Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like Peptide 1 by Interacting with Transient Receptor Potential Ankyrin 1 in a Porcine Ex-Vivo Intestinal Segment Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.1.1. First Experiment
2.1.2. Second and Third Experiment
2.2. First Experiment
2.2.1. Animals and Sampling Procedures
2.2.2. Tissue Viability
2.2.3. Hormone Analysis
2.2.4. Gene Expression
2.3. Second Experiment
2.3.1. Animals and Sampling Procedures
2.3.2. Hormone Analysis
2.3.3. Tissue Viability
2.4. Third Experiment
2.4.1. Animals and Sampling Procedures
2.4.2. Hormone Analysis
2.5. Statistics
3. Results
3.1. First Experiment
3.1.1. Viability of Ex-Vivo Intestinal Tissue
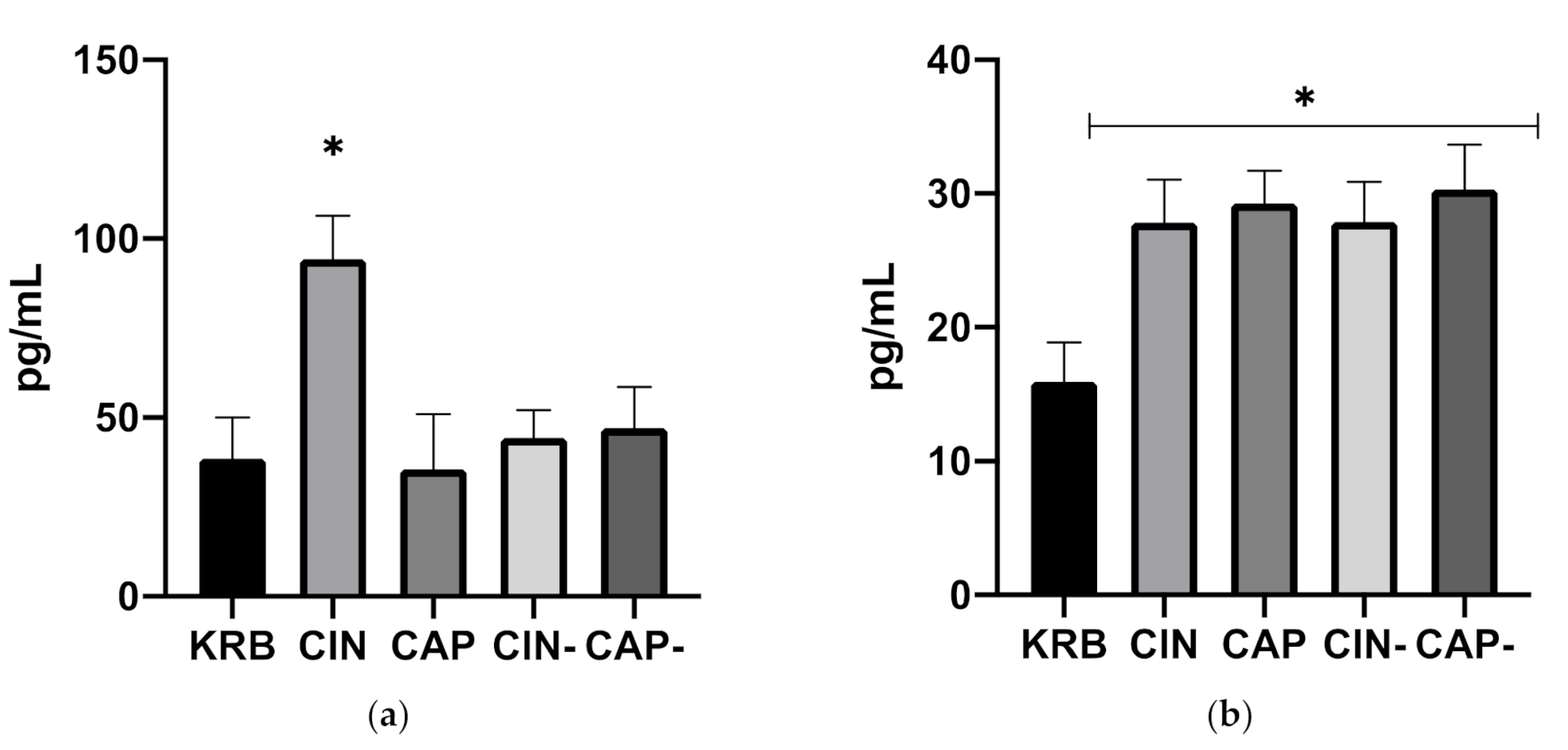
3.1.2. Release of Gastrointestinal Hormones upon Exposure to Cinnamaldehyde and Capsaicin
3.1.3. Gene Expression
3.2. Second Experiment
3.2.1. Viability of Ex-Vivo Intestinal Tissue
3.2.2. Release of Gastrointestinal Hormones upon Exposure to Cinnamaldehyde in Duodenum and Ileum
3.3. Third Experiment
Release of CCK upon Exposure to Cinnamaldehyde in Duodenum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Indian J. Med. Res. 2004, 119, 167–179. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Lallès, J.P.; Bosi, P.; Janczyk, P.; Koopmans, S.J.; Torrallardona, D. Impact of bioactive substances on the gastrointestinal tract and performance of weaned piglets: A review. Animal 2009, 3, 1625–1643. [Google Scholar] [CrossRef] [Green Version]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Li, P.; Piao, X.; Ru, Y.; Han, X.; Xue, L.; Zhang, H. Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australas. J. Anim. Sci. 2012, 25, 1617. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Hossain, M.; Kim, G.; Hwang, J.; Ji, H.; Yang, C. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian-Australas. J. Anim. Sci. 2013, 26, 683. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Wang, J.; Kim, H.; Meng, Q.; Ao, X.; Hong, S.; Kim, I. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower–finisher pigs. Livest. Sci. 2010, 128, 115–122. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Food/Nahrung 2000, 44, 42–46. [Google Scholar] [CrossRef]
- Aihara, E.; Hayashi, M.; Sasaki, Y.; Kobata, A.; Takeuchi, K. Mechanisms underlying capsaicin-stimulated secretion in the stomach: Comparison with mucosal acidification. J. Pharmacol. Exp. Ther. 2005, 315, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limlomwongse, L.; Chaitauchawong, C.; Tongyai, S. Effect of capsaicin on gastric acid secretion and mucosal blood flow in the rat. J. Nutr. 1979, 109, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Manzanilla, E.G.; Perez, J.F.; Martin, M.; Kamel, C.; Baucells, F.; Gasa, J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J. Anim. Sci. 2004, 82, 3210–3218. [Google Scholar] [CrossRef] [Green Version]
- Fledderus, J.; Bikker, P.; Kluess, J.W. Increasing diet viscosity using carboxymethylcellulose in weaned piglets stimulates protein digestibility. Livest. Sci. 2007, 109, 89–92. [Google Scholar] [CrossRef]
- Rist, V.; Weiss, E.; Eklund, M.; Mosenthin, R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal 2013, 7, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Pieper, R.; Villodre Tudela, C.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Roura, E.; Koopmans, S.-J.; Lallès, J.-P.; Le Huerou-Luron, I.; de Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Legrand, C.; Merlini, J.M.; de Senarclens-Bezençon, C.; Michlig, S. New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, L.J.; Callaghan, B.; Rivera, L.R.; Lieu, T.M.; Poole, D.P.; Cho, H.J.; Bravo, D.M.; Furness, J.B. Effects of food components that activate TRPA1 receptors on mucosal ion transport in the Mouse intestine. Nutrients 2016, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Reyes, L.E.; Ladas, T.P.; Chiang, C.-C.; Durand, D.M. TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo. Exp. Neurol. 2013, 250, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Van Liefferinge, E.; Van Noten, N.; Degroote, J.; Vrolix, G.; Van Poucke, M.; Peelman, L.; Van Ginneken, C.; Roura, E.; Michiels, J. Expression of Transient Receptor Potential Ankyrin 1 and Transient Receptor Potential Vanilloid 1 in the Gut of the Peri-Weaning Pig Is Strongly Dependent on Age and Intestinal Site. Animals 2020, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar] [CrossRef] [Green Version]
- Purhonen, A.K.; Louhivuori, L.M.; Kiehne, K.; Åkerman, K.E.O.; Herzig, K.H. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008, 582, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Camacho, S.; Michlig, S.; De Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; Le Coutre, J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, E.C.; Diakogiannaki, E.; Gentry, C.; Psichas, A.; Habib, A.M.; Bevan, S.; Fischer, M.J.M.; Reimann, F.; Gribble, F.M. Stimulation of GLP-1 secretion downstream of the ligand-gated ion channel TRPA1. Diabetes 2015, 64, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripken, D.; Van Der Wielen, N.; Wortelboer, H.M.; Meijerink, J.; Witkamp, R.F.; Hendriks, H.F.J. Steviol glycoside rebaudioside A induces glucagon-like peptide-1 and peptide YY release in a porcine ex vivo intestinal model. J. Agric. Food Chem. 2014, 62, 8365–8370. [Google Scholar] [CrossRef] [PubMed]
- Voortman, T.; Hendriks, H.F.J.; Witkamp, R.F.; Wortelboer, H.M. Effects of long- and short-chain fatty acids on the release of gastrointestinal hormones using an ex vivo porcine intestinal tissue model. J. Agric. Food Chem. 2012, 60, 9035–9042. [Google Scholar] [CrossRef]
- Boudry, G.; Perrier, C. Thyme and cinnamon extracts induce anion secretion in piglet small intestine via cholinergic pathways. J. Physiol. Pharmacol. 2008, 59, 543–552. [Google Scholar]
- Weber, E.; Neunlist, M.; Schemann, M.; Frieling, T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. J. Physiol. 2001, 536, 741–751. [Google Scholar] [CrossRef]
- Alvarez-Berdugo, D.; Jiménez, M.; Clavé, P.; Rofes, L. Pharmacodynamics of TRPV1 agonists in a bioassay using human PC-3 cells. Sci. World J. 2014, 2014, 184526. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, i.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, R. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ruijter, J.; Ramakers, C.; Hoogaars, W.; Karlen, Y.; Bakker, O.; Van den Hoff, M.; Moorman, A. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nygard, A.-B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Park, M.; Kim, K.; Lee, Y.M.; Rhyu, M.R. Hesperetin stimulates cholecystokinin secretion in enteroendocrine STC-1 cells. Biomol. Ther. 2013, 21, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toschi, A.; Tugnoli, B.; Rossi, B.; Piva, A.; Grilli, E. Thymol modulates the endocannabinoid system and gut chemosensing of weaning pigs. BMC Vet. Res. 2020, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Michiels, J.; Missotte, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal. J. Sci. Food Agric. 2008, 88, 125–135. [Google Scholar] [CrossRef]
- Malandrino, N.; Smith, R.J. Synthesis, secretion, and transport of peptide hormones. In Principles of Endocrinology and Hormone Action; Belfiore, A., LeRoith, D., Eds.; Springer: New York, NY, USA, 2018; pp. 29–42. [Google Scholar]
- Maljaars, J.; Haddeman, E.; Peters, H.; Masclee, A.A. Comparison of ileal and duodenal brake mechanisms on satiety and gastrointestinal transport. Gastroenterology 2007, 132, A207–A208. [Google Scholar]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Effects of GLP-1 and incretin-based therapies on gastrointestinal motor function. Exp. Diabetes Res. 2011, 2011, 279530. [Google Scholar] [CrossRef]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Schirra, J.; Göke, B. The physiological role of GLP-1 in human: Incretin, ileal brake or more? Regul. Pept. 2005, 128, 109–115. [Google Scholar] [CrossRef]
- Maljaars, P.; Peters, H.; Mela, D.; Masclee, A. Ileal brake: A sensible food target for appetite control. A review. Physiol. Behav. 2008, 95, 271–281. [Google Scholar] [CrossRef]
- Lin, H.C.; Zhao, X.-T.; Wang, L. Intestinal transit is more potently inhibited by fat in the distal (ileal brake) than in the proximal (jejunal brake) gut. Dig. Dis. Sci. 1997, 42, 19–25. [Google Scholar] [CrossRef]
- Davis, M.A.; Bynum, J.P.W.; Sirovich, B.E.; Practice, C.; Science, E.; Medical, A.; Junction, W.R. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 175, 777–783. [Google Scholar] [CrossRef]
- Sadofsky, L.R.; Boa, A.N.; Maher, S.A.; Birrell, M.A.; Belvisi, M.G.; Morice, A.H. TRPA1 is activated by direct addition of cysteine residues to the N-hydroxysuccinyl esters of acrylic and cinnamic acids. Pharmacol. Res. 2011, 63, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lieder, B.; Hoi, J.; Burian, N.; Hans, J.; Holik, A.-K.; Beltran Marquez, L.R.; Ley, J.P.; Hatt, H.; Somoza, V. Structure-Dependent Effects of Cinnamaldehyde Derivatives on TRPA1-Induced Serotonin Release in Human Intestinal Cell Models. J. Agric. Food Chem. 2020, 68, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Hira, T.; Yahagi, A.; Nishiyama, C.; Yamashita, T.; Imagi, J.; Hara, H. Unsaturated aldehydes induce CCK secretion via TRPA1 in STC-1 cells. Mol. Nutr. Food Res. 2014, 58, 1042–1051. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol | Accession Number | Nucleotide Sequence of Primers, 5′-3′ | Ta (°C) | Product Length (bp) |
|---|---|---|---|---|
| HPRT1 | DQ178126 | Forward: CCGAGGATTTGGAAAAGGT Reverse: CTATTTCTGTTCAGTGCTTTGATGT | 60 | 181 |
| YWHAZ | DQ178130 | Forward: ATGCAACCAACACATCCTATC Reverse: GCATTATTAGCGTGCTGTCTT | 60 | 178 |
| GADPH | NM_001206359.1 | Forward: TGGTGAAGGTCGGAGTGAAC Reverse: GAAGGGGTCATTGATGGCGA | 60 | 104 |
| TRPA1 | XM_021089237.1 | Forward: GAATTTACTCATTGGTTTGGCAGTTGGTG Reverse: CGGTGATGGATTTCTGATCGACCTTG | 58 | 155 |
| TRPV1 | XM_013981216.2 | Forward: GGACAGCGAGTTCAAAGACC Reverse: CCGTTTTCCACCAGAAGTGT | 63 | 240 |
| CCK | XM_021068544.1 | Forward: CAGGCTCGAAAAGCACCTTC Reverse: GCGGGGTCTTCTAGGAGGTA | 60 | 157 |
| GLP-1 | XM_005671882.3 | Forward: AGAACTCCGCCGCAGACA Reverse: TAAAGTCTCGGGTGGCAAGATT | 60 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Liefferinge, E.; Müller, M.; Van Noten, N.; Degroote, J.; Niknafs, S.; Roura, E.; Michiels, J. Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like Peptide 1 by Interacting with Transient Receptor Potential Ankyrin 1 in a Porcine Ex-Vivo Intestinal Segment Model. Animals 2021, 11, 2262. https://doi.org/10.3390/ani11082262
Van Liefferinge E, Müller M, Van Noten N, Degroote J, Niknafs S, Roura E, Michiels J. Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like Peptide 1 by Interacting with Transient Receptor Potential Ankyrin 1 in a Porcine Ex-Vivo Intestinal Segment Model. Animals. 2021; 11(8):2262. https://doi.org/10.3390/ani11082262
Chicago/Turabian StyleVan Liefferinge, Elout, Maximiliano Müller, Noémie Van Noten, Jeroen Degroote, Shahram Niknafs, Eugeni Roura, and Joris Michiels. 2021. "Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like Peptide 1 by Interacting with Transient Receptor Potential Ankyrin 1 in a Porcine Ex-Vivo Intestinal Segment Model" Animals 11, no. 8: 2262. https://doi.org/10.3390/ani11082262
APA StyleVan Liefferinge, E., Müller, M., Van Noten, N., Degroote, J., Niknafs, S., Roura, E., & Michiels, J. (2021). Cinnamaldehyde Induces Release of Cholecystokinin and Glucagon-Like Peptide 1 by Interacting with Transient Receptor Potential Ankyrin 1 in a Porcine Ex-Vivo Intestinal Segment Model. Animals, 11(8), 2262. https://doi.org/10.3390/ani11082262






