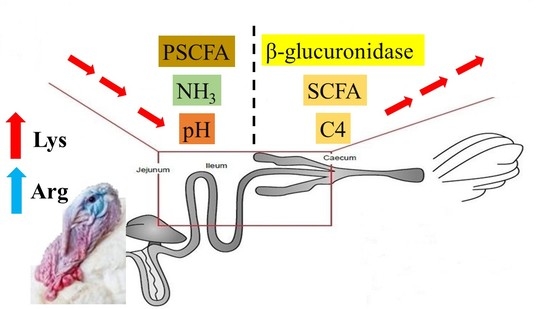
Increased Dietary Inclusion Levels of Lysine Are More Effective than Arginine in Supporting the Functional Status of the Gut in Growing Turkeys
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Parameters Analyzed
2.2.1. Performance Analysis
2.2.2. Analysis of Carcass Characteristics
2.2.3. Functional Status of the Gut
2.3. Statistical Analysis
3. Results and Discussion
3.1. Bird Performance, Carcass Characteristics and the Associated Traits
3.2. Functional Status of the Gut
3.3. Activity of Cecal Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Item | 1–4 wk | 5–8 wk | 9–12 wk | 13–16 wk | 1–16 wk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBWG (g) | DFI (g) | FCR (g/g) | DBWG (g) | DFI (g) | FCR (g/g) | DBWG (g) | DFI (g) | FCR (g/g) | DBWG (g) | DFI (g) | FCR (g/g) | DBWG (g) | DFI (g) | FCR (g/g) | |
| Treatment 1 | |||||||||||||||
| LLowALow | 37.9 | 52.7 | 1.39 | 108.1 | 185.9 | 1.72 | 151.1 | 362.0 | 2.40 | 116.4 | 410.0 | 3.53 | 99.3 | 235.3 | 2.37 |
| LLowAHigh | 38.4 | 53.6 | 1.40 | 110.3 | 188.3 | 1.71 | 151.5 | 361.2 | 2.39 | 112.7 | 411.9 | 3.67 | 99.1 | 235.5 | 2.38 |
| LHighALow | 37.3 | 51.8 | 1.39 | 106.8 | 181.5 | 1.70 | 152.6 | 357.6 | 2.35 | 116.8 | 415.5 | 3.57 | 98.7 | 233.0 | 2.36 |
| LHighAHigh | 38.0 | 52.7 | 1.39 | 111.9 | 191.8 | 1.71 | 150.4 | 361.5 | 2.41 | 112.7 | 406.6 | 3.61 | 99.5 | 236.8 | 2.38 |
| SEM | 0.245 | 0.36 | 0.004 | 0.934 | 1.510 | 0.007 | 1.060 | 1.821 | 0.011 | 1.302 | 2.553 | 0.031 | 0.471 | 1.188 | 0.008 |
| Lysine (Lys) | |||||||||||||||
| Low | 38.2 | 53.2 | 1.39 | 109.2 | 187.1 | 1.72 | 151.3 | 361.6 | 2.39 | 114.6 | 411.0 | 3.60 | 99.2 | 235.4 | 2.37 |
| High | 37.7 | 52.3 | 1.39 | 109.4 | 186.6 | 1.71 | 151.5 | 359.5 | 2.38 | 114.7 | 411.0 | 3.59 | 99.1 | 234.9 | 2.37 |
| Arginine (Arg) | |||||||||||||||
| Low | 37.6 | 52.3 | 1.39 | 107.5 | 183.7 b | 1.71 | 151.8 | 359.8 | 2.37 | 116.6 | 412.7 | 3.55 | 99.0 | 234.1 | 2.37 |
| High | 38.2 | 53.2 | 1.39 | 111.1 | 190.1 a | 1.71 | 150.9 | 361.4 | 2.40 | 112.7 | 409.2 | 3.64 | 99.3 | 236.1 | 2.38 |
| p value | |||||||||||||||
| Lys | 0.317 | 0.216 | 0.496 | 0.917 | 0.865 | 0.589 | 0.937 | 0.580 | 0.496 | 0.948 | 0.989 | 0.893 | 0.921 | 0.844 | 0.854 |
| Arg | 0.238 | 0.226 | 0.805 | 0.057 | 0.032 | 0.884 | 0.688 | 0.678 | 0.280 | 0.152 | 0.509 | 0.157 | 0.764 | 0.422 | 0.460 |
| Lys×Arg | 0.877 | 0.958 | 0.819 | 0.433 | 0.173 | 0.378 | 0.573 | 0.538 | 0.127 | 0.954 | 0.309 | 0.488 | 0.660 | 0.474 | 0.666 |
| BW before Slaughter, kg | Carcass | Edible Giblets | Muscle | Abdominal Fat | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Gizzard | Heart | Breast | Thigh | Drumstick | Total Muscle | ||||
| Treatment 1 | ||||||||||
| LLowALow | 11.32 | 81.51 | 1.29 | 0.51 | 0.32 | 21.18 | 10.55 | 7.71 | 39.45 | 1.71 |
| LLowAHigh | 11.30 | 81.68 | 1.35 | 0.49 | 0.31 | 21.11 | 10.80 | 8.02 | 39.92 | 1.76 |
| LHighALow | 11.39 | 81.68 | 1.48 | 0.49 | 0.29 | 21.24 | 10.55 | 8.18 | 39.97 | 2.02 |
| LHighAHigh | 11.39 | 81.82 | 1.32 | 0.44 | 0.32 | 21.57 | 10.36 | 7.89 | 39.81 | 1.91 |
| SEM | 0.057 | 0.199 | 0.039 | 0.012 | 0.005 | 0.183 | 0.104 | 0.122 | 0.271 | 0.069 |
| Lysine (Lys) | ||||||||||
| Low | 11.31 | 81.60 | 1.32 | 0.50 | 0.32 | 21.15 | 10.67 | 7.86 | 39.68 | 1.73 |
| High | 11.39 | 81.75 | 1.40 | 0.47 | 0.31 | 21.40 | 10.45 | 8.03 | 39.89 | 1.96 |
| Arginine (Arg) | ||||||||||
| Low | 11.35 | 81.60 | 1.39 | 0.50 | 0.31 | 21.21 | 10.55 | 7.94 | 39.71 | 1.86 |
| High | 11.35 | 81.75 | 1.33 | 0.47 | 0.31 | 21.34 | 10.58 | 7.95 | 39.87 | 1.83 |
| p value | ||||||||||
| Lys | 0.501 | 0.716 | 0.328 | 0.136 | 0.325 | 0.500 | 0.304 | 0.501 | 0.717 | 0.105 |
| Arg | 0.959 | 0.717 | 0.472 | 0.177 | 0.345 | 0.742 | 0.917 | 0.970 | 0.783 | 0.819 |
| Lys × Arg | 0.917 | 0.972 | 0.163 | 0.643 | 0.131 | 0.604 | 0.312 | 0.245 | 0.582 | 0.565 |
| Item | pH24 | Color | Protein, % | ||
|---|---|---|---|---|---|
| Lightness, L* | Redness, a* | Yellowness, b* | |||
| Treatment 1 | |||||
| LLowALow | 5.75 | 56.28 | 4.25 | 10.93 | 25.34 |
| LLowAHigh | 5.72 | 56.05 | 3.74 | 11.33 | 25.13 |
| LHighALow | 5.58 | 55.23 | 4.35 | 11.59 | 25.34 |
| LHighAHigh | 5.70 | 54.92 | 4.14 | 11.42 | 25.20 |
| SEM | 0.041 | 0.419 | 0.121 | 0.163 | 0.071 |
| Lysine (Lys) | |||||
| Low | 5.73 | 56.16 | 4.00 | 11.13 | 25.23 |
| High | 5.64 | 55.08 | 4.24 | 11.51 | 25.27 |
| Arginine (Arg) | |||||
| Low | 5.66 | 55.76 | 4.30 | 11.26 | 25.34 |
| High | 5.71 | 55.48 | 3.94 | 11.38 | 25.16 |
| p value | |||||
| Lys | 0.266 | 0.216 | 0.310 | 0.260 | 0.823 |
| Arg | 0.562 | 0.754 | 0.143 | 0.724 | 0.231 |
| Lys × Arg | 0.365 | 0.961 | 0.522 | 0.389 | 0.792 |
References
- Ravindran, V.; Adeola, O.; Rodehutscord, M.; Kluth, H.; van der Klis, J.D.; van Eerden, E.; Helmbrecht, A. Determination of ileal digestibility of amino acids in raw materials for broiler chickens—Results of collaborative studies and assay recommendations. Anim. Feed Sci. Technol. 2017, 225, 62–72. [Google Scholar] [CrossRef]
- Sohel, M.M.H. Macronutrient modulation of mRNA and microRNA function in animals: A review. Anim. Nutr. 2020, 6, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.L.; Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Effect of protein sources on performance characteristics of turkeys in the first three weeks of life. Anim. Nutr. 2019, 5, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Lemme, A.; Frackenpohl, U.; Petri, A.; Meyer, H. Effects of reduced dietary protein concentrations with amino acid supplementation on performance and carcass quality in Turkey toms 14 to 140 days of age. Int. J. Poult. Sci. 2004, 3, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Applegate, T.; Powers, W.; Angel, R.; Hoehler, D. Effect of amino acid formulation and amino acid supplementation on performance and nitrogen excretion in Turkey toms. Poult. Sci. 2008, 87, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet. Q. 2021, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Keerqin, C.; Wu, S.-B.; Svihus, B.; Swick, R.; Morgan, N.; Choct, M. An early feeding regime and a high-density amino acid diet on growth performance of broilers under subclinical necrotic enteritis challenge. Anim. Nutr. 2017, 3, 25–32. [Google Scholar] [CrossRef]
- British United Turkeys (BUT). Aviagen Turkeys. Feeding Guidelines for Nicholas and B.U.T. Heavy Lines. Available online: https://www.aviagenturkeys.com/uploads/2015/11/20/NU06%20Feeding%20Guidelines%20for%20Nicholas%20&%20BUT%20Heavy%20Lines%20EN.pdf (accessed on 2 February 2021).
- National Research Council. Nutrient Requirements of Poultry; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Ognik, K.; Całyniuk, Z.; Mikulski, D.; Stępniowska, A.; Konieczka, P.; Jankowski, J. The effect of different dietary ratios of lysine, arginine and methionine on biochemical parameters and hormone secretion in turkeys. J. Anim. Physiol. Anim. Nutr. 2021, 105, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Beski, S.S.M.; Swick, R.A.; Iji, P.A. Specialized protein products in broiler chicken nutrition: A review. Anim. Nutr. 2015, 1, 47–53. [Google Scholar] [CrossRef]
- Jankowski, J.; Mikulski, D.; Mikulska, M.; Ognik, K.; Całyniuk, Z.; Mróz, E.; Zduńczyk, Z. The effect of different dietary ratios of arginine, methionine, and lysine on the performance, carcass traits, and immune status of turkeys. Poult. Sci. 2020, 99, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Ognik, K.; Konieczka, P.; Mikulski, D. Effects of different levels of arginine and methionine in a high-lysine diet on the immune status, performance, and carcass traits of turkeys. Poult. Sci. 2020, 99, 4730–4740. [Google Scholar] [CrossRef]
- Jankowski, J.; Ognik, K.; Całyniuk, Z.; Stępniowska, A.; Konieczka, P.; Mikulski, D. The effect of different dietary ratios of lysine, arginine and methionine on protein nitration and oxidation reactions in turkey tissues and DNA. Animal 2021, 15, 100183. [Google Scholar] [CrossRef]
- Ognik, K.; Konieczka, P.; Mikulski, D.; Jankowski, J. The effect of different dietary ratios of lysine and arginine in diets with high or low methionine levels on oxidative and epigenetic DNA damage, the gene expression of tight junction proteins and selected metabolic parameters in Clostridium perfringens-challenged turkeys. Vet. Res. 2020, 51, 50. [Google Scholar] [CrossRef] [Green Version]
- Aviagen. Management Guidelines for Growing Commercial Turkeys. Available online: https://www.aviagenturkeys.com/uploads/2016/08/30/Management%20Guidelines%20for%20Growing%20Commercial%20Turkeys_UK.pdf (accessed on 2 February 2021).
- Ognik, K.; Mikulski, D.; Konieczka, P.; Tykałowski, B.; Krauze, M.; Stępniowska, A.; Nynca, A.; Jankowski, J. The immune status, oxidative and epigenetic changes in tissues of turkeys fed diets with different ratios of arginine and lysine. Sci. Rep. 2021, 11, 15975. [Google Scholar] [CrossRef]
- Konieczka, P.; Mikulski, D.; Ognik, K.; Juśkiewicz, J.; Zduńczyk, Z.; Józefiak, D.; Jankowski, J. Chemically preserved high-moisture corn in the turkey diet does not compromise performance and maintains the functional status of the gut. Anim. Feed Sci. Technol. 2020, 263, 114483. [Google Scholar] [CrossRef]
- AOAC. Agricultural Chemicals Official Methods of Analysis; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2007. [Google Scholar]
- ISO 1871. Food and Feed Products–General Guidelines for the Determination of Nitrogen by the Kjeldahl Method; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Juskiewicz, J.; Gruzauskas, R.; Zdunczyk, Z.; Semaskaite, A.; Jankowski, J.; Totilas, Z.; Jarule, V.; Sasyte, V.; Zdunczyk, P.; Raceviciute-Stupeliene, A.; et al. Effects of dietary addition of Macleaya cordata alkaloid extract on growth performance, caecal indices and breast meat fatty acids profile in male broilers. J. Anim. Physiol. Anim. Nutr. 2011, 95, 171–178. [Google Scholar] [CrossRef]
- Gugołek, A.; Juśkiewicz, J.; Strychalski, J.; Zwoliński, C.; Żary-Sikorska, E.; Konstantynowicz, M. The effects of rapeseed meal and legume seeds as substitutes for soybean meal on productivity and gastrointestinal function in rabbits. Arch. Anim. Nutr. 2017, 71, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Konieczka, P.; Szkopek, D.; Kinsner, M.; Fotschki, B.; Juśkiewicz, J.; Banach, J. Cannabis-derived cannabidiol and nanoselenium improve gut barrier function and affect bacterial enzyme activity in chickens subjected to C. perfringens challenge. Vet. Res. 2020, 51, 141. [Google Scholar] [CrossRef] [PubMed]
- StatSoft Inc. Statistica (Data Analysis Software System), Version 13. Available online: www.statsoft.com (accessed on 16 December 2020).
- Oso, A.O.; Williams, G.A.; Oluwatosin, O.O.; Bamgbose, A.M.; Adebayo, A.O.; Olowofeso, O.; Pirgozliev, V.; Adegbenjo, A.A.; Osho, S.O.; Alabi, J.O.; et al. Effect of dietary supplementation with arginine on haematological indices, serum chemistry, carcass yield, gut microflora, and lymphoid organs of growing turkeys. Livest. Sci. 2017, 198, 58–64. [Google Scholar] [CrossRef]
- Veldkamp, T.; Kwakkel, R.P.; Ferket, P.R.; Simons, P.C.M.; Noordhuizen, J.P.T.M.; Pijpers, A. Effects of ambient temperature, arginine-to-lysine ratio, and electrolyte balance on performance, carcass, and blood parameters in commercial male turkeys. Poult. Sci. 2000, 79, 1608–1616. [Google Scholar] [CrossRef]
- Veldkamp, T.; Kwakkel, R.; Ferket, P.; Kogut, J.; Verstegen, M. Growth responses to dietary lysine at high and low ambient temperature in male turkeys. Poult. Sci. 2003, 82, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Guan, S.; Li, T.; Huang, R.; Wu, G.; Ruan, Z.; Yin, Y. Dietary l-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br. J. Nutr. 2011, 105, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, M.; Kong, Y.; Ma, H.; Zou, S. Comparison of the novel compounds creatine and pyruvateon lipid and protein metabolism in broiler chickens. Animal 2011, 5, 1082–1089. [Google Scholar] [CrossRef] [Green Version]
- Kidd, M.T.; Kerr, B.J. Dietary arginine and lysine ratios in Large White toms. 2. Lack of interaction between arginine: Lysine ratios and electrolyte balance. Poult. Sci. 1998, 77, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Passantino, A.; Khan, R.U.; Tufarelli, V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, K.D.; Callaway, T.R.; Chalova, V.I.; McReynolds, J.L.; Hume, M.E.; Dunkley, C.S.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Foodborne Salmonella ecology in the avian gastrointestinal tract. Anaerobe 2009, 15, 26–35. [Google Scholar] [CrossRef]
- Waititu, S.M.; Yitbarek, A.; Matini, E.; Echeverry, H.; Kiarie, E.; Rodriguez-Lecompte, J.C.; Nyachoti, C.M. Effect of supplementing direct-fed microbials on broiler performance, nutrient digestibilities, and immune responses. Poult. Sci. 2014, 93, 625–635. [Google Scholar] [CrossRef]
- Shazali, N.; Loh, T.C.; Foo, H.L.; Samsudin, A.A. Gut microflora and intestinal morphology changes of broiler chickens fed reducing dietary protein supplemented with lysine, methionine, and threonine in tropical environment. Rev. Bras. Zootec. 2019, 48. [Google Scholar] [CrossRef]
- Tellez, G., Jr.; Arreguin-Nava, M.A.; Maguey, J.A.; Michel, M.A.; Latorre, J.D.; Merino-Guzman, R.; Hernandez-Velasco, X.; Moore, P.A., Jr.; Hargis, B.M.; Tellez-Isaias, G. Effect of Bacillus-direct-fed microbial on leaky gut, serum peptide YY concentration, bone mineralization, and ammonia excretion in neonatal female turkey poults fed with a rye-based diet. Poult. Sci. 2020, 99, 4514–4520. [Google Scholar] [CrossRef]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Balnave, D.; Barke, J. Re-evaluation of the classical dietary arginine:lysine interaction for modern poultry diets: A review. World’s Poult. Sci. J. 2002, 58, 275–289. [Google Scholar] [CrossRef]
- Ball, R.O.; Urschel, K.L.; Pencharz, P.B. Nutritional consequences of interspecies differences in arginine and lysine metabolism. J. Nutr. 2007, 137, 1626S–1641S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, F.L.d.S.; Kim, W.K. Secondary functions of arginine and sulfur amino acids in poultry health: Review. Animals 2020, 10, 2106. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, G.; Kobayashi, H.; Shibata, M.; Kubota, M.; Kadowaki, M.; Fujimura, S. Reduction in dietary lysine increases muscle free amino acids through changes in protein metabolism in chickens. Poult. Sci. 2020, 99, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Drażbo, A.A.; Juśkiewicz, J.; Józefiak, A.; Konieczka, P. The fermentation process improves the nutritional value of rapeseed cake for Turkeys-effects on performance, gut bacterial population and its fermentative activity. Animals 2020, 10, 1711. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.L.; Keita, A.V.; Parsons, B.N.; Prorok-Hamon, M.; Knight, P.; Winstanley, C.; O’Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Soluble plantain fibre blocks adhesion and M-cell translocation of intestinal pathogens. J. Nutr. Biochem. 2013, 24, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Guyard-Nicodème, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P.; et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef]
- Bjerrum, L.; Engberg, R.M.; Leser, T.D.; Jensen, B.B.; Finster, K.; Pedersen, K. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poult. Sci. 2006, 85, 1151–1164. [Google Scholar] [CrossRef]
- Zhang, B.; Gan, L.; Shahid, M.S.; Lv, Z.; Fan, H.; Liu, D.; Guo, Y. In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. J. Anim. Sci. Biotechnol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Bederska-Łojewska, D.; Świątkiewicz, S.; Arczewska-Włosek, A.; Schwarz, T. Rye non-starch polysaccharides: Their impact on poultry intestinal physiology, nutrients digestibility and performance indices—A review. Ann. Anim. Sci. 2017, 17, 351–369. [Google Scholar] [CrossRef] [Green Version]
- Beaud, D.; Tailliez, P.; Anba-Mondoloni, J. Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 2005, 151, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
| Item | Feeding Period, wk | |||
|---|---|---|---|---|
| 1–4 | 5–8 | 9–12 | 13–16 | |
| Ingredients | ||||
| Wheat | 56.454 | 56.576 | 64.037 | 64.472 |
| Maize | - | - | - | 10.000 |
| Soybean meal, 48% CP 2 | 25.380 | 27.722 | 21.121 | 9.324 |
| Rapeseed meal | 4.566 | 5.000 | 6.000 | 7.000 |
| Potato protein | 5.000 | - | - | - |
| Soybean oil | 0.892 | 3.088 | 4.725 | 4.371 |
| Maize gluten meal | 3.000 | 2.769 | - | 2.000 |
| Sodium bicarbonate | 0.200 | 0.200 | 0.100 | 0.200 |
| Sodium chloride | 0.158 | 0.161 | 0.217 | 0.095 |
| Limestone | 2.046 | 1.766 | 1.391 | 1.075 |
| Monocalcium phosphate | 1.707 | 1.390 | 1.409 | 0.558 |
| L-lysine HCl | - | 0.317 | 0.347 | 0.300 |
| DL-methionine | 0.195 | 0.231 | 0.163 | 0.072 |
| L-threonine | 0.052 | 0.131 | 0.139 | 0.182 |
| Choline chloride | 0.100 | 0.100 | 0.100 | 0.100 |
| Vitamin–mineral premix 3 | 0.250 | 0.250 | 0.250 | 0.250 |
| Titanium oxide | - | 0.300 | - | - |
| Calculated nutrient content 4 | ||||
| Metabolizable energy, kcal/kg | 2800 | 2900 | 3000 | 3150 |
| Crude protein | 26.35 (26.79) | 24.0 (24.33) | 20.3 (20.31) | 17.0 (17.35) |
| Arginine | 1.52 (1.50) | 1.42 (1.38) | 1.21 (1.25) | 0.92 (0.89) |
| Lysine | 1.30 (1.23) | 1.35 (1.36) | 1.20 (1.18) | 0.90 (0.76) |
| Methionine | 0.62 (0.61) | 0.59 (0.56) | 0.46 (0.45) | 0.35 (0.38) |
| Methionine + Cysteine | 1.09 (1.06) | 1.03 (0.97) | 0.85 (0.83) | 0.71 (0.72) |
| Threonine | 1.05 (1.03) | 0.97 (0.94) | 0.84 (0.83) | 0.76 (0.78) |
| Calcium | 1.25 | 1.10 | 0.95 | 0.65 |
| Available phosphorus | 0.65 | 0.55 | 0.47 | 0.32 |
| Item | Treatment 2 | |||
|---|---|---|---|---|
| LLowALow | LLowAHigh | LHighALow | LHighAHigh | |
| 1–4 wk | ||||
| L-lysine HCl | 0.47 | 0.47 | 0.67 | 0.67 |
| L-arginine HCl | 0.02 | 0.18 | 0.17 | 0.35 |
| DL-methionine | 0 | 0 | 0 | 0 |
| 5–8 wk | ||||
| L-lysine HCl | 0.18 | 0.18 | 0.37 | 0.37 |
| L-arginine HCl | 0.05 | 0.20 | 0.19 | 0.35 |
| DL-methionine | 0.03 | 0.03 | 0.03 | 0.03 |
| 9–12 wk | ||||
| L-lysine HCl | 0.15 | 0.15 | 0.32 | 0.32 |
| L-arginine HCl | 0 | 0.12 | 0.11 | 0.25 |
| DL-methionine | 0.06 | 0.06 | 0.06 | 0.06 |
| 13–16 wk | ||||
| L-lysine HCl | 0.30 | 0.30 | 0.43 | 0.43 |
| L-arginine HCl | 0.06 | 0.16 | 0.16 | 0.27 |
| DL-methionine | 0.01 | 0.01 | 0.01 | 0.01 |
| Item | Small Intestine | Ceca | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Villus Height, µm | Crypt Depth, µm | Villus Height/Crypt Depth | Mass with Digesta | Viscosity, Ileal | Dry Matter, Ileal | pH, Ileal | Tissue | Digesta | Dry Matter | NH3 | pH | |
| g/kg BW | mPa s | % | g/kg BW | g/kg BW | % | mg/g | ||||||
| Group (n = 8) 1 | ||||||||||||
| LLowALow | 1264.9 | 134.3 | 9.43 | 28.0 | 2.46 | 17.2 | 6.26 | 3.34 | 3.46 | 13.8 | 0.713 a | 6.52 |
| LLowAHigh | 1271.8 | 137.8 | 9.24 | 27.7 | 2.29 | 17.5 | 6.20 | 3.35 | 3.11 | 14.6 | 0.771 a | 6.56 |
| LHighALow | 1246.4 | 133.0 | 9.38 | 26.8 | 2.53 | 17.4 | 6.28 | 3.33 | 4.08 | 13.5 | 0.662 ab | 6.33 |
| LHighAHigh | 1267.6 | 135.0 | 9.40 | 26.9 | 2.49 | 16.7 | 6.13 | 3.64 | 4.31 | 14.9 | 0.560 b | 6.10 |
| SEM | 8.59 | 0.97 | 0.081 | 0.620 | 0.097 | 0.268 | 0.059 | 0.098 | 0.211 | 0.330 | 0.023 | 0.066 |
| Lysine (Lys) | ||||||||||||
| Low | 1268.3 | 136.0 | 9.34 | 27.9 | 2.37 | 17.4 | 6.23 | 3.34 | 3.28 b | 14.2 | 0.742 | 6.54 a |
| High | 1257.0 | 134.0 | 9.39 | 26.8 | 2.51 | 17.0 | 6.21 | 3.49 | 4.19 a | 14.2 | 0.611 | 6.21 b |
| SEM | ||||||||||||
| Arginine (Arg) | ||||||||||||
| Low | 1255.6 | 133.6 | 9.41 | 27.4 | 2.50 | 17.3 | 6.27 | 3.33 | 3.77 | 13.7 | 0.688 | 6.42 |
| High | 1269.7 | 136.4 | 9.32 | 27.3 | 2.39 | 17.1 | 6.17 | 3.49 | 3.71 | 14.8 | 0.666 | 6.33 |
| SEM | 48.85 | 5.35 | 0.465 | |||||||||
| p value | ||||||||||||
| Lys | 0.528 | 0.307 | 0.751 | 0.441 | 0.490 | 0.577 | 0.835 | 0.482 | 0.033 | 0.998 | <0.001 | 0.012 |
| Arg | 0.434 | 0.163 | 0.615 | 0.942 | 0.604 | 0.731 | 0.405 | 0.435 | 0.885 | 0.108 | 0.571 | 0.461 |
| Lys × Arg | 0.688 | 0.699 | 0.555 | 0.828 | 0.742 | 0.386 | 0.749 | 0.479 | 0.484 | 0.645 | 0.044 | 0.277 |
| Concentration, μmol/g | SCFA Profile, % | SCFA Pool | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C4i | C4 | C5i | C5 | PSCFA | Total SCFA | C2 | C3 | C4 | ||
| Group (n = 8) 1 | ||||||||||||
| LLowALow | 48.5 a | 5.17 | 0.472 | 12.6 | 0.699 | 0.588 | 1.76 | 68.1 | 71.3 a | 7.63 | 18.4 b | 236 |
| LLowAHigh | 39.9 b | 5.23 | 0.385 | 16.5 | 0.679 | 0.764 | 1.83 | 63.5 | 62.8 b | 8.13 | 26.2 a | 199 |
| LHighALow | 47.9 a | 5.27 | 0.308 | 18.6 | 0.410 | 0.810 | 1.53 | 73.3 | 65.4 b | 7.06 | 25.4 a | 298 |
| LHighAHigh | 53.2 a | 5.87 | 0.245 | 20.7 | 0.288 | 0.664 | 1.20 | 80.9 | 65.7 b | 7.16 | 25.7 a | 350 |
| SEM | 1.380 | 0.460 | 0.039 | 0.806 | 0.047 | 0.045 | 0.086 | 1.909 | 0.855 | 0.587 | 0.904 | 18.2 |
| Lysine (Lys) | ||||||||||||
| Low | 44.2 | 5.20 | 0.428 | 14.6 b | 0.689 a | 0.676 | 1.79 a | 65.8 b | 67.1 | 7.88 | 22.3 | 217 b |
| High | 50.5 | 5.57 | 0.276 | 19.6 a | 0.349 b | 0.737 | 1.36 b | 77.1 a | 65.6 | 7.11 | 25.5 | 323 a |
| Arginine (Arg) | ||||||||||||
| Low | 48.2 | 5.22 | 0.390 | 15.6 b | 0.555 | 0.699 | 1.64 | 70.7 | 68.4 | 7.34 | 21.9 | 267 |
| High | 46.6 | 5.55 | 0.315 | 18.6 a | 0.484 | 0.714 | 1.51 | 72.2 | 64.3 | 7.64 | 25.9 | 274 |
| p value | ||||||||||||
| Lys | 0.009 | 0.704 | 0.052 | <0.001 | <0.001 | 0.500 | 0.010 | <0.001 | 0.275 | 0.535 | 0.036 | 0.002 |
| Arg | 0.473 | 0.734 | 0.325 | 0.029 | 0.349 | 0.867 | 0.408 | 0.646 | 0.005 | 0.805 | 0.012 | 0.819 |
| Lys × Arg | 0.005 | 0.776 | 0.876 | 0.475 | 0.500 | 0.083 | 0.210 | 0.066 | 0.003 | 0.872 | 0.017 | 0.168 |
| Glucosidase | Galactosidase | β-Glucuronidase | α-Arabinopyranosidase | α-Arabinofuranosidase | β-Xylosidase | β-Cellobiosidase | |||
|---|---|---|---|---|---|---|---|---|---|
| α- | β- | α- | β- | ||||||
| Group (n = 8) 1 | |||||||||
| LLowALow | 27.2 | 7.09 b | 60.5 b | 35.2 | 61.8 | 3.30 | 2.52 | 6.13 | 0.143 |
| LLowAHigh | 34.4 | 8.59 b | 72.4 b | 57.4 | 43.1 | 4.66 | 5.00 | 9.52 | 0.199 |
| LHighALow | 29.2 | 8.78 b | 58.9 b | 26.1 | 67.5 | 3.69 | 3.18 | 8.66 | 0.156 |
| LHighAHigh | 48.7 | 17.7 a | 104.0 a | 44.9 | 66.0 | 6.75 | 6.19 | 16.6 | 0.257 |
| SEM | 2.333 | 1.116 | 4.917 | 3.430 | 3.310 | 0.363 | 0.300 | 0.954 | 0.012 |
| Lysine (Lys) | |||||||||
| Low | 30.8 b | 7.83 | 66.5 | 46.3 | 52.4 b | 3.98 b | 3.76 b | 7.82 b | 0.171 |
| High | 38.9 a | 13.2 | 81.3 | 35.5 | 66.7 a | 5.22 a | 4.68 a | 12.6 a | 0.207 |
| Arginine (Arg) | |||||||||
| Low | 28.2 b | 7.93 | 59.7 | 30.7 b | 64.7 | 3.49 b | 2.85 b | 7.39 b | 0.149 b |
| High | 41.6 a | 13.2 | 88.0 | 51.1 a | 54.5 | 5.71 a | 5.60 a | 13.1 a | 0.228 a |
| p value | |||||||||
| Lys | 0.039 | 0.004 | 0.067 | 0.069 | 0.022 | 0.039 | 0.005 | <0.001 | 0.067 |
| Arg | <0.001 | 0.005 | <0.001 | <0.001 | 0.096 | <0.001 | <0.001 | <0.001 | <0.001 |
| Lys × Arg 2 | 0.111 | 0.041 | 0.044 | 0.770 | 0.157 | 0.147 | 0.398 | 0.104 | 0.236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konieczka, P.; Mikulski, D.; Ognik, K.; Juśkiewicz, J.; Zduńczyk, Z.; Jankowski, J. Increased Dietary Inclusion Levels of Lysine Are More Effective than Arginine in Supporting the Functional Status of the Gut in Growing Turkeys. Animals 2021, 11, 2351. https://doi.org/10.3390/ani11082351
Konieczka P, Mikulski D, Ognik K, Juśkiewicz J, Zduńczyk Z, Jankowski J. Increased Dietary Inclusion Levels of Lysine Are More Effective than Arginine in Supporting the Functional Status of the Gut in Growing Turkeys. Animals. 2021; 11(8):2351. https://doi.org/10.3390/ani11082351
Chicago/Turabian StyleKonieczka, Paweł, Dariusz Mikulski, Katarzyna Ognik, Jerzy Juśkiewicz, Zenon Zduńczyk, and Jan Jankowski. 2021. "Increased Dietary Inclusion Levels of Lysine Are More Effective than Arginine in Supporting the Functional Status of the Gut in Growing Turkeys" Animals 11, no. 8: 2351. https://doi.org/10.3390/ani11082351
APA StyleKonieczka, P., Mikulski, D., Ognik, K., Juśkiewicz, J., Zduńczyk, Z., & Jankowski, J. (2021). Increased Dietary Inclusion Levels of Lysine Are More Effective than Arginine in Supporting the Functional Status of the Gut in Growing Turkeys. Animals, 11(8), 2351. https://doi.org/10.3390/ani11082351