In Vitro Production of Embryos from Prepubertal Holstein Cattle and Mediterranean Water Buffalo: Problems, Progress and Potential
Abstract
Simple Summary
Abstract
1. Introduction
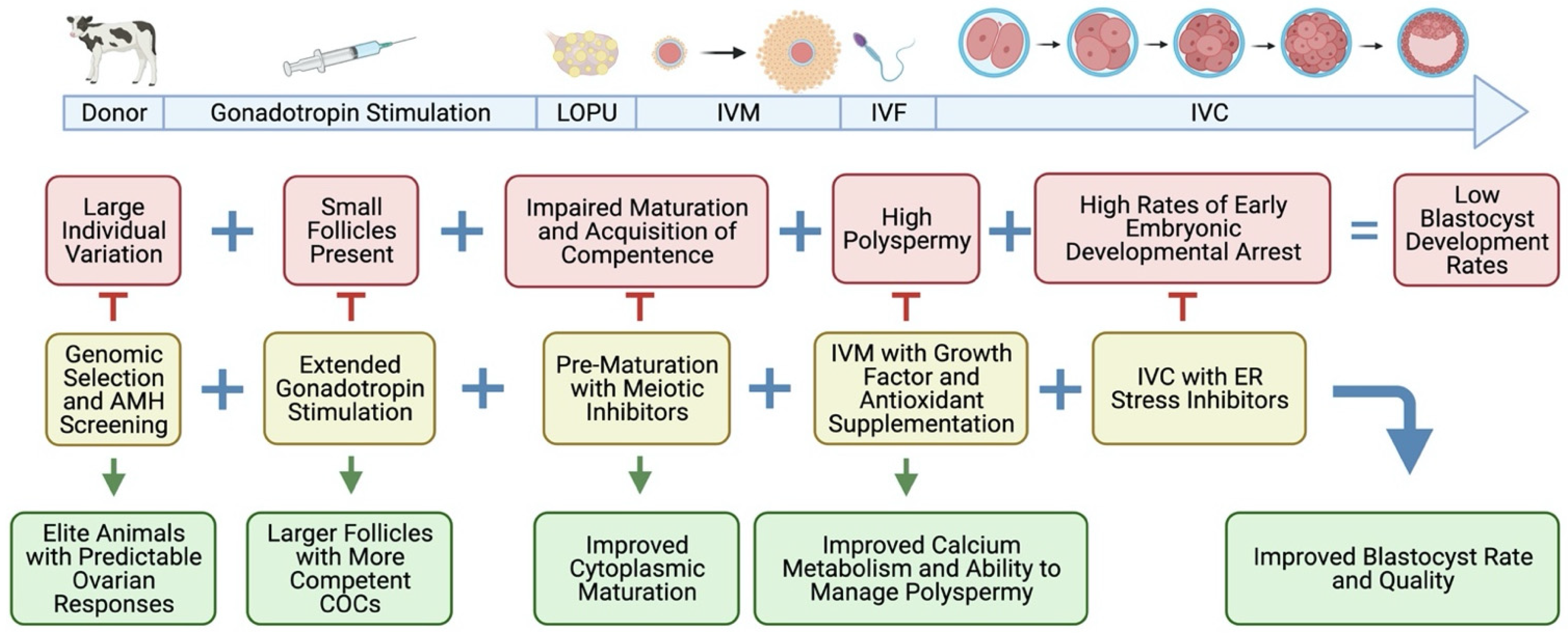
2. Increasing the Rate of Genetic Gain by Shortening Generation Intervals
3. History of LOPU-IVEP in Prepubertal Calves
4. Understanding Developmental Competence of Oocytes
4.1. The Hypothalamic–Pituitary–Ovarian (HPO) Axis
4.2. Follicular Microenvironment
4.3. Oocyte and Granulosa Cell Crosstalk
5. Hormonal Stimulation
6. LOPU and COC Quality
7. Individual Variation
Seasonality
8. In Vitro Embryo Production
8.1. Oocyte In Vitro Maturation (IVM)
8.2. In Vitro Fertilization (IVF)
8.3. Embryo In Vitro Culture (IVC) and Transfer
8.4. Embryo Cryopreservation
9. Future Perspectives: What Can We Do Better?
9.1. Optimized Gonadotropin Stimulation
9.2. Oxidative Stress and the Importance of Antioxidants
9.3. Endoplasmic Reticulum Stress
9.4. Cytokines and Growth Factors
9.5. Oocyte Pre-Maturation In Vitro
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viana, J. Statistics of Embryo Production and Transfer in Domestic Farm Animals; International Embryo Transfer Society: Champaign, IL, USA, 2019. [Google Scholar]
- Ferré, L.; Kjelland, M.; Strøbech, L.; Hyttel, P.; Mermillod, P.; Ross, P. Recent advances in bovine in vitro embryo production: Reproductive biotechnology history and methods. Animal 2020, 14, 991–1004. [Google Scholar] [CrossRef]
- Gasparrini, B. In vitro embryo production in buffalo species: State of the art. Theriogenology 2002, 57, 237–256. [Google Scholar] [CrossRef]
- Wani, N.A. In vitro embryo production (IVEP) in camelids: Present status and future perspectives. Reprod. Biol. 2020, 21, 100471. [Google Scholar] [CrossRef]
- Gil, M.; Cuello, C.; Parrilla, I.; Vazquez, J.; Roca, J.; Martinez, E. Advances in swine in vitro embryo production technologies. Reprod. Domest. Anim. 2010, 45, 40–48. [Google Scholar] [CrossRef]
- Paramio, M.T.; Izquierdo, D. Current status of in vitro embryo production in sheep and goats. Reprod. Domest. Anim. 2014, 49, 37–48. [Google Scholar] [CrossRef]
- Baldassarre, H. Laparoscopic Ovum Pick-Up Followed by In Vitro Embryo Production and Transfer in Assisted Breeding Programs for Ruminants. Animals 2021, 11, 216. [Google Scholar] [CrossRef]
- Berg, D.; Asher, G. New developments reproductive technologies in deer. Theriogenology 2003, 59, 189–205. [Google Scholar] [CrossRef]
- Blondin, P. Status of embryo production in the world. Anim. Reprod. 2015, 12, 356–358. [Google Scholar]
- Baldassarre, H.; De Matos, D.; Furnus, C.; Castro, T.; Fischer, E.C. Technique for efficient recovery of sheep oocytes by laparoscopic folliculocentesis. Anim. Reprod. Sci. 1994, 35, 145–150. [Google Scholar] [CrossRef]
- Stangl, M.; Kühholzer, B.; Besenfelder, U.; Brem, G. Repeated endoscopic ovum pick-up in sheep. Theriogenology 1999, 52, 709–716. [Google Scholar] [CrossRef]
- Baldassarre, H.; Wang, B.; Kafidi, N.; Keefer, C.; Lazaris, A.; Karatzas, C. Advances in the production and propagation of transgenic goats using laparoscopic ovum pick-up and in vitro embryo production technologies. Theriogenology 2002, 57, 275–284. [Google Scholar] [CrossRef]
- Baldassarre, H.; Keefer, C.; Wang, B.; Lazaris, A.; Karatzas, C.N. Nuclear transfer in goats using in vitro matured oocytes recovered by laparoscopic ovum pick-up. Cloning Stem Cells 2003, 5, 279–285. [Google Scholar] [CrossRef]
- Baldassarre, H.; Carelli, J.; Requena, L.; Rodrigues, M.; Ferreira, S.; Salomão, J.; Neto, P.J. Efficient recovery of oocytes from “onça parda” (Puma Concolor) by laparoscopic ovum pick-up of gonadotropin-stimulated females. Anim. Reprod. 2015, 12, 717. [Google Scholar]
- Miller, A.M.; Roelke, M.E.; Goodrowe, K.L.; Howard, J.; Wildt, D.E. Oocyte recovery, maturation and fertilization in vitro in the puma (Felis concolor). Reproduction 1990, 88, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Crichton, E.G.; Bedows, E.; Miller-Lindholm, A.K.; Baldwin, D.M.; Armstrong, D.L.; Graham, L.H.; Ford, J.J.; Gjorret, J.O.; Hyttel, P.; Pope, C.E. Efficacy of porcine gonadotropins for repeated stimulation of ovarian activity for oocyte retrieval and in vitro embryo production and cryopreservation in Siberian tigers (Panthera tigris altaica). Biol. Reprod. 2003, 68, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, A.M.; Johnston, L.A.; Seal, U.S.; Armstrong, D.L.; Tilson, R.L.; Wolf, P.; Petrini, K.; Simmons, L.G.; Gross, T.; Wildt, D.E. In vitro fertilization and embryo development in vitro and in vivo in the tiger (Panthera tigris). Biol. Reprod. 1990, 43, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Jorge Neto, P.N.; Requena, L.; Pizzutto, C.; Baldassarre, H. Laparoscopic Ovum Pick-Up (LOPU): From animal production to conservation. Spermova 2018, 8, 61–67. [Google Scholar] [CrossRef]
- Tervit, H. Laparoscopy/laparotomy oocyte recovery and juvenile breeding. Anim. Reprod. Sci. 1996, 42, 227–238. [Google Scholar] [CrossRef]
- Pierson, J.; Wang, B.; Neveu, N.; Sneek, L.; Cote, F.; Karatzas, C.; Baldassarre, H. Effects of repetition, interval between treatments and season on the results from laparoscopic ovum pick-up in goats. Reprod. Fertil. Dev. 2004, 16, 795–799. [Google Scholar] [CrossRef][Green Version]
- Taneja, M.; Bols, P.E.; Van de Velde, A.; Ju, J.-C.; Schreiber, D.; Tripp, M.W.; Levine, H.; Echelard, Y.; Riesen, J.; Yang, X. Developmental competence of juvenile calf oocytes in vitro and in vivo: Influence of donor animal variation and repeated gonadotropin stimulation. Biol. Reprod. 2000, 62, 206–213. [Google Scholar] [CrossRef]
- Khatir, H.; Lonergan, P.; Carolan, C.; Mermillod, P. Prepubertal bovine oocyte: A negative model for studying oocyte developmental competence. Mol. Reprod. Dev. 1996, 45, 231–239. [Google Scholar] [CrossRef]
- Revel, F.; Mermillod, P.; Peynot, N.; Renard, J.; Heyman, Y. Low developmental capacity of in vitro matured and fertilized oocytes from calves compared with that of cows. J. Reprod. Fertil. 1995, 103, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Duby, R.T.; Damiani, P.; Looney, C.R.; Fissore, R.A.; Robl, J.M. Prepuberal calves as oocyte donors: Promises and problems. Theriogenology 1996, 45, 121–130. [Google Scholar] [CrossRef]
- Levesque, J.; Sirard, M. Proteins in oocytes from calves and adult cows before maturation: Relationship with their development capacity. Reprod. Nutr. Dev. 1994, 34, 133–139. [Google Scholar] [CrossRef]
- Steeves, T.; Gardner, D.; Zuelke, K.; Squires, T.; Fry, R. In vitro development and nutrient uptake by embryos derived from oocytes of pre-pubertal and adult cows. Mol. Reprod. Dev. 1999, 54, 49–56. [Google Scholar] [CrossRef]
- Baruselli, P.S.; Soares, J.G.; Bayeux, B.M.; Silva, J.C.; Mingoti, R.D.; Carvalho, N.A. Assisted reproductive technologies (ART) in water buffaloes. Anim. Reprod. 2018, 15, 971–983. [Google Scholar] [CrossRef]
- Mogas, T.; Palomo, M.; Izquierdo, M.; Paramio, M. Developmental capacity of in vitro matured and fertilized oocytes from prepubertal and adult goats. Theriogenology 1997, 47, 1189–1203. [Google Scholar] [CrossRef]
- Koeman, J.; Keefer, C.L.; Baldassarre, H.; Downey, B.R. Developmental competence of prepubertal and adult goat oocytes cultured in semi-defined media following laparoscopic recovery. Theriogenology 2003, 60, 879–889. [Google Scholar] [CrossRef]
- O’Brien, J.; Catt, S.; Ireland, K.; Maxwell, W.; Evans, G. In vitro and in vivo developmental capacity of oocytes from prepubertal and adult sheep. Theriogenology 1997, 47, 1433–1443. [Google Scholar] [CrossRef]
- O’Brien, J.; Dwarte, D.; Ryan, J.; Maxwell, W.; Evans, G. Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep. Reprod. Fertil. Dev. 1996, 8, 1029–1037. [Google Scholar] [CrossRef]
- Sherrer, E.; Rathbun, T.; Davis, D. Fertilization and blastocyst development in oocytes obtained from prepubertal and adult pigs. J. Anim. Sci. 2004, 82, 102–108. [Google Scholar] [CrossRef]
- Ikeda, K.; Takahashi, Y. Comparison of maturational and developmental parameters of oocytes recovered from prepubertal and adult pigs. Reprod. Fertil. Dev. 2003, 15, 215–221. [Google Scholar] [CrossRef]
- De Paz, P.; Sánchez, A.; De la Fuente, J.; Chamorro, C.; Alvarez, M.; Anel, E.; Anel, L. Ultrastructural and cytochemical comparison between calf and cow oocytes. Theriogenology 2001, 55, 1107–1116. [Google Scholar] [CrossRef]
- Kauffold, J.; Amer, H.A.H.; Bergfeld, U.; Weber, W.; Sobiraj, A. The In Vitro Developmental Competence of Oocytes from Juvenile Calves is Related to Follicular Diameter. J. Reprod. Dev. 2005, 51, 325–332. [Google Scholar] [CrossRef]
- Li, J.; Liang, A.; Li, Z.; Du, C.; Hua, G.; Salzano, A.; Campanile, G.; Gasparrini, B.; Yang, L. An association analysis between PRL genotype and milk production traits in Italian Mediterranean river buffalo. J. Dairy Res. 2017, 84, 430. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, P.; Wei, H.; Xiang, T.; Molina, J.A.; Metzger, J.; Broek, D.; Kasinathan, S.; Faber, D.C.; Allan, M.F. Acceleration of genetic gain in cattle by reduction of generation interval. Sci. Rep. 2015, 5, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Seidel, G.; Larson, L.; Spilman, C.; Hahn, J.; Foote, R. Culture and transfer of calf ova. J. Dairy Sci. 1971, 54, 923–926. [Google Scholar] [CrossRef]
- Onuma, H.; Foote, R. In vitro development of ova from prepuberal cattle. J. Dairy Sci. 1969, 52, 1085–1087. [Google Scholar] [CrossRef]
- Onuma, H.; Hahn, J.; Foote, R. Factors affecting superovulation, fertilization and recovery of superovulated ova in prepuberal cattle. J. Reprod. Fertil. 1970, 21, 119–126. [Google Scholar] [CrossRef][Green Version]
- Marden, W.G. The hormone control of ovulation in the calf. Endocrinology 1952, 50, 456–461. [Google Scholar] [CrossRef]
- Black, W.; Ulberg, L.; Christian, R.; Casida, L. Ovulation and Fertilization in the Hormone-Stimulated Calf 1, 2. J. Dairy Sci. 1953, 36, 274–280. [Google Scholar] [CrossRef]
- Armstrong, D.T.; Holm, P.; Irvine, B.; Petersen, B.A.; Stubbings, R.; McLean, D.; Stevens, G.; Seamark, R.F. Pregnancies and live birth from in vitro fertilization of calf oocytes collected by laparoscopic follicular aspiration. Theriogenology 1992, 38, 667–678. [Google Scholar] [CrossRef]
- Kajihara, Y.; Blakewood, E.; Myers, M.; Kometani, N.; Goto, K.; Godke, R. In vitro maturation and fertilization of follicular oocytes obtained from calves. Theriogenology 1991, 35, 220. [Google Scholar] [CrossRef]
- Palma, G.; Clement-Sengewald, A.; Krefft, H. In vitro production of cattle embryos from calf oocytes. Theriogenology 1993, 39, 278. [Google Scholar] [CrossRef]
- Looney, C.; Damiani, P.; Lindsey, B.; Long, C.; Gonseth, C.; Johnson, D.; Duby, R. Use of prepuberal heifers as oocyte donors for IVF: Effect of age and gonadotrophin treatment. Theriogenology 1995, 43, 269. [Google Scholar] [CrossRef]
- Damiani, P.; Fissore, R.; Cibelli, J.; Long, C.; Balise, J.; Robl, J.; Duby, R. Evaluation of developmental competence, nuclear and ooplasmic maturation of calf oocytes. Mol. Reprod. Dev. 1996, 45, 521–534. [Google Scholar] [CrossRef]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Genomic selection in dairy cattle: Progress and challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Ponsart, C.; Le Bourhis, D.; Knijn, H.; Fritz, S.; Guyader-Joly, C.; Otter, T.; Lacaze, S.; Charreaux, F.; Schibler, L.; Dupassieux, D. Reproductive technologies and genomic selection in dairy cattle. Reprod. Fertil. Dev. 2014, 26, 12–21. [Google Scholar] [CrossRef]
- Moore, S.; Hasler, J. A 100-Year Review: Reproductive technologies in dairy science. J. Dairy Sci. 2017, 100, 10314–10331. [Google Scholar] [CrossRef]
- Leoni, G.G.; Succu, S.; Satta, V.; Paolo, M.; Bogliolo, L.; Bebbere, D.; Spezzigu, A.; Madeddu, M.; Berlinguer, F.; Ledda, S. In vitro production and cryotolerance of prepubertal and adult goat blastocysts obtained from oocytes collected by laparoscopic oocyte-pick-up (LOPU) after FSH treatment. Reprod. Fertil. Dev. 2009, 21, 901–908. [Google Scholar] [CrossRef]
- Marchal, R.; Feugang, J.; Perreau, C.; Venturi, E.; Terqui, M.; Mermillod, P. Meiotic and developmental competence of prepubertal and adult swine oocytes. Theriogenology 2001, 56, 17–29. [Google Scholar] [CrossRef]
- Currin, L.; Michalovic, L.; Bellefleur, A.-M.; Gutierrez, K.; Glanzner, W.; Schuermann, Y.; Bohrer, R.C.; Dicks, N.; da Rosa, P.R.; De Cesaro, M.P. The effect of age and length of gonadotropin stimulation on the in vitro embryo development of Holstein calf oocytes. Theriogenology 2017, 104, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kauffold, J.; Amer, H.A.H.; Bergfeld, U.; Müller, F.; Weber, W.; Sobiraj, A. Offspring from Non-stimulated Calves at an Age Younger than Two Months: A Preliminary Report. J. Reprod. Dev. 2005, 51, 527–532. [Google Scholar] [CrossRef] [PubMed]
- de Roux, N.; Genin, E.; Carel, J.-C.; Matsuda, F.; Chaussain, J.-L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef]
- Roa, J.; Navarro, V.M.; Tena-Sempere, M. Kisspeptins in reproductive biology: Consensus knowledge and recent developments. Biol. Reprod. 2011, 85, 650–660. [Google Scholar] [CrossRef]
- García-Galiano, D.; Pinilla, L.; Tena-Sempere, M. Sex steroids and the control of the Kiss1 system: Developmental roles and major regulatory actions. J. Neuroendocrinol. 2012, 24, 22–33. [Google Scholar] [CrossRef]
- Atkins, J.A.; Pohler, K.G.; Smith, M.F. Physiology and endocrinology of puberty in heifers. Vet. Clin. North Am. Food Anim. Pract. 2013, 29, 479–492. [Google Scholar] [CrossRef]
- Redmond, J.; Baez-Sandoval, G.; Spell, K.; Spencer, T.; Lents, C.; Williams, G.; Amstalden, M. Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. J. Neuroendocrinol. 2011, 23, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Schallenberger, E.; Gombe, S.; Karg, H. Endocrine patterns associated with puberty in male and female cattle. J. Reprod. Fertil. Suppl. 1980, 30, 103–110. [Google Scholar] [CrossRef]
- Day, M.; Imakawa, K.; Wolfe, P.; Kittok, R.; Kinder, J. Endocrine mechanisms of puberty in heifers. Role of hypothalamo-pituitary estradiol receptors in the negative feedback of estradiol on luteinizing hormone secretion. Biol. Reprod. 1987, 37, 1054–1065. [Google Scholar] [CrossRef]
- Michalovic, L.; Currin, L.; Gutierrez, K.; Bellefleur, A.M.; Glanzner, W.G.; Schuermann, Y.; de Macedo, M.P.; Bohrer, R.C.; Dicks, N.; Lopez, R. Granulosa cells of prepubertal cattle respond to gonadotropin signaling and upregulate genes that promote follicular growth and prevent cell apoptosis. Mol. Reprod. Dev. 2018, 85, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Pawlak, P.; Pszczola, M.; Cieslak, A.; Lechniak, D. Prepubertal heifers versus cows–the differences in the follicular environment. Theriogenology 2016, 87, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Khatir, H.; Carolan, C.; Lonergan, P.; Mermillod, P. Characterization of calf follicular fluid and its ability to support cytoplasmic maturation of cow and calf oocytes. J. Reprod. Fertil. 1997, 111, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Dodson, S.; McLeod, B.; Haresign, W.; Peters, A.; Lamming, G. Endocrine changes from birth to puberty in the heifer. J. Reprod. Fertil. 1988, 82, 527–538. [Google Scholar] [CrossRef]
- Murayama, C.; Miyazaki, H.; Miyamoto, A.; Shimizu, T. Luteinizing hormone (LH) regulates production of androstenedione and progesterone via control of histone acetylation of StAR and CYP17 promoters in ovarian theca cells. Mol. Cell. Endocrinol. 2012, 350, 1–9. [Google Scholar] [CrossRef]
- Sen, A.; Hammes, S.R. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol. Endocrinol. 2010, 24, 1393–1403. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Albertini, D.F.; Rider, V. Patterns of intercellular connectivity in the mammalian cumulus-oocyte complex. Microsc. Res. Tech. 1994, 27, 125–133. [Google Scholar] [CrossRef]
- de Ávila, A.; da Silveira, J. Role of extracellular vesicles during oocyte maturation and early embryo development. Reprod. Fertil. Dev. 2020, 32, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Albertini, D.F.; Combelles, C.; Benecchi, E.; Carabatsos, M.J. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction 2001, 121, 647–653. [Google Scholar] [CrossRef]
- Lonergan, P.; Monaghan, P.; Rizos, D.; Boland, M.; Gordon, I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol. Reprod. Dev. 1994, 37, 48–53. [Google Scholar] [CrossRef]
- Otoi, T.; Yamamoto, K.; Koyama, N.; Tachikawa, S.; Suzuki, T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997, 48, 769–774. [Google Scholar] [CrossRef]
- Gandolfi, F.; Milanesi, E.; Pocar, P.; Luciano, A.; Brevini, T.; Acocella, F.; Lauria, A.; Armstrong, D. Comparative analysis of calf and cow oocytes during in vitro maturation. Mol. Reprod. Dev. 1998, 49, 168–175. [Google Scholar] [CrossRef]
- Fair, T.; Hyttel, P.; Greve, T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol. Reprod. Dev. 1995, 42, 437–442. [Google Scholar] [CrossRef]
- Clarke, H.J. History, origin, and function of transzonal projections: The bridges of communication between the oocyte and its environment. Anim. Reprod. 2018, 15, 215–223. [Google Scholar] [CrossRef]
- Alam, M.H.; Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 2020, 19, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, A.D.; Gilbert, I.; Scantland, S.; Fournier, E.; Ashkar, F.; Bastien, A.; Saadi, H.A.S.; Gagné, D.; Sirard, M.-A.; Khandjian, É.W.; et al. Cumulus Cell Transcripts Transit to the Bovine Oocyte in Preparation for Maturation1. Biol. Reprod. 2016, 94, 94. [Google Scholar] [CrossRef]
- Palmerini, M.G.; Nottola, S.A.; Leoni, G.G.; Succu, S.; Borshi, X.; Berlinguer, F.; Naitana, S.; Bekmukhambetov, Y.; Macchiarelli, G. In vitro maturation is slowed in prepubertal lamb oocytes: Ultrastructural evidences. Reprod. Biol. Endocrinol. 2014, 12, 1–13. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Veeramachaneni, D.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef]
- Sohel, M.M.H.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: Implications for bovine oocyte developmental competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef]
- Matsuno, Y.; Onuma, A.; Fujioka, Y.A.; Yasuhara, K.; Fujii, W.; Naito, K.; Sugiura, K. Effects of exosome-like vesicles on cumulus expansion in pigs in vitro. J. Reprod. Dev. 2017, 63, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.d.A.M.M.; Fujihara, M.; Nagashima, J.B.; Noonan, M.J.; Inoue-Murayama, M.; Songsasen, N. Follicular extracellular vesicles enhance meiotic resumption of domestic cat vitrified oocytes. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Hung, W.-T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. Extracellular vesicles from bovine follicular fluid support cumulus expansion. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef]
- Hung, W.-T.; Navakanitworakul, R.; Khan, T.; Zhang, P.; Davis, J.S.; McGinnis, L.K.; Christenson, L.K. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol. Reprod. 2017, 97, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Navakanitworakul, R.; Hung, W.-T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and small RNA content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Winger, Q.A.; Bouma, G.J.; Carnevale, E.M. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-β signalling during follicle development in the mare. Reprod. Fertil. Dev. 2015, 27, 897–905. [Google Scholar] [CrossRef]
- Diez-Fraile, A.; Lammens, T.; Tilleman, K.; Witkowski, W.; Verhasselt, B.; De Sutter, P.; Benoit, Y.; Espeel, M.; D’Herde, K. Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro fertilization. Hum. Fertil. 2014, 17, 90–98. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- Hendriksen, P.; Vos, P.; Steenweg, W.; Bevers, M.; Dieleman, S. Bovine follicular development and its effect on the in vitro competence of oocytes. Theriogenology 2000, 53, 11–20. [Google Scholar] [CrossRef]
- Ledda, S.; Bogliolo, L.; Leoni, G.; Naitana, S. Follicular size affects the meiotic competence of in vitro matured prepubertal and adult oocytes in sheep. Reprod. Nutr. Dev. 1999, 39, 503–508. [Google Scholar] [CrossRef][Green Version]
- Crozet, N.; Ahmed-Ali, M.; Dubos, M. Developmental competence of goat oocytes from follicles of different size categories following maturation, fertilization and culture in vitro. Reproduction 1995, 103, 293–298. [Google Scholar] [CrossRef][Green Version]
- Han, Z.-B.; Lan, G.-C.; Wu, Y.-G.; Han, D.; Feng, W.-G.; Wang, J.-Z.; Tan, J.-H. Interactive effects of granulosa cell apoptosis, follicle size, cumulus–oocyte complex morphology, and cumulus expansion on the developmental competence of goat oocytes: A study using the well-in-drop culture system. Reproduction 2006, 132, 749–758. [Google Scholar] [CrossRef][Green Version]
- de Carvalho, J.G.S.; de Carvalho, N.A.T.; Bayeux, B.M.; Watanabe, Y.F.; Watanabe, O.Y.; Mingoti, R.D.; Baruselli, P.S. Superstimulation prior to the ovum pick-up improves the in vitro embryo production in nulliparous, primiparous and multiparous buffalo (Bubalus bubalis) donors. Theriogenology 2019, 138, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Bagg, M.A.; Nottle, M.B.; Armstrong, D.T.; Grupen, C.G. Relationship between follicle size and oocyte developmental competence in prepubertal and adult pigs. Reprod. Fertil. Dev. 2007, 19, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Marchal, R.; Vigneron, C.; Perreau, C.; Bali-Papp, A.; Mermillod, P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology 2002, 57, 1523–1532. [Google Scholar] [CrossRef]
- Raghu, H.; Nandi, S.; Reddy, S. Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro. Reprod. Fertil. Dev. 2002, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, H.; Currin, L.; Michalovic, L.; Bellefleur, A.-M.; Gutierrez, K.; Mondadori, R.G.; Glanzner, W.G.; Schuermann, Y.; Bohrer, R.C.; Dicks, N. Interval of gonadotropin administration for in vitro embryo production from oocytes collected from Holstein calves between 2 and 6 months of age by repeated laparoscopy. Theriogenology 2018, 116, 64–70. [Google Scholar]
- Armstrong, D.; Kotaras, P.; Earl, C. Advances in production of embryos in vitro from juvenile and prepubertal oocytes from the calf and lamb. Reprod. Fertil. Dev. 1997, 9, 333–339. [Google Scholar] [CrossRef]
- Stubbings, R.B.; Wosik, C.; Armstrong, D.T. Ovarian response in calves to multiple versus a single subcutaneous injection of folltropin. Theriogenology 1993, 39, 321. [Google Scholar] [CrossRef]
- Armstrong, D.; Irvine, B.; Earl, C.; McLean, D.; Seamark, R. Gonadotropin stimulation regimens for follicular aspiration and in vitro embryo production from calf oocytes. Theriogenology 1994, 42, 1227–1236. [Google Scholar] [CrossRef]
- Moore, W.; Ward, D.N. Pregnant mare serum gonadotropin. An in vitro biological characterization of the lutropin-follitropin dual activity. J. Biol. Chem. 1980, 255, 6930–6936. [Google Scholar] [CrossRef]
- Baldassarre, H.; Bordignon, V. Laparoscopic ovum pick-up for in vitro embryo production from dairy bovine and buffalo calves. Anim. Reprod. 2018, 15, 191–196. [Google Scholar] [CrossRef]
- Armstrong, D. Recent advances in superovulation of cattle. Theriogenology 1993, 39, 7–24. [Google Scholar] [CrossRef]
- Liang, A.; Salzano, A.; D’Esposito, M.; Comin, A.; Montillo, M.; Yang, L.; Campanile, G.; Gasparrini, B. Anti-Mullerian hormone (AMH) concentration in follicular fluid and mRNA expression of AMH receptor type II and LH receptor in granulosa cells as predictive markers of good buffalo (Bubalus bubalis) donors. Theriogenology 2016, 86, 963–970. [Google Scholar] [CrossRef]
- Baruselli, P.; Batista, E.; Vieira, L.; Souza, A. Relationship between follicle population, AMH concentration and fertility in cattle. Anim. Reprod. 2018, 12, 487–497. [Google Scholar]
- Alward, K.J.; Bohlen, J.F. Overview of Anti-Müllerian hormone (AMH) and association with fertility in female cattle. Reprod. Domest. Anim. 2020, 55, 3–10. [Google Scholar] [CrossRef]
- Batista, E.; Guerreiro, B.; Freitas, B.; Silva, J.; Vieira, L.; Ferreira, R.; Rezende, R.; Basso, A.; Lopes, R.; Rennó, F. Plasma anti-Müllerian hormone as a predictive endocrine marker to select Bos taurus (Holstein) and Bos indicus (Nelore) calves for in vitro embryo production. Domest. Anim. Endocrinol. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- Zicarelli, L. Influence of seasonality on buffalo production. World 2013, 1961, 1961. [Google Scholar]
- Shahzad, Q.; Waqas, M.; Pu, L.; Wadood, A.A.; Xie, L.; Husna, A.U.; Yang, K.; Wang, J.; Xu, H.; Lu, K. Seasonality and photoperiod influence in vitro production of buffalo embryos. Reprod. Domest. Anim. 2020, 55, 1115–1123. [Google Scholar] [CrossRef]
- Di Francesco, S.; Boccia, L.; Campanile, G.; Di Palo, R.; Vecchio, D.; Neglia, G.; Zicarelli, L.; Gasparrini, B. The effect of season on oocyte quality and developmental competence in Italian Mediterranean buffaloes (Bubalus bubalis). Anim. Reprod. Sci. 2011, 123, 48–53. [Google Scholar] [CrossRef]
- Gasparrini, B. Effects of reproductive season on embryo development in the buffalo. Reprod. Fertil. Dev. 2019, 31, 68–81. [Google Scholar] [CrossRef]
- Plansky, V.; Dimitrov, D. Puberty age and body weight of the water buffalo heifers. Tradit. Mod. Vet. Med. 2020, 5, 25–28. [Google Scholar]
- Di Francesco, S.; Novoa, M.V.S.; Vecchio, D.; Neglia, G.; Boccia, L.; Campanile, G.; Zicarelli, L.; Gasparrini, B. Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian buffalo performed in different seasons. Theriogenology 2012, 77, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E. Effects of heat stress on reproduction. J. Dairy Sci. 2003, 86, E104–E114. [Google Scholar] [CrossRef]
- De Rensis, F.; Scaramuzzi, R.J. Heat stress and seasonal effects on reproduction in the dairy cow—A review. Theriogenology 2003, 60, 1139–1151. [Google Scholar] [CrossRef]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef]
- Majerus, V.; Lequarré, A.-S.; Ferguson, E.; Kaidi, S.; Massip, A.; Dessy, F.; Donnay, I. Characterization of embryos derived from calf oocytes: Kinetics of cleavage, cell allocation to inner cell mass, and trophectoderm and lipid metabolism. Mol. Reprod. Dev. 2000, 57, 346–352. [Google Scholar] [CrossRef]
- Brackett, B.; Bousquet, D.; Boice, M.; Donawick, W.; Evans, J.; Dressel, M. Normal development following in vitro fertilization in the cow. Biol. Reprod. 1982, 27, 147–158. [Google Scholar] [CrossRef]
- Reader, K.L.; Cox, N.R.; Stanton, J.-A.L.; Juengel, J.L. Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reprod. Fertil. Dev. 2015, 27, 513–522. [Google Scholar] [CrossRef]
- Mermillod, P.; Le Bourhis, D.; Lonergan, P.; Khatir, H.; Heyman, Y. Assessment of cytoplasmic competence of prepubertal calf oocytes by use of nuclear transfer. Theriogenology 1998, 49, 187. [Google Scholar] [CrossRef]
- Gasparrini, B.; Neglia, G.; Di Palo, R.; Vecchio, D.; Albero, G.; Esposito, L.; Campanile, G.; Zicarelli, L. Influence of oocyte donor on in vitro embryo production in buffalo. Anim. Reprod. Sci. 2014, 144, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, F.; Caetano, A.; Watanabe, Y.; Ripamonte, P.; Carambula, S.; Merighe, G.; Garcia, S. Genome activation and developmental block in bovine embryos. Anim. Reprod. Sci. 2004, 82, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sirard, M.-A. The influence of in vitro fertilization and embryo culture on the embryo epigenetic constituents and the possible consequences in the bovine model. J. Dev. Orig. Health Dis. 2017, 8, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.M. Consequences of assisted reproductive techniques on the embryonic epigenome in cattle. Reprod. Fertil. Dev. 2020, 32, 65–81. [Google Scholar] [CrossRef]
- Chen, Z.; Hagen, D.E.; Elsik, C.G.; Ji, T.; Morris, C.J.; Moon, L.E.; Rivera, R.M. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 4618–4623. [Google Scholar] [CrossRef]
- Young, L.E.; Fernandes, K.; McEvoy, T.G.; Butterwith, S.C.; Gutierrez, C.G.; Carolan, C.; Broadbent, P.J.; Robinson, J.J.; Wilmut, I.; Sinclair, K.D. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat. Genet. 2001, 27, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Duan, J.; Gao, X.; Zhu, W.; Lu, X.; Yang, L.; Zhang, J.; Li, G.; Ci, W. Programming and inheritance of parental DNA methylomes in mammals. Cell 2014, 157, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Blondin, P.; Vigneault, C.; Labrecque, R.; Sirard, M.-A. The age of the bull influences the transcriptome and epigenome of blastocysts produced by IVF. Theriogenology 2020, 144, 122–131. [Google Scholar] [CrossRef]
- Morin-Doré, L.; Blondin, P.; Vigneault, C.; Grand, F.-X.; Labrecque, R.; Sirard, M.-A. Transcriptomic evaluation of bovine blastocysts obtained from peri-pubertal oocyte donors. Theriogenology 2017, 93, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Baruselli, P.S.; de Carvalho, J.G.S.; Elliff, F.M.; da Silva, J.C.B.; Chello, D.; de Carvalho, N.A.T. Embryo transfer in buffalo (Bubalus bubalis). Theriogenology 2020, 150, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.B.; Rezende, R.G.; Colli, M.H.A.; Bayeux, B.M.; Mingoti, R.D.; Ojeda-Rojas, O.A.; Basso, A.C.; Naves, J.; Baruselli, P.S. In vitro embryo production in buffalo: Comparison between calves, prepubertal Heifers and lactating cows. Anim. Reprod. 2017, 14, 766. [Google Scholar]
- Seidel, G.E., Jr. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology 2006, 65, 228–235. [Google Scholar] [CrossRef]
- Ferré, L.B.; Kjelland, M.E.; Taiyeb, A.M.; Campos-Chillon, F.; Ross, P.J. Recent progress in bovine In vitro-derived embryo cryotolerance: Impact of In vitro culture systems, advances in cryopreservation and future considerations. Reprod. Domest. Anim. 2020, 55, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef]
- Zolini, A.M.; Carrascal-Triana, E.; de King, A.R.; Hansen, P.J.; Torres, C.A.A.; Block, J. Effect of addition of L-carnitine to media for oocyte maturation and embryo culture on development and cryotolerance of bovine embryos produced in vitro. Theriogenology 2019, 133, 135–143. [Google Scholar] [CrossRef]
- Takahashi, T.; Inaba, Y.; Somfai, T.; Kaneda, M.; Geshi, M.; Nagai, T.; Manabe, N. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2013, 25, 589–599. [Google Scholar] [CrossRef]
- Verma, M.; Pandey, S.; Bhat, I.A.; Mukesh, B.; Anand, J.; Chandra, V.; Sharma, G.T. Impact of l-carnitine on lipid content and post thaw survivability of buffalo embryos produced in vitro. Cryobiology 2018, 82, 99–105. [Google Scholar] [CrossRef]
- Boccia, L.; De Blasi, M.; Zullo, G.; Longobardi, V.; Vecchio, D.; Gasparrini, B. L-Carnitine during in vitro culture enhances the cryotolerance of buffalo (bubalus bubalis) in vitro-derived embryos. Reprod. Fertil. Dev. 2012, 25, 214. [Google Scholar] [CrossRef]
- Krause, A.R.T.; Dias, F.C.; Adams, G.P.; Mapletoft, R.J.; Singh, J. Effect of dose and duration of FSH treatment on ovarian response in prepubertal calves. Anim. Reprod. Sci. 2020, 219, 106471. [Google Scholar] [CrossRef]
- Krause, A.R.T.; Dias, F.C.; Adams, G.P.; Mapletoft, R.J.; Singh, J. Antral follicle counts and association with ovarian superstimulatory response to gonadotropins in prepubertal calves. Anim. Reprod. Sci. 2021, 227, 106730. [Google Scholar] [CrossRef]
- Presicce, G.A.; Senatore, E.M.; De Santis, G.; Stecco, R.; Terzano, G.M.; Borghese, A.; De Mauro, G.J. Hormonal stimulation and oocyte maturational competence in prepuberal Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology 2002, 57, 1877–1884. [Google Scholar] [CrossRef]
- Lafleur, M.V.M.; Hoorweg, J.J.; Joenje, H.; Westmijze, E.J.; Retèl, J. The ambivalent role of glutathione in the protection of DNA against singlet oxygen. Free Radic. Res. 1994, 21, 9–17. [Google Scholar] [CrossRef]
- Miyamura, M.; Yoshida, M.; Hamano, S.; Kuwayama, M. Glutathione concentration during maturation and fertilization in bovine oocytes. Theriogenology 1995, 1, 282. [Google Scholar] [CrossRef]
- Gasparrini, B.; Sayoud, H.; Neglia, G.; de Matos, D.G.; Donnay, I.; Zicarelli, L. Glutathione synthesis during in vitro maturation of buffalo (Bubalus bubalis) oocytes: Effects of cysteamine on embryo development. Theriogenology 2003, 60, 943–952. [Google Scholar] [CrossRef]
- Rodríguez-González, E.; López-Bejar, M.; Mertens, M.J.; Paramio, M.T. Effects on in vitro embryo development and intracellular glutathione content of the presence of thiol compounds during maturation of prepubertal goat oocytes. Mol. Reprod. Dev. Inc. Gamete Res. 2003, 65, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. Role of glutathione in the maturation and fertilization of pig oocytes in vitro. Mol. Reprod. Dev. 1993, 35, 76–81. [Google Scholar] [CrossRef]
- Yoshida, M.; Ishigaki, K.; Nagai, T.; Chikyu, M.; Pursel, V.G. Glutathione concentration during maturation and after fertilization in pig oocytes: Relevance to the ability of oocytes to form male pronucleus. Biol. Reprod. 1993, 49, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.-T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Jiao, G.-Z.; Cao, X.-Y.; Cui, W.; Lian, H.-Y.; Miao, Y.-L.; Wu, X.-F.; Han, D.; Tan, J.-H. Developmental Potential of Prepubertal Mouse Oocytes Is Compromised Due Mainly to Their Impaired Synthesis of Glutathione. PLoS ONE 2013, 8, e58018. [Google Scholar] [CrossRef]
- Gasparrini, B.; Neglia, G.; Di Palo, R.; Campanile, G.; Zicarelli, L. Effect of cysteamine during in vitro maturation on buffalo embryo development. Theriogenology 2000, 54, 1537–1542. [Google Scholar] [CrossRef]
- Gasparrini, B.; Boccia, L.; Marchandise, J.; Di Palo, R.; George, F.; Donnay, I.; Zicarelli, L. Enrichment of in vitro maturation medium for buffalo (Bubalus bubalis) oocytes with thiol compounds: Effects of cystine on glutathione synthesis and embryo development. Theriogenology 2006, 65, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Roura, M.; Catalá, M.G.; Menéndez-Blanco, I.; Izquierdo, D.; Fouladi-Nashta, A.A.; Paramio, M.T. Beneficial effects of melatonin on in vitro embryo production from juvenile goat oocytes. Reprod. Fertil. Dev. 2018, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Catalá, M.G.; Roura, M.; Menéndez-Blanco, I.; Piras, A.R.; Izquierdo, D.; Paramio, M.T. Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 2019, 54, 381–390. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; He, C.; Zhang, L.; Tan, D.; Reiter, R.J.; Xu, J.; Ji, P.; Liu, G. Beneficial effects of melatonin on bovine oocytes maturation: A mechanistic approach. J. Pineal Res. 2014, 57, 239–247. [Google Scholar] [CrossRef]
- Shi, J.M.; Tian, X.Z.; Zhou, G.B.; Wang, L.; Gao, C.; Zhu, S.E.; Zeng, S.M.; Tian, J.H.; Liu, G.S. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J. Pineal Res. 2009, 47, 318–323. [Google Scholar] [CrossRef]
- Soni, N.; Pandey, A.; Kumar, A.; Verma, A.; Kumar, S.; Gunwant, P.; Phogat, J.; Kumar, V.; Singh, V. Expression of MTNR1A, steroid (ERα, ERβ, and PR) receptor gene transcripts, and the concentration of melatonin and steroid hormones in the ovarian follicles of buffalo. Domest. Anim. Endocrinol. 2020, 72, 106371. [Google Scholar] [CrossRef]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Devaraj, M.; Gupta, P.; Ravindra, J.; Nandi, S. Effect of taurine and melatonin in the culture medium on buffalo in vitro embryo development. Reprod. Domest. Anim. 2009, 44, 12–16. [Google Scholar] [CrossRef]
- Tian, H.; Qi, Q.; Yan, F.; Wang, C.; Hou, F.; Ren, W.; Zhang, L.; Hou, J. Enhancing the developmental competence of prepubertal lamb oocytes by supplementing the in vitro maturation medium with sericin and the fibroblast growth factor 2-leukemia inhibitory factor-Insulin-like growth factor 1 combination. Theriogenology 2021, 159, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Añez, J.C.; Lucas-Hahn, A.; Hadeler, K.-G.; Aldag, P.; Niemann, H. Melatonin enhances in vitro developmental competence of cumulus-oocyte complexes collected by ovum pick-up in prepubertal and adult dairy cattle. Theriogenology 2021, 161, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Latham, K.E. Endoplasmic reticulum stress signaling in mammalian oocytes and embryos: Life in balance. Int. Rev. Cell Mol. Biol. 2015, 316, 227–265. [Google Scholar] [PubMed]
- Zhang, J.Y.; Diao, Y.F.; Kim, H.R.; Jin, D.I. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS ONE 2012, 7, e40433. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Agrawal, H.; Mullani, N.; Sandhu, A.; Singh, M.K.; Chauhan, M.S.; Singla, S.K.; Palta, P.; Manik, R.S. Supplementation of tauroursodeoxycholic acid during IVC did not enhance in vitro development and quality of buffalo IVF embryos but combated endoplasmic reticulum stress. Theriogenology 2015, 84, 200–207. [Google Scholar] [CrossRef]
- Kim, J.S.; Song, B.S.; Lee, K.S.; Kim, D.H.; Kim, S.U.; Choo, Y.K.; Chang, K.T.; Koo, D.B. Tauroursodeoxycholic acid enhances the pre-implantation embryo development by reducing apoptosis in pigs. Reprod. Domest. Anim. 2012, 47, 791–798. [Google Scholar] [CrossRef]
- Dicks, N.; Bohrer, R.C.; Gutierrez, K.; Michalak, M.; Agellon, L.B.; Bordignon, V. Relief of endoplasmic reticulum stress enhances DNA damage repair and improves development of pre-implantation embryos. PLoS ONE 2017, 12, e0187717. [Google Scholar] [CrossRef]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.-i. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Khatun, H.; Ihara, Y.; Takakura, K.; Egashira, J.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.-i. Role of endoplasmic reticulum stress on developmental competency and cryo-tolerance in bovine embryos. Theriogenology 2020, 142, 131–137. [Google Scholar] [CrossRef]
- Yoon, S.-B.; Choi, S.-A.; Sim, B.-W.; Kim, J.-S.; Mun, S.-E.; Jeong, P.-S.; Yang, H.-J.; Lee, Y.; Park, Y.-H.; Song, B.-S. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol. Reprod. 2014, 90, 104. [Google Scholar] [CrossRef]
- Dicks, N.; Gutierrez, K.; Currin, L.; de Macedo, M.P.; Glanzner, W.G.; Mondadori, R.G.; Michalak, M.; Agellon, L.B.; Bordignon, V. Tauroursodeoxycholic acid/TGR5 signaling promotes survival and early development of glucose-stressed porcine embryos. Biol. Reprod. 2021, 105, 76–86. [Google Scholar] [CrossRef]
- Pioltine, E.M.; Costa, C.B.; Barbosa Latorraca, L.; Franchi, F.F.; Dos Santos, P.H.; Mingoti, G.Z.; Paula-Lopes, F.F.d.; Nogueira, M.F.G. Treatment of in vitro-Matured Bovine Oocytes With Tauroursodeoxycholic Acid Modulates the Oxidative Stress Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 296. [Google Scholar] [CrossRef]
- Beuers, U.; Nathanson, M.H.; Boyer, J.L. Effects of tauroursodeoxycholic acid on cytosolic Ca2+ signals in isolated rat hepatocytes. Gastroenterology 1993, 104, 604–612. [Google Scholar] [CrossRef]
- Xie, Q.; Khaoustov, V.I.; Chung, C.C.; Sohn, J.; Krishnan, B.; Lewis, D.E.; Yoffe, B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress–induced caspase-12 activation. Hepatology 2002, 36, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 201703998. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.D.; Gilchrist, R.B.; Kelly, J.M.; Thompson, J.G.; Sutton-McDowall, M.L. Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology 2013, 79, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Thompson, J.G. Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007, 67, 6–15. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, X.; Li, R.; Zhang, C.; Zhang, J. Effects of pre-incubation with C-type natriuretic peptide on nuclear maturation, mitochondrial behavior, and developmental competence of sheep oocytes. Biochem. Biophys. Res. Commun. 2018, 497, 200–206. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Fan, X.; Li, R.; Zhang, J. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 199–206. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Menéndez-Blanco, I.; Catalá, M.-G.; Izquierdo, D.; Thompson, J.G.; Paramio, M.-T. Biphasic in vitro maturation with C-type natriuretic peptide enhances the developmental competence of juvenile-goat oocytes. PLoS ONE 2019, 14, e0221663. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, P.R.A.; De Cesaro, M.P.; Dau, A.M.P.; Duggavathi, R.; Bordignon, V.; Gonçalves, P.B.D. Reversible meiotic arrest of bovine oocytes by EGFR inhibition and follicular hemisections. Theriogenology 2017, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]

| Species | Number of Animals | Number of COCs Recovered | |||
|---|---|---|---|---|---|
| Total | Mean ± SD All Calves (Total Per Calf) | Mean ± SD Bottom Calf (Total) | Mean ± SD Top Calf (Total) | ||
| Holstein | 11 | 1393 | 22.2 ± 14 (126.6) | 12.7 ± 4 (72) | 38.2 ± 11 (229) |
| Buffalo | 8 | 774 | 16.2 ± 9 (81) | 10.1 ± 3 (50) | 26.6 ± 6 (130) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currin, L.; Baldassarre, H.; Bordignon, V. In Vitro Production of Embryos from Prepubertal Holstein Cattle and Mediterranean Water Buffalo: Problems, Progress and Potential. Animals 2021, 11, 2275. https://doi.org/10.3390/ani11082275
Currin L, Baldassarre H, Bordignon V. In Vitro Production of Embryos from Prepubertal Holstein Cattle and Mediterranean Water Buffalo: Problems, Progress and Potential. Animals. 2021; 11(8):2275. https://doi.org/10.3390/ani11082275
Chicago/Turabian StyleCurrin, Luke, Hernan Baldassarre, and Vilceu Bordignon. 2021. "In Vitro Production of Embryos from Prepubertal Holstein Cattle and Mediterranean Water Buffalo: Problems, Progress and Potential" Animals 11, no. 8: 2275. https://doi.org/10.3390/ani11082275
APA StyleCurrin, L., Baldassarre, H., & Bordignon, V. (2021). In Vitro Production of Embryos from Prepubertal Holstein Cattle and Mediterranean Water Buffalo: Problems, Progress and Potential. Animals, 11(8), 2275. https://doi.org/10.3390/ani11082275






