to Assess Thermoregulatory Reactions of Female Buffaloes in a Humid Tropical Environment. Front. Vet. Sci. 2020, 7, 180. [Google Scholar] [CrossRef]
- Bleul, U.; Hässig, M.; Kluser, F. Screening of Febrile Cows Using a Small Handheld Infrared Thermography Device. Tierarztl. Prax. Ausg. G Grosstiere/Nutztiere 2021, 49, 12–20. [Google Scholar] [CrossRef]
- Seixas, A.; Ammer, K. Utility of Infrared Thermography When Monitoring Autonomic Activity. Eur. J. Appl. Physiol. 2019, 119, 1455–1457. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of Infrared Thermography as a Non-Invasive Method of Measuring the Autonomic Nervous Response in Sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef]
- Jansson, A.; Lindgren, G.; Velie, B.D.; Solé, M. An Investigation into Factors Influencing Basal Eye Temperature in the Domestic Horse (Equus Caballus) When Measured Using Infrared Thermography in Field Conditions. Physiol. Behav. 2021, 228. [Google Scholar] [CrossRef]
- Strutzke, S.; Fiske, D.; Hoffmann, G.; Ammon, C.; Heuwieser, W.; Amon, T. Technical Note: Development of a Noninvasive Respiration Rate Sensor for Cattle. J. Dairy Sci. 2019, 102, 690–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques da Silva, D.C. Termografia Infravermelho e Medidas de Eficiência de Bubalinos de Três Grupos Genéticos Sob Condições Tropicais. Ph.D. Thesis, Universidade Estadual Paulista, Botucatu, Brasil, 2019. [Google Scholar]
- Abbas, A.K.; Heimann, K.; Jergus, K.; Orlikowsky, T.; Leonhardt, S. Neonatal Non-Contact Respiratory Monitoring Based on Real-Time Infrared Thermography. Biomed. Eng. Online 2011, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Machado, N.A.F.; Da Costa, L.B.S.; Barbosa-Filho, J.A.D.; De Oliveira, K.P.L.; De Sampaio, L.C.; Peixoto, M.S.M.; Damasceno, F.A. Using Infrared Thermography to Detect Subclinical Mastitis in Dairy Cows in Compost Barn Systems. J. Therm. Biol. 2021, 97, 102881. [Google Scholar] [CrossRef]
- Sarubbi, F.; Grazioli, G.; Auriemma, G. A Potential Application of Infrared Thermography ( IRT ) in Mediterranean A Potential Application of Infrared Thermography ( IRT ) in Mediterranean Lactating Buffalo. Asian Basic Appl. Res. J. 2020, 2, 11–16. [Google Scholar]
- Fox, L.K.; Gay, J.M. Contagious Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 1993, 9, 475–487. [Google Scholar] [CrossRef]
- Hovinen, M.; Siivonen, J.; Taponen, S.; Hänninen, L.; Pastell, M.; Aisla, A.-M.; Pyörälä, S. Detection of Clinical Mastitis with the Help of a Thermal Camera. J. Dairy Sci. 2008, 91, 4592–4598. [Google Scholar] [CrossRef]
- Polat, B.; Colak, A.; Cengiz, M.; Yanmaz, L.E.; Oral, H.; Bastan, A.; Kaya, S.; Hayirli, A. Sensitivity and Specificity of Infrared Thermography in Detection of Subclinical Mastitis in Dairy Cows. J. Dairy Sci. 2010, 93, 3525–3532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos-Hernández, A.; Ghezzi, M.D.; Napolitano, F.; Cuibus, A.; Álvarez-Macías, A.; Braghieri, A.; Mota-Rojas, D. Anatomophysiology of the mammary gland: Neuroendocrinology of milk ejection in the river buffalo. In El Búfalo de Agua en Latinoamérica, Hallazgos Recientes; Napolitano, F., Mota-Rojas, D., Guerrero-Legarreta, I., Orihuela, A., Eds.; BM Editores: Mexico City, Mexico, 2020; pp. 721–771. [Google Scholar]
- Budras, K.D.; Habel, R.E. Bovine Anatomy an Illustrated Text, 1st ed.; Schulutersche: Hanover, Germany, 2003; pp. 1–440. [Google Scholar]
- Radigonda, V.L.; Pereira, G.R.; da Cruz Favaro, P.; Barca Júnior, F.A.; Borges, M.H.F.; Galdioli, V.H.G.; Júnior, C.K. Infrared Thermography Relationship between the Temperature of the Vulvar Skin, Ovarian Activity, and Pregnancy Rates in Braford Cows. Trop. Anim. Health Prod. 2017, 49, 1787–1791. [Google Scholar] [CrossRef]
- de Ruediger, F.R.; Yamada, P.H.; Bicas Barbosa, L.G.; Mungai Chacur, M.G.; Pinheiro Ferreira, J.C.; de Carvalho, N.A.T.; Milani Soriano, G.A.; Codognoto, V.M.; Oba, E. Effect of Estrous Cycle Phase on Vulvar, Orbital Area and Muzzle Surface Temperatures as Determined Using Digital Infrared Thermography in Buffalo. Anim. Reprod. Sci. 2018, 197, 154–161. [Google Scholar] [CrossRef] [Green Version]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared Thermography in Animal Production: An Overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Yadav, S.K.; Singh, P.; Kumar, P.; Singh, S.V.; Singh, A.; Kumar, S. Scrotal Infrared Thermography and Testicular Biometry: Indicator of Semen Quality in Murrah Buffalo Bulls. Anim. Reprod. Sci. 2019, 209, 106145. [Google Scholar] [CrossRef]
- Chacur, M.G.M. Termografia Por Infravermelho Na Reprodução de Bubalinos. Rev. Bras. Reprod. Anim. 2017, 41, 180–187. [Google Scholar]
- Gianesella, M.; Arfuso, F.; Fiore, E.; Giambelluca, S.; Giudice, E.; Armato, L.; Piccione, G. Infrared Thermography as a Rapid and Non-Invasive Diagnostic Tool to Detect Inflammatory Foot Diseases in Dairy Cows. Pol. J. Vet. Sci. 2018, 21, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mendo, O.; von Keyserlingk, M.A.G.; Veira, D.M.; Weary, D.M. Effects of Pasture on Lameness in Dairy Cows. J. Dairy Sci. 2007, 90, 1209–1214. [Google Scholar] [CrossRef]
- LokeshBabu, D.S.; Jeyakumar, S.; Vasant, P.J.; Sathiyabarathi, M.; Manimaran, A.; Kumaresan, A.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Kataktalware, M.A.; et al. Monitoring Foot Surface Temperature Using Infrared Thermal Imaging for Assessment of Hoof Health Status in Cattle: A Review. J. Therm. Biol. 2018, 78, 10–21. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An Investigation into the Use of Infrared Thermography (IRT) as a Rapid Diagnostic Tool for Foot Lesions in Dairy Cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Orman, A.; Endres, M.I. Use of Thermal Imaging for Identification of Foot Lesions in Dairy Cattle. Act. Agric. Scand. A Anim. Sci. 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Alsaaod, M.; Büscher, W. Detection of Hoof Lesions Using Digital Infrared Thermography in Dairy Cows. J. Dairy Sci. 2012, 95, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bleul, U.; Hässig, M.; Kluser, F. Screening of Febrile Cows Using Infrared Thermography. Tierarztliche Praxis Ausgabe G Grosstiere Nutztiere 2019, 49, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Metzner, M.; Sauter-Louis, C.; Seemueller, A.; Petzl, W.; Klee, W. Infrared Thermography of the Udder Surface of Dairy Cattle: Characteristics, Methods, and Correlation with Rectal Temperature. Vet. J. 2014, 199, 57–62. [Google Scholar] [CrossRef]
- Schaefer, A.L.; Cook, N.J.; Bench, C.; Chabot, J.B.; Colyn, J.; Liu, T.; Okine, E.K.; Stewart, M.; Webster, J.R. The Non-Invasive and Automated Detection of Bovine Respiratory Disease Onset in Receiver Calves Using Infrared Thermography. Res. Vet. Sci. 2012, 93, 928–935. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Wang, D.; Gonçalves Titto, C.; Gómez-Prado, J.; Carvajal-de la Fuenta, V.; Ghezzi, M.; Boscato-Funes, L.; Barrios-García, H.; Torres-Bernal, F.; Casas-Alvarado, A.; et al. Pathophysiology of fever and application of infrared thermography (IRT) in the detection of sick domestic animals: Recent advances. Animals 2021. in revision. [Google Scholar]
- Fernández-Cuevas, I.; Bouzas Marins, J.C.; Arnáiz Lastras, J.; Gómez Carmona, P.M.; Piñonosa Cano, S.; García-Concepción, M.Á.; Sillero-Quintana, M. Classification of Factors Influencing the Use of Infrared Thermography in Humans: A Review. Infrared Phys. Technol. 2015, 71, 28–55. [Google Scholar] [CrossRef]
- Loughmiller, J.A.; Spire, M.E.; Dritz, S.S.; Fenwick, B.W.; Hosni, M.H.; Hogge, S.B. Relationship between Mean Body Surface Temperature Measured by Use of Infrared Thermography and Ambient Temperature in Clinically Normal Pigs and Pigs Inoculated with Actinobacillus Pleuropneumoniae. Am. J. Vet. Res. 2001. [Google Scholar] [CrossRef] [PubMed]
- Soroko, M.; Howell, K.; Dudek, K. The Effect of Ambient Temperature on Infrared Thermographic Images of Joints in the Distal Forelimbs of Healthy Racehorses. J. Therm. Biol. 2017, 66, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Chen, S.; Li, G.; Chen, J.; Wang, J.; Gu, X. Infrared Thermography Measured Body Surface Temperature and Its Relationship with Rectal Temperature in Dairy Cows under Different Temperature-Humidity Indexes. Int. J. Biometeorol. 2019, 63, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Upadhyay, R. Heat Stress and Animal Productivity; Springer: New Dheli, India, 2013; ISBN 978-81-322-0878-5. [Google Scholar]
- Robinson, E.B. Thermoregulation. In Textbook of Veterinary Physiology; Klein, B.G., Ed.; Elsevier: London, UK, 2014; pp. 533–542. [Google Scholar]
- Khongdee, T.; Sripoon, S.; Vajrabukka, C. The Effects of High Temperature and Wallow on Physiological Responses of Swamp Buffaloes (Bubalus Bubalis) during Winter Season in Thailand. J. Therm. Biol. 2011, 36, 417–421. [Google Scholar] [CrossRef]
- Oliveira, J.P.F.; Rangel, A.H.N.; Barreto, M.L.J.; Araújo, V.M.; Lima Júnior, D.M.; Novaes, L.P.; Aureliano, I.P.L. Temperamento de Búfalas Em Sala de Ordenha Sobre Índices Produtivos e Adaptabilidade Ao Ambiente: Uma Revisão. J. Anim. Behav. Biometeorol. 2013, 1, 21–30. [Google Scholar] [CrossRef]
- Das, G.; Khan, F. Summer Anoestrus in Buffalo—A Review. Reprod. Domest. Anim. 2010, 45, e483–e494. [Google Scholar] [CrossRef]
- De Rosa, G.; Napolitano, F.; Grasso, F.; Pacelli, C.; Bordi, A. On the Development of a Monitoring Scheme of Buffalo Welfare at Farm Level. Ital. J. Anim. Sci. 2005, 4, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Bertoni, A.; Álvarez-Macías, A.; Mota-Rojas, D. Productive Performance of Buffaloes and Their Development Options in Tropical Regions. Soc. Rural. Prod. Med. Ambiente 2019, 19, 59–80. [Google Scholar]
- Isola, J.V.V.; Menegazzi, G.; Busanello, M.; dos Santos, S.B.; Agner, H.S.S.; Sarubbi, J. Differences in Body Temperature between Black-and-White and Red-and-White Holstein Cows Reared on a Hot Climate Using Infrared Thermography. J. Therm. Biol. 2020, 94, 102775. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s Biological Functions as Affected by Heat Stress—A Review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Debbarma, D.; Uppal, V.; Bansal, N.; Gupta, A. Histomorphometrical Study on Regional Variation in Distribution of Sweat Glands in Buffalo Skin. Dermatol. Res. Pract. 2018, 2018, 5345390. [Google Scholar] [CrossRef]
- Hafez, E.S.E.; Badreldin, A.L.; Shafei, M.M. Skin Structure of Egyptian Buffaloes and Cattle with Particular Reference to Sweat Glands. J. Agric. Sci. 1955, 46, 19–30. [Google Scholar] [CrossRef]
- Presicce, G.A. (Ed.) The Buffalo (Bubalus Bubalis)—Production and Research; Bentham Science Publishers: Padua, Italy, 2017; ISBN 9781681084176. [Google Scholar]
- Desta, T.T. Introduction of Domestic Buffalo (Bubalus Bubalis) into Ethiopia Would Be Feasible. Renew. Agric. Food Syst. 2012, 27, 305–313. [Google Scholar] [CrossRef]
- Castro, A.C.; Lourenço Júnior, J.D.; Santos, N.D.; Monteiro, E.M.; Aviz, M.A.; Garcia, A.R. Sistema Silvipastoril Na Amazônia: Ferramenta Para Elevar o Desempenho Produtivo de Búfalos. Ciênc. Rural 2008, 38, 2395–2402. [Google Scholar] [CrossRef]
- Das, S.; Upadhyay, R.; Madan, M. Heat Stress in Murrah Buffalo Calves. Livest. Prod. Sci. 1999, 61, 71–78. [Google Scholar] [CrossRef]
- Dimri, U.; Ranjan, R.; Sharma, M.C.; Varshney, V.P. Effect of Vitamin E and Selenium Supplementation on Oxidative Stress Indices and Cortisol Level in Blood in Water Buffaloes during Pregnancy and Early Postpartum Period. Trop. Anim. Health Pro. 2010, 42, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zhengkang, H.; Zhenzhong, C.; Shaohua, Z.; Vale, W.G.; Barnabe, V.H.; Mattos, J.C.A. Rumen Metabolism, Blood Corgisol and T3, T4 Levels and Other Physiological Paramaters of Swamp Buffalo Subjected to Solar Radiation. In Proceedings of the IVth World Buffalo Congress, San Paulo, Brazil, 27–30 June 1994; pp. 39–40. [Google Scholar]
- Berdugo- Gutiérrez, J.; Napolitano, F.; Mota- Rojas, D.; González, N.J.; Ruíz- Buitrago, J.; Guerrero-Legarreta, I. El Búfalo de Agua y El Estrés Calórico; BM Editores Special Section, Let´s Learn Animal Welfare Together: Mexico City, Mexico, 2017. [Google Scholar]
- Mora-Medina, P.; Berdugo-Gutiérrez, J.A.; Mota-Rojas, D.; Ruiz-Buitrago, J.D.; Nava-Adame, J.; Guerrero-Legarreta, I. Behaviour and Welfare of Dairy Buffaloes: Pasture or Confinement? J. Buffalo Sci. 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Mora-Medina, P.; Napolitano, F.; Mota-Rojas, D.; Berdugo-Gutiérrez, J.; Ruiz-Buitrago, J.; Guerrero-Legarreta, I. Imprinting, Sucking and Allosucking Behaviors in Buffalo Calves. J. Buffalo Sci. 2018, 7, 49–57. [Google Scholar] [CrossRef]
- Napolitano, F.; Pacelli, C.; Grasso, F.; Braghieri, A.; De Rosa, G. The Behaviour and Welfare of Buffaloes (Bubalus bubalis) in Modern Dairy Enterprises. Animal 2013, 7, 1704–1713. [Google Scholar] [CrossRef] [Green Version]
- Sevegnani, K.B.; Fernandes, D.P.B.; da Silva, S.H.M.-G. Evaluation of Thermorregulatory Capacity of Dairy Buffaloes Using Infrared Thermography. Eng. Agric. 2016, 36, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lendez, P.A.; Nieto, M.V.; Martínez, L.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Heat Stress: Its Effect on the Immune Status of Dairy Cows. Rev. Med. Vet. 2020, 101, 7–12. [Google Scholar]
- Lendez, P.A.; Martinez Cuesta, L.; Nieto Farias, M.V.; Vater, A.A.; Ghezzi, M.D.; Mota-Rojas, D.; Dolcini, G.L.; Ceriani, M.C. Alterations in TNF-α and Its Receptors Expression in Cows Undergoing Heat Stress. Vet. Immunol. Immunopathol. 2021, 235, 110232. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Wang, W.; Guo, L.; Bindelle, J. Recent Advances on Early Detection of Heat Strain in Dairy Cows Using Animal-Based Indicators: A Review. Animals 2021, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.S.V.; da Silva, S.C.; Salles, F.A.; Roma, L.C.; El Faro, L.; Bustos Mac Lean, P.A.; Lins de Oliveira, C.E.; Martello, L.S. Mapping the Body Surface Temperature of Cattle by Infrared Thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy Cow Reproduction under the Influence of Heat Stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, H.; Tarr, G.; Loudon, K.; Lomax, S.; White, P.; McGreevy, P.; Polkinghorne, R.; González, L.A. Using Infrared Thermography on Farm of Origin to Predict Meat Quality and Physiological Response in Cattle (Bos taurus) Exposed to Transport and Marketing. Meat Sci. 2020, 169, 108173. [Google Scholar] [CrossRef]
- Bertoni, A.; Napolitano, F.; Mota-Rojas, D.; Sabia, E.; Álvarez-Macías, A.; Mora-Medina, P.; Morales-Canela, A.; Berdugo-Gutiérrez, J.; Guerrero- Legarreta, I. Similarities and Differences between River Buffaloes and Cattle: Health, Physiological, Behavioral and Productivity Aspects. J. Buffalo Sci. 2020, 9, 92–109. [Google Scholar] [CrossRef]
- Wang, F.-K.; Shih, J.-Y.; Juan, P.-H.; Su, Y.-C.; Wang, Y.-C. Non-Invasive Cattle Body Temperature Measurement Using Infrared Thermography and Auxiliary Sensors. Sensors 2021, 21, 2425. [Google Scholar] [CrossRef]
- de Lima, V.; Piles, M.; Rafel, O.; López-Béjar, M.; Ramón, J.; Velarde, A.; Dalmau, A. Use of Infrared Thermography to Assess the Influence of High Environmental Temperature on Rabbits. Res. Vet. Sci. 2013, 95, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Takemura, K.; Sato, S. Investigation of Various Essential Factors for Optimum Infrared Thermography. J. Vet. Med. Sci. 2013, 75, 1349–1353. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Rojas, D.; Pereira, A.M.F.; Wang, D.; Martínez-Burnes, J.; Ghezzi, M.; Hernández-Avalos, I.; Lendez, P.; Mora-Medina, P.; Casas, A.; Olmos-Hernández, A.; et al. Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals 2021, 11, 2247. https://doi.org/10.3390/ani11082247
Mota-Rojas D, Pereira AMF, Wang D, Martínez-Burnes J, Ghezzi M, Hernández-Avalos I, Lendez P, Mora-Medina P, Casas A, Olmos-Hernández A, et al. Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals. 2021; 11(8):2247. https://doi.org/10.3390/ani11082247
Chicago/Turabian StyleMota-Rojas, Daniel, Alfredo M. F. Pereira, Dehua Wang, Julio Martínez-Burnes, Marcelo Ghezzi, Ismael Hernández-Avalos, Pamela Lendez, Patricia Mora-Medina, Alejandro Casas, Adriana Olmos-Hernández, and et al. 2021. "Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity" Animals 11, no. 8: 2247. https://doi.org/10.3390/ani11082247
APA StyleMota-Rojas, D., Pereira, A. M. F., Wang, D., Martínez-Burnes, J., Ghezzi, M., Hernández-Avalos, I., Lendez, P., Mora-Medina, P., Casas, A., Olmos-Hernández, A., Domínguez, A., Bertoni, A., & Geraldo, A. d. M. (2021). Clinical Applications and Factors Involved in Validating Thermal Windows Used in Infrared Thermography in Cattle and River Buffalo to Assess Health and Productivity. Animals, 11(8), 2247. https://doi.org/10.3390/ani11082247












