Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress
Abstract
Simple Summary
Abstract
1. Introduction
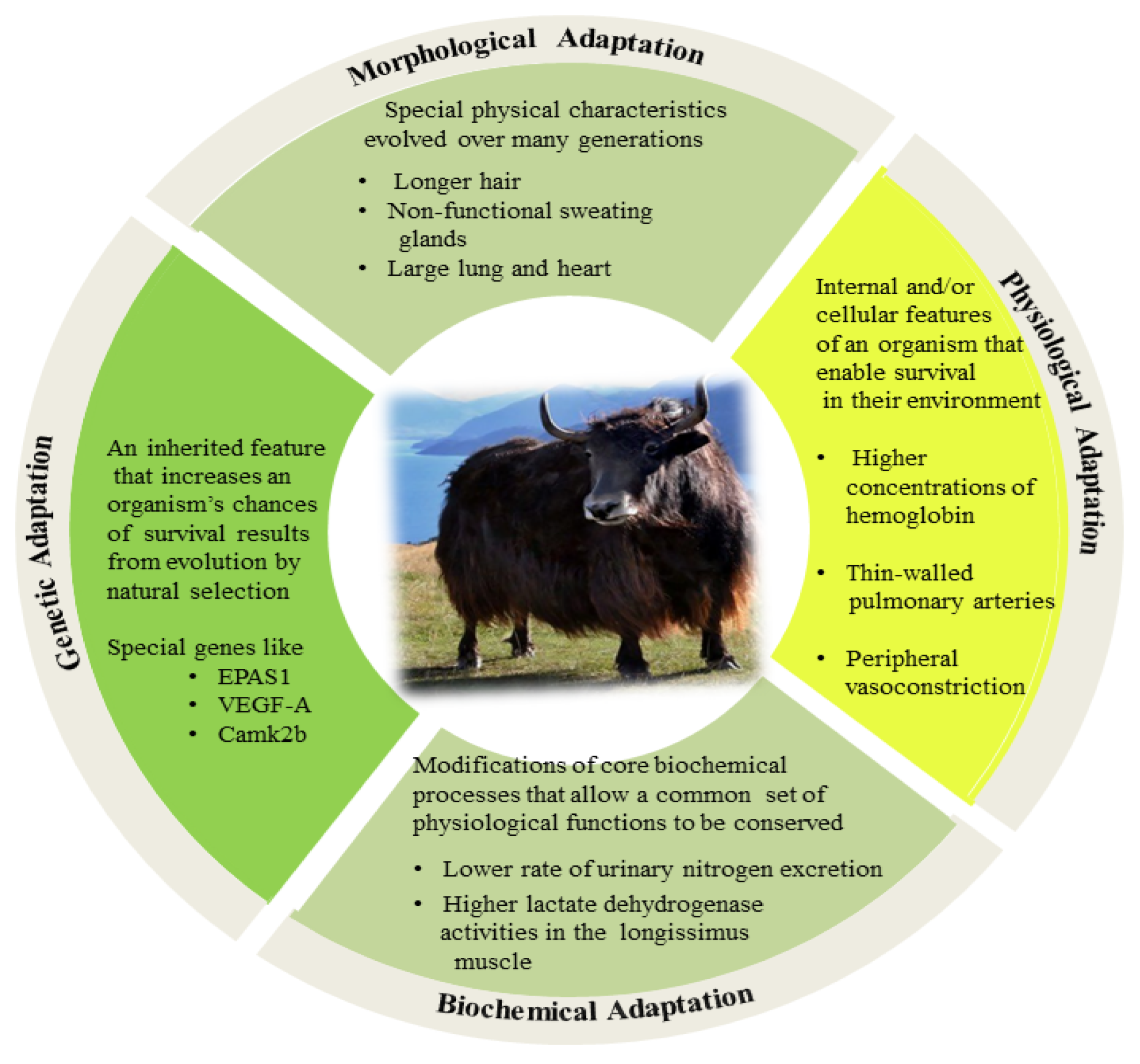
2. High Altitude Adaptation Mechanisms of Yak
2.1. Morphological Adaptations
2.2. Physiological Adaptations
2.3. Biochemical Adaptations
2.4. Genetic Background of High-Altitude Adaptations
3. Transcriptomic Changes in Yaks Living in High-altitude Environments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Miao, F.; Guo, Z.; Xue, R.; Wang, X.; Shen, Y. Effects of Grazing and Precipitation on Herbage Biomass, Herbage Nutritive Value, and Yak Performance in an Alpine Meadow on the Qinghai–Tibetan Plateau. PLoS ONE 2015, 10, e0127275. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. Recent Advances in High Altitude Medicine and Biology. High Alt. Med. Biol. 2015, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, D.; Sinsky, E.; Miller, J. Role of snow-albedo feedback in higher elevation warming over the Himalayas, Tibetan Plateau and Central Asia. Environ. Res. Lett. 2014, 9, 114008. [Google Scholar] [CrossRef]
- Vuille, M. Climate variability and high altitude temperature and precipitation. In Encyclopedia of Snow, Ice and Glaciers; Singh, V.P., Singh, P., Haritashya, U.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 153–156. [Google Scholar]
- Han, X.T.; Xie, A.Y.; Bi, X.C.; Liu, S.J.; Hu, L.H. Effects of high altitude and season on fasting heat production in the yak Bos grunniens or Poephagus grunniens. Br. J. Nutr. 2002, 88, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wiener, G.; Han, J.; Long, R. The Yak; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2003. [Google Scholar]
- Key, N.; Sneeringer, S. Potential Effects of Climate Change on the Productivity of U.S. Dairies. Am. J. Agric. Econ. 2014, 96, 1136–1156. [Google Scholar] [CrossRef]
- Burtscher, M.; Gatterer, H.; Burtscher, J.; Mairbäurl, H. Extreme Terrestrial Environments: Life in Thermal Stress and Hypoxia. A Narrative Review. Front. Physiol. 2018, 9, 572. [Google Scholar] [CrossRef]
- Qiu, Q.; Wang, L.; Wang, K.; Yang, Y.; Ma, T.; Wang, Z.; Zhang, X.; Ni, Z.; Hou, F.; Long, R.; et al. Yak whole-genome resequencing reveals domestication signatures and prehistoric population expansions. Nat. Commun. 2015, 6, 10283. [Google Scholar] [CrossRef]
- Ma, Z.-J.; Zhong, J.-C.; Han, J.-L.; Xu, J.-T.; Liu, Z.-N.; Bai, W.-L. Research progress on molecular genetic diversity of the yak (Bos grunniens). Yi Chuan Hered. 2013, 35, 151–160. [Google Scholar] [CrossRef]
- Wang, H.; Long, R.; Liang, J.B.; Guo, X.; Ding, L.; Shang, Z. Comparison of Nitrogen Metabolism in Yak (Bos grunniens) and Indigenous Cattle (Bos taurus) on the Qinghai-Tibetan Plateau. Asian-Australas. J. Anim. Sci. 2011, 24, 766–773. [Google Scholar] [CrossRef]
- Lan, D.; Xiong, X.; Huang, C.; Mipam, T.D.; Li, J. Toward understanding the genetic basis of yak ovary reproduction: A characterization and comparative analyses of estrus ovary transcriptiome in yak and cattle. PLoS ONE 2016, 11, 0152675. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.I.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef]
- Lan, D.; Xiong, X.; Ji, W.; Li, J.; Mipam, T.D.; Ai, Y.; Chai, Z. Transcriptome profile and unique genetic evolution of positively selected genes in yak lungs. Genetica 2018, 146, 151–160. [Google Scholar] [CrossRef]
- Ding, X.; Liang, C.; Guo, X.; Wu, X.; Wang, H.; Johnson, K.; Yan, P. Physiological insight into the high-altitude adaptations in domesticated yaks (Bos grunniens) along the Qinghai-Tibetan Plateau altitudinal gradient. Livest. Sci. 2014, 162, 233–239. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.-R.; Lv, F.-H.; He, S.-G.; Tian, S.; Peng, W.-F.; Sun, Y.-W.; Zhao, Y.-X.; Tu, X.-L.; Zhang, M.; et al. Whole-Genome Sequencing of Native Sheep Provides Insights into Rapid Adaptations to Extreme Environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef]
- Friedrich, J.; Wiener, P. Selection signatures for high-altitude adaptation in ruminants. Anim. Genet. 2020, 51, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Guang-Xin, E.; Basang, W.D.; Zhu, Y.B. Whole-genome analysis identifying candidate genes of altitude adaptive ecological thresholds in yak populations. J. Anim. Breed. Genet. 2019, 136, 371–377. [Google Scholar] [CrossRef]
- WU, X.Y.; DING, X.Z.; Min, C.H.U.; Xian, G.U.O.; BAO, P.J.; Liang, C.N.; Ping, Y.A.N. Novel SNP of EPAS1 gene associated with higher hemoglobin concentration revealed the hypoxia adaptation of yak (Bos grunniens). J. Integr. Agric. 2015, 14, 741–748. [Google Scholar] [CrossRef]
- Guang-Xin, E.; Yang, B.-G.; Basang, W.-D.; Zhu, Y.-B.; An, T.-W.; Luo, X.-L. Screening for signatures of selection of Tianzhu white yak using genome-wide re-sequencing. Anim. Genet. 2019, 50, 534–538. [Google Scholar] [CrossRef]
- Mishra, K.P.; Ganju, L. Influence of High Altitude Exposure on the Immune System: A Review. Immunol. Investig. 2010, 39, 219–234. [Google Scholar] [CrossRef]
- Parraguez, V.H.; Atlagich, M.; Díaz, R.; Bruzzone, M.E.; Behn, C.; Raggi, L.A. Effect of hypobaric hypoxia on lamb intrauterine growth: Comparison between high- and low-altitude native ewes. Reprod. Fertil. Dev. 2005, 17, 497–505. [Google Scholar] [CrossRef]
- Parraguez, V.H.; Urquieta, B.; Perez, L.; Castellaro, G.; De los Reyes, M.; Torres-Rovira, L.; Aguado-Martínez, A.; Astiz, S.; González-Bulnes, A. Fertility in a high-altitude environment is compromised by luteal dysfunction: The relative roles of hypoxia and oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 24. [Google Scholar] [CrossRef]
- Colditz, I.G.; Hine, B.C. Resilience in farm animals: Biology, management, breeding and implications for animal welfare. Anim. Prod. Sci. 2016, 56, 1961. [Google Scholar] [CrossRef]
- Fu, M.; Chen, Y.; Xiong, X.; Lan, D.; Li, J. Establishment of Mammary Gland Model In Vitro: Culture and Evaluation of a Yak Mammary Epithelial Cell Line. PLoS ONE 2014, 9, e113669. [Google Scholar] [CrossRef]
- Durmowicz, A.G.; Hofmeister, S.; Kadyraliev, T.K.; Aldashev, A.A.; Stenmark, K.R. Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J. Appl. Physiol. 1993, 74, 2276–2285. [Google Scholar] [CrossRef]
- Guan, J.; Long, K.; Ma, J.; Zhang, J.; He, D.; Jin, L.; Tang, Q.; Jiang, A.; Wang, X.; Hu, Y.; et al. Comparative analysis of the microRNA transcriptome between yak and cattle provides insight into high-altitude adaptation. PeerJ 2017, 5, e3959. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Li, H.G.; Li, Y.J.; Guo, S.C.; Yang, J.; Qi, D.L.; Jin, C.; Zhao, X.Q. Hypoxia-inducible factor 1α cDNA cloning and its mRNA and protein tissue specific expression in domestic yak (Bos grunniens) from Qinghai-Tibetan plateau. Biochem. Biophys. Res. Commun. 2006, 348, 310–319. [Google Scholar] [CrossRef]
- Shao, B.; Long, R.; Ding, Y.; Wang, J.; Ding, L.; Wang, H. Morphological adaptations of yak (Bos grunniens) tongue to the foraging environment of the Qinghai-Tibetan Plateau1. J. Anim. Sci. 2010, 88, 2594–2603. [Google Scholar] [CrossRef]
- Krishnan, G.; Paul, V.; Hanah, S.S.; Bam, J.; Das, P.J. Effects of climate change on yak production at high altitude. Indian J. Anim. Sci. 2016, 86, 621–626. [Google Scholar]
- Roth, G.; Wake, D.B. Conservatism and innovation in the evolution of feeding in vertebrates. In Complex Organismal Functions: Integration and Evolution in Vertebrates; Wake, D.B., Roth, G., Eds.; John Wiley and Sons: New York, NY, USA, 1989; pp. 7–21. [Google Scholar]
- Long, R.J.; Ding, L.M.; Shang, Z.H.; Guo, X.H. The yak grazing system on the Qinghai-Tibetan plateau and its status. Rangel. J. 2008, 30, 241–246. [Google Scholar] [CrossRef]
- Wu, X.Y.; Liang, C.N.; Ding, X.Z.; Guo, X.; Bao, P.J.; Chu, M.; Liu, W.B.; Yan, P. Association of novel single-nucleotide polymorphisms of the vascular endothelial growth factor-A gene with high-altitude adaptation in yak (Bos grunniens). Genet. Mol. Res. 2013, 12, 5506–5515. [Google Scholar] [CrossRef]
- Ivy, C.M.; Scott, G.R. Control of breathing and the circulation in high-altitude mammals and birds. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 186, 66–74. [Google Scholar] [CrossRef]
- Wei, Q.; Yu, H. Comparison of histological structure of pulmonary alveoli between 180 days old yak and plain cattle. J. Qinghai Univ. Nat. Sci. 2008, 26, 36–39, (In Chinese with English Abstract). [Google Scholar]
- Claydon, V.E.; Norcliffe, L.J.; More, J.P.; Rivera, M.; Leon-Velarde, F.; Appenzeller, O.; Hainsworth, R. Orthostatic tolerance and blood volume in Adens high-altitude dwellers. Exp. Physiol. 2004, 89, 565–571. [Google Scholar] [CrossRef]
- West, J.B. Human responses to extreme altitudes. Integr. Comp. Biol. 2006, 46, 25–34. [Google Scholar] [CrossRef]
- West, J.B. Physiological Effects of Chronic Hypoxia. N. Engl. J. Med. 2017, 376, 1965–1971. [Google Scholar] [CrossRef]
- Gaughan, J.B.; Sejian, V.; Mader, T.L.; Dunshea, F.R. Adaptation strategies: Ruminants. Anim. Front. 2019, 9, 47–53. [Google Scholar] [CrossRef]
- Manou-Stathopoulou, V.; Goodwin, C.D.; Patterson, T.; Redwood, S.R.; Marber, M.S.; Williams, R.P. The effects of cold and exercise on the cardiovascular system. Heart 2015, 101, 808–820. [Google Scholar] [CrossRef]
- Ruf, T.; Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015, 90, 891–926. [Google Scholar] [CrossRef]
- Zou, H.; Hu, R.; Wang, Z.; Shah, A.M.; Zeng, S.; Peng, Q.; Xue, B.; Wang, L.; Zhang, X.; Wang, X.; et al. Effects of Nutritional Deprivation and Re-Alimentation on the Feed Efficiency, Blood Biochemistry, and Rumen Microflora in Yaks (Bos grunniens). Animals 2019, 9, 807. [Google Scholar] [CrossRef]
- Zhou, J.W.; Zhong, C.L.; Liu, H.; Degen, A.A.; Titgemeyer, E.C.; Ding, L.M.; Shang, Z.H.; Guo, X.S.; Qiu, Q.; Li, Z.P.; et al. Comparison of nitrogen utilization and urea kinetics between yaks (Bos grunniens) and indigenous cattle (Bos taurus). J. Anim. Sci. 2017, 95, 4600–4612. [Google Scholar] [CrossRef]
- Han, X.T.; Chen, J.; Han, Z.K. Ruminal nitrogen metabolism and the flows of nitrogen fractions reaching the duodenum of growing yaks fed diets containing different levels of crude protein. Acta Zoonutrimenta Sin. 1998, 10, 34–43. [Google Scholar]
- Wang, H.C.; Long, R.J.; Zhou, W.; Li, X.P.; Zhou, J.W.; Guo, X.S. A comparative study on urinary purine derivative excretion of yak (Bos grunniens), cattle (Bos taurus), and crossbred (Bos taurus x Bos grunniens) in the Qinghai-Tibetan plateau, China. J. Anim. Sci. 2009, 87, 2355–2362. [Google Scholar] [CrossRef][Green Version]
- Xue, B.; Chai, S.T.; Liu, S.J.; Wang, W.B. Study on the protein requirement of growing yaks. Chinese Qinghai. J. Anim. Vet. 1994, 24, 1–6. [Google Scholar]
- Hu, L.H.; Xie, A.Y.; Han, X.T. Study on the body surface areas of growing yaks and cattle. Chin. J. Anim. Sci. 1994, 30, 9–10. [Google Scholar]
- Markert, C.L. Biochemistry and function of lactate dehydrogenase. Cell Biochem. Funct. 1984, 2, 131–134. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Wang, G.S.; Feng, J.; Huang, J.Q.; Xu, Y.O.; Jin, S.Y.; Li, Y.P.; Jiang, Z.R.; Zheng, Y.C. Comparison of enzyme activi-ties and gene expression profiling between yak and bovine skeletal muscles. Livest. Sci. 2011, 135, 93–97. [Google Scholar] [CrossRef]
- Gnecchi-Ruscone, G.A.; Abondio, P.; De Fanti, S.; Sarno, S.; Sherpa, M.G.; Sherpa, P.T.; Marinelli, G.; Natali, L.; Di Marcello, M.; Peluzzi, D.; et al. Evidence of Polygenic Adaptation to High Altitude from Tibetan and Sherpa Genomes. Genome Biol. Evol. 2018, 10, 2919–2930. [Google Scholar] [CrossRef]
- Amos, W.; Harwood, J. Factors affecting levels of genetic diversity in natural populations. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 177–186. [Google Scholar] [CrossRef]
- Pritchard, J.; Di Rienzo, A. Adaptation not by sweeps alone. Nat. Rev. Genet. 2010, 11, 665–667. [Google Scholar] [CrossRef]
- Höllinger, I.; Pennings, P.S.; Hermisson, J. Polygenic adaptation: From sweeps to subtle frequency shifts. PLoS Genet. 2019, 15, e1008035. [Google Scholar] [CrossRef]
- Ding, X.; Yang, C.; Bao, P.; Wu, X.; Pei, J.; Yan, P.; Guo, X. Population genetic variations of the matrix metalloproteinases-3 gene revealed hypoxia adaptation in domesticated yaks (Bos grunniens). Asian-Australas. J. Anim. Sci. 2019, 32, 1801–1808. [Google Scholar] [CrossRef]
- Dolt, K.S.; Mishra, M.K.; Karar, J.; Baig, M.A.; Ahmed, Z.; Pasha, M.Q. cDNA cloning, gene organization and variant specific expression of HIF-1α in high altitude yak (Bos grunniens). Gene 2007, 386, 73–80. [Google Scholar] [CrossRef]
- Allen, M.S.; Bradford, B.J.; Oba, M. Board-Invited Review: The hepatic oxidation theory of the control of feed intake and its application to ruminants. J. Anim. Sci. 2009, 87, 3317–3334. [Google Scholar] [CrossRef]
- Weimer, P.J.; Russell, J.B.; Muck, R.E. Lessons from the cow: What the ruminant animal can teach us about consolidated bio-processing of cellulosic biomass. Bioresour. Technol. 2009, 100, 5323–5331. [Google Scholar] [CrossRef]
- Moon, Y.A.; Horton, J.D. Identification of two mammalian reductases involved in the two-carbon fatty acyl elongation cascade. J. Biol. Chem. 2003, 278, 7335–7343. [Google Scholar] [CrossRef]
- Li, Y.; Trojer, P.; Xu, C.F.; Cheung, P.; Kuo, A.; Drury III, W.J.; Qiao, Q.; Neubert, T.A.; Xu, R.M.; Gozani, O.; et al. The target of the NSD family of histone lysine methyltransferases depends on the nature of the substrate. J. Biol. Chem. 2009, 284, 34283–34295. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Xu, Y.; Dong, X.; Shao, B. Evolutionary Adaptation of Aquaporin-4 in Yak (Bos grunniens) Brain to High-Altitude Hypoxia of Qinghai-Tibetan Plateau. High Alt. Med. Biol. 2020, 21, 167–175. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Elzo, M.A.; Dang, S.; Jia, X.; Lai, S. Genetic diversity of ATP8 and ATP6 genes is associated with high-altitude adaptation in yak. Mitochondrial DNA Part A 2017, 29, 385–393. [Google Scholar] [CrossRef]
- Wang, H.; Chai, Z.; Hu, D.; Ji, Q.; Xin, J.; Zhang, C.; Zhong, J. A global analysis of CNVs in diverse yak populations using whole-genome resequencing. BMC Genom. 2019, 20, 61. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Wang, J.; Elzo, M.A.; Yang, X.; Lai, S. Genetic diversities of MT-ND1 and MT-ND2 genes are associated with high-altitude adaptation in yak. Mitochondrial DNA Part A 2017, 29, 485–494. [Google Scholar] [CrossRef]
- Guang-Xin, E.; Yang, B.-G.; Zhu, Y.-B.; Duang, X.-H.; Basang, W.-D.; Luo, X.-L.; An, T.-W. Genome-wide selective sweep analysis of the high-altitude adaptability of yaks by using the copy number variant. 3 Biotech 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Somero, G.N. Linking biogeography to physiology: Evolutionary and acclamatory adjustments of thermal limits. Front Zool. 2005, 2, 1–9. [Google Scholar] [CrossRef]
- Gracey, A.Y.; Chaney, M.L.; Boomhower, J.P.; Tyburczy, W.R.; Connor, K.; Somero, G.N. Rhythms of Gene Expression in a Fluctuating Intertidal Environment. Curr. Biol. 2008, 18, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Q.; He, Y.; Yang, L.; Zhang, X.; Shi, P.; Yang, L.; Liu, Z.; Zhang, F.; Liu, F.; et al. The Transcriptomic Landscape of Yaks Reveals Molecular Pathways for High Altitude Adaptation. Genome Biol. Evol. 2018, 11, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Lisy, K.; Peet, D.J. Turn me on: Regulating HIF transcriptional activity. Cell Death Differ. 2008, 15, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.D.; Coleman, M.; Pugh, C.W. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell. Mol. Life Sci. 2009, 66, 3539–3554. [Google Scholar] [CrossRef]
- Xiong, X.; Fu, M.; Lan, D.; Li, J.; Zi, X.; Zhong, J. Yak response to high-altitude hypoxic stress by altering mRNA expression and DNA methylation of hypoxia-inducible factors. Anim. Biotech. 2015, 26, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Wang, G.L. A Nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Schofield, C.; Ratcliffe, P. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H. Hypoxia-inducible factor as a physiological regulator. Exp. Physiol. 2005, 90, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.B.; Ning, H.X.; Zhu, S.S.; Sun, P.; Xu, S.X.; Chang, Z.J.; Zhao, X.Q. Cloning of hypoxia-inducible factor 1alpha cDNA from a high hypoxia tolerant mammal-plateau pika (Ochotona curzoniae). Biochem. Biophys. Res. Commun. 2004, 316, 565–572. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Y.; Wang, L.; Ma, T.; Shang, H.; Ding, L.; Han, J.; Qiu, Q. Different gene expressions between cattle and yak provide insights into high-altitude adaptation. Anim. Genet. 2015, 47, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.-W.; Chai, Z.-X.; Zhang, C.-F.; Zhang, Q.; Zhu, Y.; Cao, H.-W.; Ji, Q.-M.; Zhong, J.-C. Transcriptome profiles revealed the mechanisms underlying the adaptation of yak to high-altitude environments. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
| Special Morphological Structures | Function | References |
|---|---|---|
| Compact body, thick outer hair covering, and nonexistence of functional sweat glands | Minimize dissipation of body heat during winter | [6] |
| Thin-walled pulmonary arteries with little smooth muscles | Facilitate superefficient O2 flow under hypobaric hypoxia | [16] |
| Larger lungs and hearts | Aid oxygen uptake | [17] |
| Shorter tongue and greater lingual prominence | Improve forage digestibility through efficient grinding of food | [31] |
| Candidate Genes | Functions | References |
|---|---|---|
| Camk2b, Gcnt3, Hsd17b12, Whsc1, and Glul | High level of nutrition utilization in high altitudes | [13] |
| HIF1A, MMP3, ADAM17, ARG2 | High-altitude adaptation | [13] |
| DEXI, DCC, and MRP4 | Adaptation to high-altitude environments | [14] |
| PDE4D, RPS6KA6, ITPR1, and GNAO1 | Environmental information processing and environmental adaptability | [20] |
| EPAS1 | Key transcription factor that activates the expression of oxygen-regulated genes | [21] |
| ABCG8, COL4A1, LOC102287650, PDCD1, and NUP210 | Adaptation to high-altitude environments | [22] |
| VEGF-A | Regulation of blood vessel size | [35] |
| MMP3 | Regulator of the cellular response to hypoxia | [58] |
| HIF-1α | Transcription of genes involved in oxygen homeostasis | [59] |
| AQP4 | Resistance to cerebral edema | [62] |
| ATP8 and ATP6 | Mitochondrial ATPase assembly | [63] |
| DCC, GSTCD, MRPS28, and MOGAT2 | Adaptation to high-altitude environments | [64] |
| MT-ND1 and MT-ND2 | Electron transport chain of oxidative phosphorylation | [65] |
| GRIK4, IFNLR1, LOC102275985, GRHL3, and LOC102275713 | Physiological regulation under a hypoxic environment | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayalew, W.; Chu, M.; Liang, C.; Wu, X.; Yan, P. Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress. Animals 2021, 11, 2344. https://doi.org/10.3390/ani11082344
Ayalew W, Chu M, Liang C, Wu X, Yan P. Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress. Animals. 2021; 11(8):2344. https://doi.org/10.3390/ani11082344
Chicago/Turabian StyleAyalew, Wondossen, Min Chu, Chunnian Liang, Xiaoyun Wu, and Ping Yan. 2021. "Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress" Animals 11, no. 8: 2344. https://doi.org/10.3390/ani11082344
APA StyleAyalew, W., Chu, M., Liang, C., Wu, X., & Yan, P. (2021). Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress. Animals, 11(8), 2344. https://doi.org/10.3390/ani11082344






