The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review
Abstract
:Simple Summary
Abstract
1. Introduction
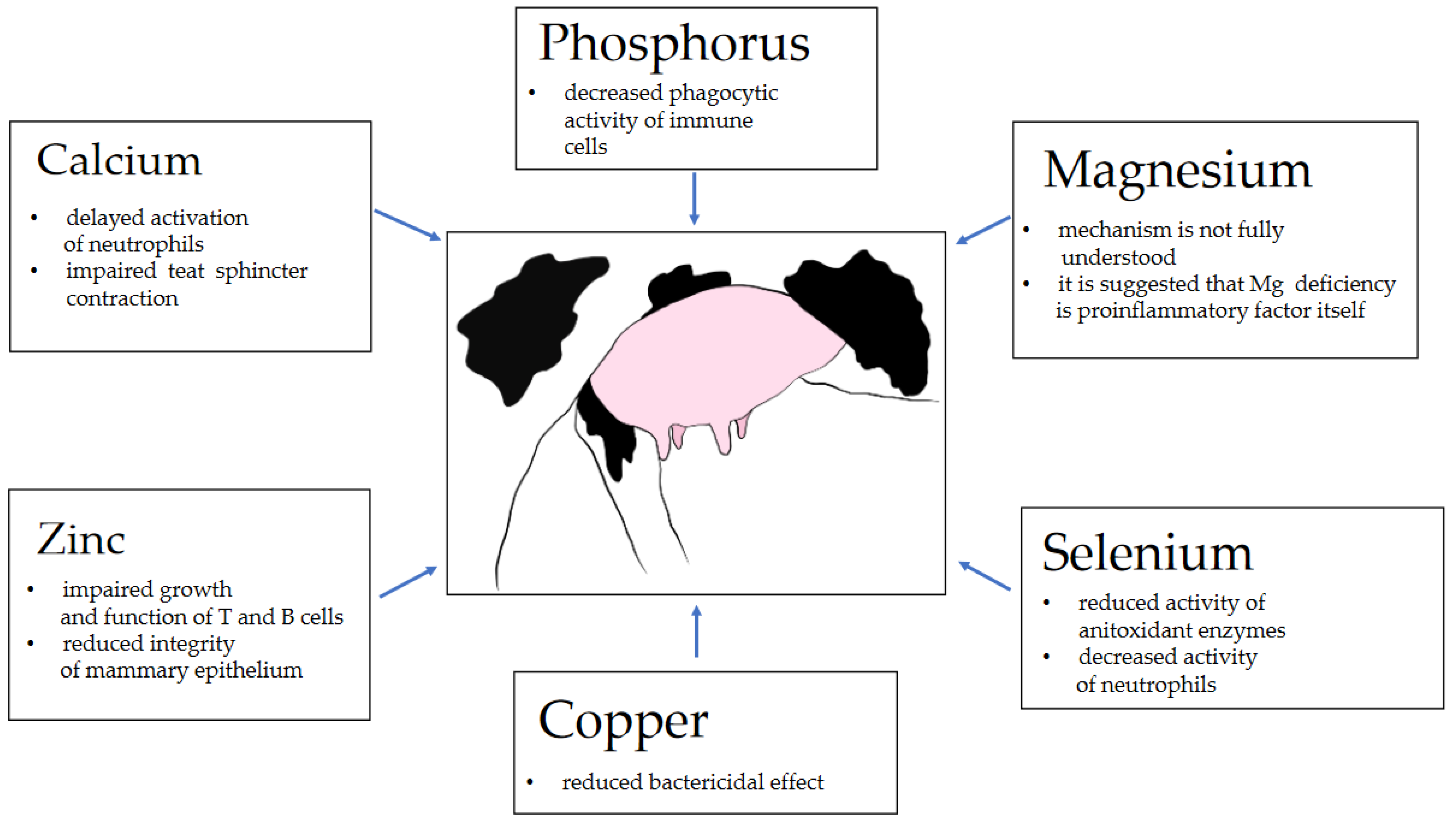
2. Calcium
3. Phosphorus
4. Magnesium
5. Selenium
6. Copper
7. Zinc
8. Mineral Nanoparticles—A Promising Tool in Udder Inflammation Management
9. Mineral Supplementation as an Auxiliary Tool in Mastitis Treatment—Field Trials
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero, J.; Benavides, E.; Meza, C. Assessing Financial Impacts of Subclinical Mastitis on Colombian Dairy Farms. Front. Vet. Sci. 2018, 5, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine Mastitis: Prevalence, Risk Factors and Isolation of Staphylococcus Aureus in Dairy Herds at Hawassa Milk Shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, W.P. A 100-Year Review: From Ascorbic Acid to Zinc—Mineral and Vitamin Nutrition of Dairy Cows. J. Dairy Sci. 2017, 100, 10045–10060. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001.
- Cozzi, G. Short Communication: Reference Values for Blood Parameters in Holstein Dairy Cows: Effects of Parity, Stage of Lactation, and Season of Production. J. Dairy Sci. 2011, 94, 7. [Google Scholar] [CrossRef] [PubMed]
- Zelal, A. Hypomagnesemia Tetany in Cattle. J. Adv. Dairy Res. 2017, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [Green Version]
- Laven, R.; Lawrence, K.; Livesey, C. The Assessment of Blood Copper Status in Cattle: A Comparison of Measurements of Caeruloplasmin and Elemental Copper in Serum and Plasma. N. Z. Vet. J. 2007, 55, 171–176. [Google Scholar] [CrossRef]
- Spolders, M.; Holtershinken, M.; Meyer, U.; Rehage, J.; Flachowsky, G. Assessment of Reference Values for Copper and Zinc in Blood Serum of First and Second Lactating Dairy Cows. Vet. Med. Int. 2010, 2010, 8. [Google Scholar] [CrossRef]
- Chester-Jones, H.; Vermeire, D.; Brommelsiek, W.; Brokken, K.; Marx, G.; Linn, J.G. Effect of Trace Mineral Source on Reproduction and Milk Production in Holstein Cows. Prof. Anim. Sci. 2013, 29, 289–297. [Google Scholar] [CrossRef]
- Molefe, K.; Mwanza, M. Effects of Mineral Supplementation on Reproductive Performance of Pregnant Cross-breed Bonsmara Cows: An Experimental Study. Reprod. Domest. Anim. 2020, 55, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ganda, E.K.; Bisinotto, R.S.; Vasquez, A.K.; Teixeira, A.G.V.; Machado, V.S.; Foditsch, C.; Bicalho, M.; Lima, F.S.; Stephens, L.; Gomes, M.S.; et al. Effects of Injectable Trace Mineral Supplementation in Lactating Dairy Cows with Elevated Somatic Cell Counts. J. Dairy Sci. 2016, 99, 7319–7329. [Google Scholar] [CrossRef] [Green Version]
- Machado, V.S.; Bicalho, M.L.S.; Pereira, R.V.; Caixeta, L.S.; Knauer, W.A.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Effect of an Injectable Trace Mineral Supplement Containing Selenium, Copper, Zinc, and Manganese on the Health and Production of Lactating Holstein Cows. Vet. J. 2013, 197, 451–456. [Google Scholar] [CrossRef]
- DeGaris, P.J.; Lean, I.J. Milk Fever in Dairy Cows: A Review of Pathophysiology and Control Principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Goff, J.P. The Monitoring, Prevention, and Treatment of Milk Fever and Subclinical Hypocalcemia in Dairy Cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef]
- Horst, R.L.; Goff, J.P.; McCluskey, B. Prevalence of Subclinical Hypocalcemia in U.S. Dairy Operations; US Department of Agriculture (USDA) Agricultural Research Service: Washington, DC, USA, 2003.
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. Parturition and Hypocalcemia Blunts Calcium Signals in Immune Cells of Dairy Cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef] [Green Version]
- Vig, M.; Kinet, J.-P. Calcium Signaling in Immune Cells. Nat. Immunol 2009, 10, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Guo, H.; Yang, W.; Li, M.; Zou, Y.; Loor, J.J.; Xia, C.; Xu, C. Effects of ORAI Calcium Release-Activated Calcium Modulator 1 (ORAI1) on Neutrophil Activity in Dairy Cows with Subclinical Hypocalcemia1. J. Anim. Sci. 2019, 97, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Ducusin, R.J.T.; Uzuka, Y.; Satoh, E.; Otani, M.; Nishimura, M.; Tanabe, S.; Sarashina, T. Effects of Extracellular Ca2+ on Phagocytosis and Intracellular Ca2+ Concentrations in Polymorphonuclear Leukocytes of Postpartum Dairy Cows. Res. Vet. Sci. 2003, 75, 27–32. [Google Scholar] [CrossRef]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvão, K.; Santos, J.E.P. Evaluation of Peripartal Calcium Status, Energetic Profile, and Neutrophil Function in Dairy Cows at Low or High Risk of Developing Uterine Disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef] [Green Version]
- Hisaeda, K.; Koshiishi, T.; Sasaki, A.; Shinozuka, Y.; Isobe, N.; Kawai, K. Changes in Ionized Calcium Concentration in the Blood of Dairy Cows with Peracute Coliform Mastitis. J. Vet. Med. Sci. 2020, 82, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Meglia, G.E.; Johannisson, A.; Petersson, L.; Waller, K.P. Changes in Some Blood Micronutrients, Leukocytes and Neutrophil Expression of Adhesion Molecules in Periparturient Dairy Cows. Acta Vet. Scand. 2001, 42, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between Subclinical Hypocalcemia and Postparturient Diseases in Dairy Cows. J. Dairy Sci. 2017, 100, 7427–7434. [Google Scholar] [CrossRef] [PubMed]
- Chamberlin, W.G.; Middleton, J.R.; Spain, J.N.; Johnson, G.C.; Ellersieck, M.R.; Pithua, P. Subclinical Hypocalcemia, Plasma Biochemical Parameters, Lipid Metabolism, Postpartum Disease, and Fertility in Postparturient Dairy Cows. J. Dairy Sci. 2013, 96, 7001–7013. [Google Scholar] [CrossRef]
- Grünberg, W. Treatment of Phosphorus Balance Disorders. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 383–408. [Google Scholar] [CrossRef]
- Eisenberg, S.W.F.; Ravesloot, L.; Koets, A.P.; Grünberg, W. Effect of Dietary Phosphorus Deprivation on Leukocyte Function in Transition Cows. J. Dairy Sci. 2019, 102, 1559–1570. [Google Scholar] [CrossRef]
- Eisenberg, S.W.F.; Ravesloot, L.; Koets, A.P.; Grünberg, W. Influence of Feeding a Low-Phosphorus Diet on Leucocyte Function in Dairy Cows. J. Dairy Sci. 2014, 97, 5176–5184. [Google Scholar] [CrossRef] [Green Version]
- Mullarky, I.K.; Wark, W.A.; Dickenson, M.; Martin, S.; Petersson-Wolfe, C.S.; Knowlton, K.F. Short Communication: Analysis of Immune Function in Lactating Dairy Cows Fed Diets Varying in Phosphorus Content. J. Dairy Sci. 2009, 92, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Kiersztejn, M.; Chevru, I.; Smogorzewski, M.; Fadda, G.; Alexiewicz, J.; Massry, S. On the mechanisms of impaired phagocytotosis in phosphate depletion. J. Am. Soc. Nephrol. 1991, 2, 1484–1489. [Google Scholar] [CrossRef]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Martens, H.; Stumpff, F. Assessment of Magnesium Intake According to Requirement in Dairy Cows. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1023–1029. [Google Scholar] [CrossRef] [Green Version]
- Weglicki, W.B.; Phillips, T.M. Pathobiology of Magnesium Deficiency: A Cytokine/Neurogenic Inflammation Hypothesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 263, R734–R737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malpuech-Brugere, C.; Nowacki, W.; Daveau, M.; Gueux, E.; Linard, C.; Rock, E.; Lebreton, J.; Mazur, A.; Rayssiguier, Y. Inflammatory response following acute magnesium deficiency in the rat. Biochim. Biophys. Acta 2000, 1501, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Van Orden, R.; Eggett, D.; Franz, K. Influence of Graded Magnesium Deficiencies on White Blood Cell Counts and Lymphocyte Subpopulations in rats. Magnes. Res. 2006, 19, 93–101. [Google Scholar]
- Bussiere, F.; Gueux, E.; Rock, E.; Girardeau, J.; Tridon, A.; Mazur, A.; Rayssiguier, Y. Increased Phagocytosis And Production Of Reactive Oxygen Species By Neutrophils During Magnesium Deficiency in Rats And Inhibition by High Magnesium Concentration. Br. J. Nutr. 2002, 87, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Jelinski, M.; Waldner, C.; Penner, G. Case-Control Study of Mineral Concentrations of Hoof Horn Tissue Derived from Feedlot Cattle with Toe Tip Necrosis Syndrome (Toe Necrosis). Can. Vet. J. 2018, 59, 7. [Google Scholar]
- An, L.; Marjani, S.L.; Wang, Z.; Liu, Z.; Liu, R.; Xue, F.; Xu, J.; Nedambale, T.L.; Yang, L.; Tian, X.C.; et al. Magnesium Is a Critical Element for Competent Development of Bovine Embryos. Theriogenology 2019, 140, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, R.U.; Tufarelli, V.; Laudadio, V. Selenium: An Essential Micronutrient for Sustainable Dairy Cows Production. Sustainability 2020, 12, 693. [Google Scholar] [CrossRef]
- Machado, V.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.S.; Kacar, C.; Foditsch, C.; Felippe, M.J.; Gilbert, R.O.; Bicalho, R.C. The Effect of Injectable Trace Minerals (Selenium, Copper, Zinc, and Manganese) on Peripheral Blood Leukocyte Activity and Serum Superoxide Dismutase Activity of Lactating Holstein Cows. Vet. J. 2014, 200, 299–304. [Google Scholar] [CrossRef]
- Hogan, J.S.; Smith, K.L.; Weiss, W.P.; Todhunter, D.A.; Schockey, W.L. Relationships Among Vitamin E, Selenium, and Bovine Blood Neutrophils. J. Dairy Sci. 1990, 73, 2372–2378. [Google Scholar] [CrossRef]
- Jing, H.; Chen, Y.; Liang, W.; Chen, M.; Qiu, C.; Guo, M. Effects of Selenium on MAC-T Cells in Bovine Mastitis: Transcriptome Analysis of Exosomal MRNA Interactions. Biol. Trace Elem. Res. 2020, 199(8), 2904–2912. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bi, C.; Wang, Y.; Sun, J.; Meng, X.; Li, J. Selenium Ameliorates Staphylococcus Aureus-Induced Inflammation in Bovine Mammary Epithelial Cells by Inhibiting Activation of TLR2, NF-ΚB and MAPK Signaling Pathways. BMC Vet. Res. 2018, 14, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyne, R.; Arthur, J.R. Effects of Selenium and Copper Deficiency on Neutrophil Function in Cattle. J. Comp. Pathol. 1981, 91, 271–276. [Google Scholar] [CrossRef]
- Sripad, K.; Upendra, H.; Yathiray, S. Efficacy of Organic and Inorganic Selenium in Treatment of Bovine Subclinical Mastitis. IOSR J. Agric. Vet. Sci. 2016, 9, 31–35. [Google Scholar]
- Hoque, M.N.; Das, Z.C.; Rahman, A.N.M.A.; Hoque, M.M. Effect of Administration of Vitamin E, Selenium and Antimicrobial Therapy on Incidence of Mastitis, Productive and Reproductive Performances in Dairy Cows. Int. J. Vet. Sci. Med. 2016, 4, 63–70. [Google Scholar] [CrossRef]
- Zigo, F.; Farkasóvá, Z.; Eleko, J.; Lapin, M.; Chripková, M.; Czerski, A. Effect of Parenteral Administration of Selenium and Vitamin E on Health Status of Mammary Gland and on Selected Antioxidant Indexes in Blood of Dairy Cows. Pol. J. Vet. Sci. 2014, 17, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.L.; Harrison, J.H.; Hancock, D.D.; Todhunter, D.A.; Conrad, H.R. Effect of Vitamin E and Selenium Supplementation on Incidence of Clinical Mastitis and Duration of Clinical Symptoms. J. Dairy Sci. 1984, 67, 1293–1300. [Google Scholar] [CrossRef]
- Wang, D.; Jia, D.; He, R.; Lian, S.; Wang, J.; Wu, R. Association Between Serum Selenium Level and Subclinical Mastitis in Dairy Cattle. Biol. Trace Elem. Res. 2021, 199, 1389–1396. [Google Scholar] [CrossRef]
- Muhee, A.; Malik, H.U.; Asharaf, I.; Shah, O.S.; Muheet, A.J.; Rather, W.; Muzamil, S. Therapeutic Efficacies of Self-Formulated Anti-Oxidant Trace Minerals Formulation in Bovine Mastitis. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 4595–4599. [Google Scholar]
- Żarczyńska, K.; Sobiech, P.; Mee, J.; Illek, J. The influence of short-term selenitetriglycerides supplementation on blood selenium, and hepatic, renal, metabolic and hematological parameters in dairy cows. Pol. J. Vet. Sci. 2020, 23, 637–646. [Google Scholar]
- Pieszka, M.; Bederska-Łojewska, D.; Szczurek, P.; Pieszka, M. The Membrane Interactions of Nano-Silica and Its Potential Application in Animal Nutrition. Animals 2019, 9, 1041. [Google Scholar] [CrossRef] [Green Version]
- Gopi, M.; Pearlin, B.; Kumar, R.D.; Shanmathy, M.; Prabakar, G. Role of Nanoparticles in Animal and Poultry Nutrition: Modes of Action and Applications in Formulating Feed Additives and Food Processing. Int. J. Pharmacol. 2017, 13, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Wichtel, J.J.; Keefe, G.P.; Leeuwen, J.A.V.; Spangler, E.; McNiven, M.A.; Ogilvie, T.H. The Selenium Status of Dairy Herds in Prince Edward Island. Can. Vet. J. 2004, 45, 9. [Google Scholar]
- Olivares, R.W.I.; Postma, G.C.; Schapira, A.; Iglesias, D.E.; Valdez, L.B.; Breininger, E.; Gazzaneo, P.D.; Minatel, L. Biochemical and Morphological Alterations in Hearts of Copper-Deficient Bovines. Biol. Trace Elem. Res. 2019, 189, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Suttle, F.N. Minerals in Animal Nutrition, 4th ed.; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- Reyes-Jara, A.; Cordero, N.; Aguirre, J.; Troncoso, M.; Figueroa, G. Antibacterial Effect of Copper on Microorganisms Isolated from Bovine Mastitis. Front. Microbiol. 2016, 7, 626. [Google Scholar] [CrossRef] [Green Version]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M. Antimicrobial Properties of Gold, Silver, Copper and Platinum Nanoparticles against Selected Microorganisms Isolated from Cases of Mastitis in Cattle. Med. Weter. 2014, 70(9), 564–567. [Google Scholar]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and Copper Nanoparticles—An Alternative in Future Mastitis Treatment and Prevention? IJMS 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaletti, R.W.; Trammell, D.S.; Smith, B.A.; Harmon, R.J. Role of Dietary Copper in Enhancing Resistance to Escherichia Coli Mastitis. J. Dairy Sci. 2003, 86, 1240–1249. [Google Scholar] [CrossRef]
- Gakhar, G.; Randhawa, S.S.; Randhawa, C.S.; Bansal, B.K.; Singh, R.S. Effect of Copper on the Milk Quality and Prevention of Mastitis in Dairy Cows. Indian J. Anim. Sci. 2010, 80, 727–728. [Google Scholar]
- Babu, U.; Failla, M.L. Respiratory Burst and Candidacidal Activity of Peritoneal Macrophages Are Impaired in Copper-Deficient Rats. J. Nutr. 1990, 120, 1692–1699. [Google Scholar] [CrossRef]
- Willing, B.P.; Pepin, D.M.; Marcolla, C.S.; Forgie, A.J.; Diether, N.E.; Bourrie, B.C.T. Bacterial Resistance to Antibiotic Alternatives: A Wolf in Sheep’s Clothing? Anim. Front. 2018, 8, 39–47. [Google Scholar] [CrossRef]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Galarza, E.M.; Farnetano, N.A.; Giuliodori, M.J.; Nikoloff, N.; Fazzio, L.E.; Furnus, C.C. Parenteral Zinc Supplementation Increases Pregnancy Rates in Beef Cows. Biol. Trace Elem. Res. 2019, 192, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Cope, C.M.; Mackenzie, A.M.; Wilde, D.; Sinclair, L.A. Effects of Level and Form of Dietary Zinc on Dairy Cow Performance and Health. J. Dairy Sci. 2009, 92, 2128–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, G.; Aggarwal, A.; Singh, A.K.; Kumar, M. Effect of Vitamin E and Zinc Supplementation on Milk Yield, Milk Composition, and Udder Health in Sahiwal Cows. Anim. Nutr. Feed Tech. 2015, 15, 67. [Google Scholar] [CrossRef]
- Whitaker, D.A.; Eares, H.F.; Ait, K.; Kelly, J.M. No Effect of a Dietary Zinc Proteinate on Clinical Mastiffs, Infection Rate, Recovery Rate and Somatic Cell Count in Dairy Cows. Vet. J. 1997, 153, 197–204. [Google Scholar] [CrossRef]
- Weng, X.; Monteiro, A.P.A.; Guo, J.; Li, C.; Orellana, R.M.; Marins, T.N.; Bernard, J.K.; Tomlinson, D.J.; DeFrain, J.M.; Wohlgemuth, S.E.; et al. Effects of Heat Stress and Dietary Zinc Source on Performance and Mammary Epithelial Integrity of Lactating Dairy Cows. J. Dairy Sci. 2018, 101, 2617–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, J.; Pandalaneni, K.; Mamedova, L.; DeFrain, J.; Amamcharla, J.; Bradford, B. Effects of Dietary Zinc Source and Level on Mammary Epithelia and Dairy Food Chemistry. Kans. Agric. Exp. Stn. Res. Rep. 2016, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Hill, E.K.; Li, J. Current and Future Prospects for Nanotechnology in Animal Production. J. Anim. Sci. Biotechnol. 2017, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria: Activity of Silver Nanoparticles against MDR Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.P.; Warszyński, P. Synthesis and Antimicrobial Activity of Monodisperse Copper Nanoparticles. Colloids Surf. Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial Effects and Resistance Induction of Silver and Gold Nanoparticles against Staphylococcus Aureus-induced Mastitis and the Potential Toxicity in Rats. MicrobiologyOpen 2019, 8, e00698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozyen, H.F.; Ibrahim, E.S.; Khairy, E.A.; El-Dek, S.I. Enhanced Antibacterial Activity of Capped Zinc Oxide Nanoparticles: A Step towards the Control of Clinical Bovine Mastitis. Vet. World 2019, 12, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Jagielski, T.; Bakuła, Z.; Pleń, M.; Kamiński, M.; Nowakowska, J.; Bielecki, J.; Wolska, K.I.; Grudniak, A.M. The Activity of Silver Nanoparticles against Microalgae of the Prototheca Genus. Nanomedicine 2018, 13, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S. Short- and Long-Term Effects of Silver Nanoparticles on Human Microvascular Endothelial Cells. WJBC 2014, 5, 457. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Ruegg, P.L. Treatments of Clinical Mastitis Occurring in Cows on 51 Large Dairy Herds in Wisconsin. J. Dairy Sci. 2014, 97, 5426–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, G.M.; Petzer, I.-M. Injectable Organic and Inorganic Selenium in Dairy Cows–Effects on Milk, Blood and Somatic Cell Count Levels. Onderstepoort J. Vet. Res. 2019, 86, a1664. [Google Scholar] [CrossRef]
- Bourne, N.; Wathes, D.C.; Lawrence, K.E.; McGowan, M.; Laven, R.A. The Effect of Parenteral Supplementation of Vitamin E with Selenium on the Health and Productivity of Dairy Cattle in the UK. Vet. J. 2008, 177, 381–387. [Google Scholar] [CrossRef]
- Smulski, S.; Gehrke, M.; Libera, K.; Cieślak, A.; Huang, H.; Patra, A.K.; Szumacher-Strabel, M. Effects of Various Mastitis Treatments on the Reproductive Performance of Cows. BMC Vet. Res. 2020, 16, 99. [Google Scholar] [CrossRef]
| Mineral | Serum Concentration | Reference |
|---|---|---|
| Calcium (Ca) | 2.2–2.6 mmol/L | [6] |
| Phosphorus (P) | 1.3–2.6 mmol/L | [6] |
| Magnesium (Mg) | 0.75–1.0 mmol/L | [7] |
| Selenium (Se) | 0.73–1.08 µmol/L | [8] |
| Copper (Cu) | 1–18 µmol/L | [9] |
| Zinc (Zn) | 8–19 µmol/L | [10] |
| Mineral | Requirement for Non-Lactating Cows | Concentration in Milk (mg/kg) | Requirement for Lactating Cows |
|---|---|---|---|
| Calcium (Ca) | 0.0154 g/kg BW | 1220 | 0.106 g/kg BW |
| Phosphorus (P) | 1 g/kg DMI | 900 | 2.5 g/kg DMI |
| Magnesium (Mg) | 3 mg/kg BW | 150 | 10 mg/kg BW |
| Copper (Cu) | 152 mg/cow (15.2 mg/kg DMI) | 0.015 | 313 mg/cow (15.7 mg/kg DMI) |
| Selenium (Se) | 0.3 mg/kg DMI | 0.01–0.025 | 0.3 mg/kg DMI |
| Zinc (Zn) | 310 mg/day (31 mg/kg DMI) | 4 | 1261 mg/day (63 mg/kg DMI) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libera, K.; Konieczny, K.; Witkowska, K.; Żurek, K.; Szumacher-Strabel, M.; Cieslak, A.; Smulski, S. The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review. Animals 2021, 11, 2330. https://doi.org/10.3390/ani11082330
Libera K, Konieczny K, Witkowska K, Żurek K, Szumacher-Strabel M, Cieslak A, Smulski S. The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review. Animals. 2021; 11(8):2330. https://doi.org/10.3390/ani11082330
Chicago/Turabian StyleLibera, Kacper, Kacper Konieczny, Katarzyna Witkowska, Katarzyna Żurek, Małgorzata Szumacher-Strabel, Adam Cieslak, and Sebastian Smulski. 2021. "The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review" Animals 11, no. 8: 2330. https://doi.org/10.3390/ani11082330
APA StyleLibera, K., Konieczny, K., Witkowska, K., Żurek, K., Szumacher-Strabel, M., Cieslak, A., & Smulski, S. (2021). The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review. Animals, 11(8), 2330. https://doi.org/10.3390/ani11082330







