Effects of Condensed Tannins Supplementation on Animal Performance, Phylogenetic Microbial Changes, and In Vitro Methane Emissions in Steers Grazing Winter Wheat
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experiment 1 (In Vivo Experiment)
Condensed Tannins Preparation and Supplementation
2.3. Experiment 2
Effect of Ruminal Fluid on In Vitro Gas and Methane Production in Steers Fed Condensed Tannins
2.4. DNA Extraction and Denaturing Gradient Gel Electrophoresis
2.5. Chemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Chemical Composition of Forages
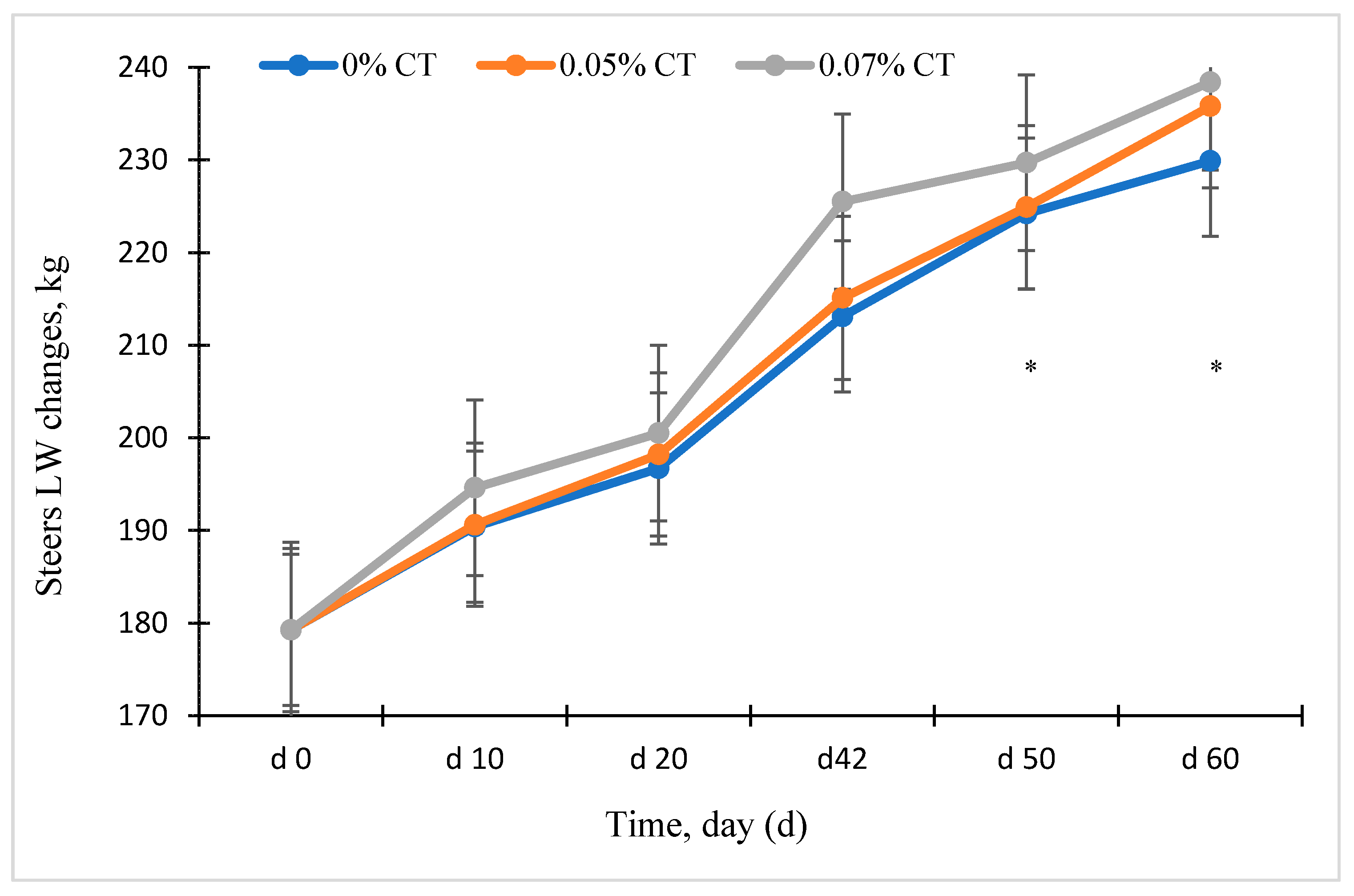
3.2. Experiment 1: Effects of Condensed Tannins on Animal Performance
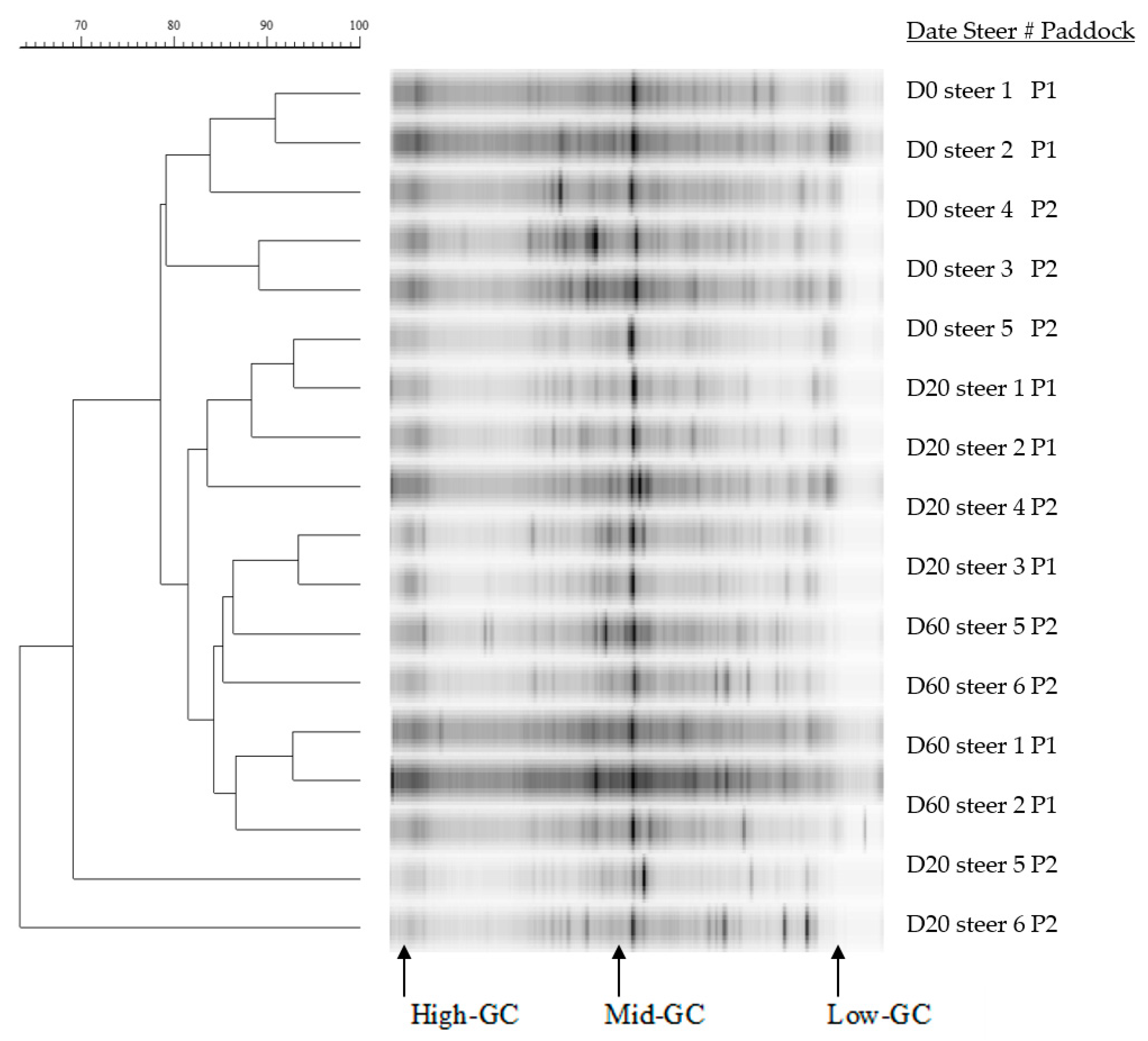
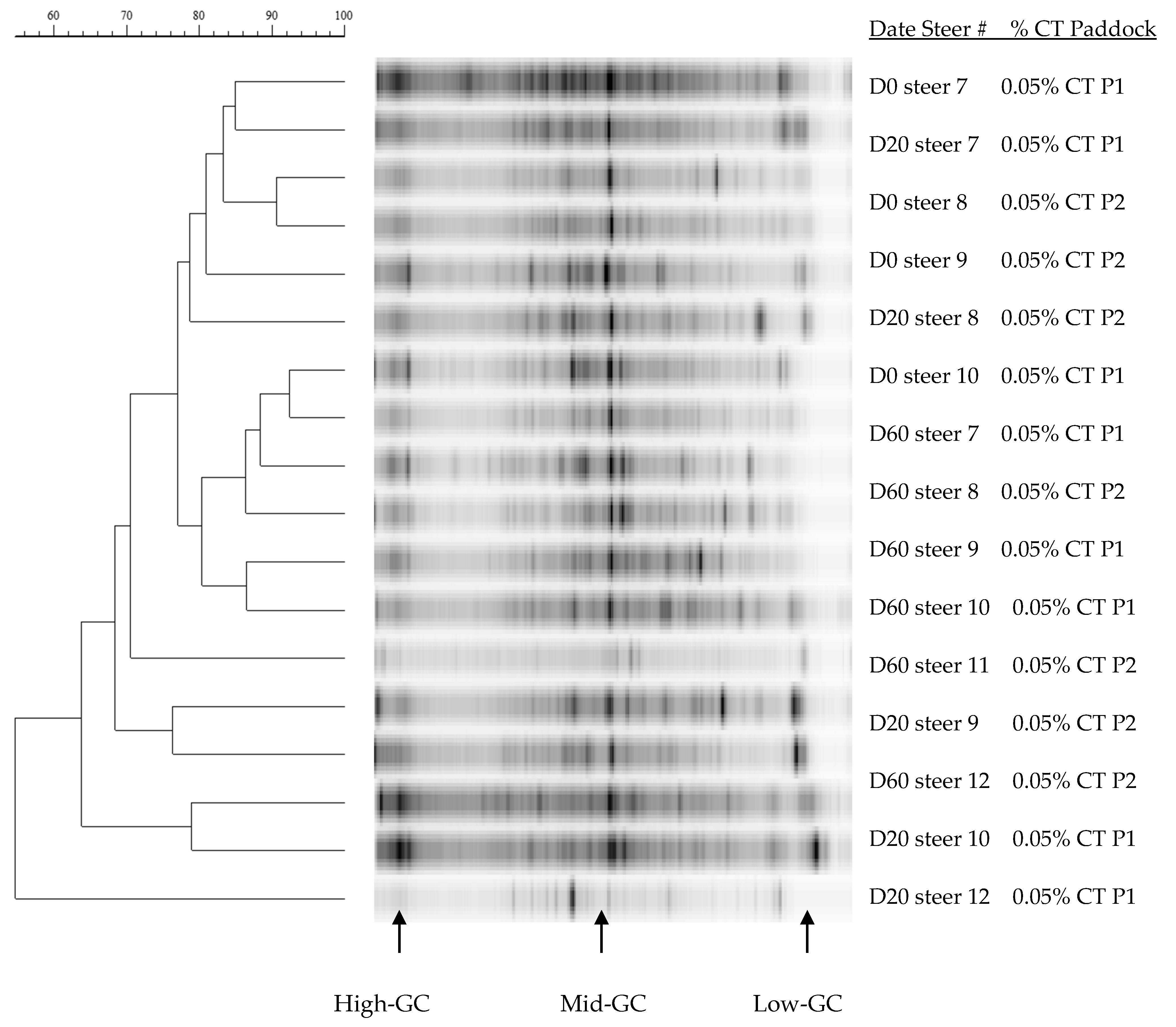
3.3. DNA Concentration and Ruminal Microbiota Changes
3.4. Experiment 2: In Vitro Gas and Methane Production in Rumen Fluid from Steers Fed Condensed Tannins
4. Discussion
4.1. Exp. 1: Animal Performance
4.2. Rumen Bacterial Diversity
4.3. Exp. 2: Condensed Tannins and In Vitro Ruminal Methane Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitta, D.W.; Pinchak, W.E.; Indugu, N.; Vecchiarelli, B.; Sinha, R.; Fulford, J.D. Metagenomic analysis of the rumen microbiome of steers with wheat-induced frothy bloat. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majak, W.; McAllister, T.A.; McCartney, D.; Stanford, K.; Cheng, K.J. Bloat in Cattle. In Alberta Agriculture Food and Rural Develop; Information Package Center Press: Edmonton, AB, Canada, 2003; pp. 1–28. [Google Scholar]
- Min, B.R.; Pinchak, W.E.; Hernandez, C.; Hume, M.E. Grazing activity and ruminal bacterial population associated with frothy bloat in steers grazing winter wheat. Prof. Anim. Sci. 2013, 29, 179–187. [Google Scholar] [CrossRef]
- Pinchak, W.E.; Min, B.R.; Malinowski, D.P.; Sij, J.W.; Gill, R.J.; Puchala, R.; Anderson, R.A. Re-evaluation of the frothy bloat complex in cattle grazing winter wheat in the southern plains: Evolution of a new integrated. In Proceedings of the 2005 Conference on Gastrointestinal Function, Chicago, IL, USA, 11–13 April 2005; p. 36. [Google Scholar]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Hume, M.E. In vitro bacterial growth and in vivo ruminal microbiota populations associated with bloat in steers grazing wheat forage. J. Anim. Sci. 2006, 84, 2873–2882. [Google Scholar] [CrossRef]
- Min, B.R.; McTear, K.; Wang, H.H.; Joakin, H.; Gurung, N.; Abrahamsen, F.; Solaiman, S.; Eun, J.S.; Lee, J.H.; Dietz, L.A.; et al. Influence of elevated protein and tannin-rich peanut skin supplementation on growth performance, blood metabolites, carcass traits, and immune-related gene expression of grazing meat goats. J. Anim. Physiol. Anim. Nutr. 2019, 104, 88–100. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannins mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Robins, C.T.; Weerasuriya, Y.; Wilson, T.C.; Mcarthur, V. Tannin chemistry in relation to digestion. J. Range Manag. 1992, 45, 57–62. [Google Scholar] [CrossRef]
- Jeronimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Baptista, E.S.; Lopes, O.; Silva, F.C. Tannins in ruminant nutrition: Impact on animal performance and quality of edible products. In Tannins Biochemistry, Food Sources and Nutritional Properties; Combs, C.A., Ed.; Biochemistry Research Trends: New York, NY, USA, 2016; pp. 121–168. [Google Scholar]
- Aboagye, I.; Beauchemin, K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Woodward, S.L.; Waghorn, G.C.; Ulyatt, M.J.; Lassey, K.R. Early indications that feeding Lotus will reduce methane emissions from ruminants. Proc. N. Z. Soc. Anim. Prod. 2001, 61, 23–26. [Google Scholar]
- Waghorn, G.C.; Tavendale, M.H.; Woodfield, D.R. Methanogenesis from forages fed to sheep. Proc. N. Z. Grass. Assoc. 2002, 64, 167–171. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S. Comparative aspects of plant tannins on digestive physiology, nutrition and microbial changes in sheep and goats: A review. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1181–1193. [Google Scholar] [CrossRef] [Green Version]
- Hume, M.E.; Kubena, L.F.; Edrington, T.S.; Donskey, C.J.; Moore, R.W.; Ricke, S.C.; Nisbet, D.J. Poultry digestive microflora biodiversity as indicated by denaturing gradient gel electrophoresis. Poult. Sci. 2003, 82, 1100–1107. [Google Scholar] [CrossRef]
- Ricke, S.C.; Hume, M.E.; Park, S.Y.; Moore, R.W.; Birkhold, S.G.; Kubena, L.F.; Nisbet, D.J. Denaturing gradient gel electrophoresis (DGGE) as a rapid method for assessing gastrointestinal tract microflora responses in laying hens fed similar zinc molt induction diets. J. Rapid Methods Autom. Microbiol. 2004, 12, 69–81. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Klenk, H.P.; Goker, M. Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. Int. J. Sys. Evol. Microbiol. 2014, 64, 352–356. [Google Scholar] [CrossRef]
- Reichenberger, E.R.; Rosen, G.; Hershberg, U.; Hershberg, R. Prokaryotic Nucleotide Composition Is Shaped by Both Phy-logeny and the Environment. Genome Biol. Evol. 2015, 7, 1380–1389. [Google Scholar] [CrossRef] [Green Version]
- Golder, H.M.; Thomson, J.M.; Denman, S.E.; McSweeney, C.S.; Lean, I.J. Genetic Markers Are Associated with the Ruminal Microbiome and Metabolome in Grain and Sugar Challenged Dairy Heifers. Front. Genet. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudelli, N.M.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Bardran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Hahnke, R.L.; Meier-Kolthoff, J.P.; Garcia-Lopez, M.; Mukheree, S.; Huntemann, M.; Ivanova, N.N.; Woyke, T.; Kyrpides, N.C.; Klenk, H.-P.; Göker, M. Genome-based taxonomic classification of Bacteroidetes. Front. Microbiol. 2016, 7, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Briggs, G.S.; Smits, N.K.; Soultanas, P. Chromosomal replication initiation machinery of low-G+C-content Firmicutes. J. Bacteriol. 2012, 194, 5162–5170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Tajima, K.; Aminov, R.I.; Nagamine, T.; Matsui, M.; Nakamura, M.; Benno, Y. Diet-dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl. Environ. Microbiol. 2001, 67, 2766–2774. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Wright, C.; Ho, P.; Eun, J.S.; Gurung, N.; Shange, R. The effect of phytochemical tannins-containing diet on rumen fermentation characteristics and microbial diversity dynamics in goats using 16S rDNA amplicon pyrosequencing. Agric. Food Anal. Bact. 2014, 4, 195–211. [Google Scholar]
- Wolf, M.; Müller, T.; Dandekar, T.; Pollack, J.D. Phylogeny of Firmicutes with special references to Mycoplasma (Mollicutes) as inferred from phosphoglycerate kinase amino acid sequence data. Int. J. Syst. Evol. Microbiol. 2004, 54, 871–875. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R. Firmicutes. In Current Protocols in Microbiology; Wiley Inter-Science, Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Wexler, H.M. Bacteroidetes: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, B.R.; Gurung, N.; Shange, R.; Solaiman, S. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing. J. Anim. Sci. 2019, 97, 3523–3534. [Google Scholar] [CrossRef] [PubMed]
- Min, B.R.; Castleberry, L.; Allen, H.; Parker, D.; Waldrop, H.; Brauer, D.; Willis, W. Associative effect of wet distillers’ grains plus solubles and tannin-rich peanut skin supplementation on in vitro rumen fermentation, greenhouse gas emissions, and microbiome changes. J. Anim. Sci. 2019, 7, 4668–4681. [Google Scholar] [CrossRef] [PubMed]
- Schodolski, J.S. Gastrointestinal microbiota. In Canine and Feline Gastroenterology; Washabau, R.J., Day, M.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 32–41. [Google Scholar] [CrossRef]
- Streit, W.; Fengel, D. Purified tannins from quebracho Colorado. Phytochemistry 1994, 36, 481–484. [Google Scholar] [CrossRef]
- Min, B.R.; McNabb, W.C.; Peters, J.S.; Barry, T.N. Solubilization and degradation of ribulose-1,5-bisphosphate carboxylase (Rubisco) protein from white clover (Trifolium repens) and Lotus corniculatus by rumen micro-organisms and the effect of condensed tannins on these processes. J. Agric. Sci. 2000, 134, 305–317. [Google Scholar] [CrossRef]
- Paisley, S.I.; Horn, G.W. Effect of Ionophore on Rumen Characteristics, Gas Production, and Occurrence of Bloat in Cattle Grazing Winter Wheat Pasture; 1998 Animal Science Research Report; Oklahoma Agricultural Experiment Station, Division of Agricultural Science and Natural Resources, Oklahoma State University: Stillwater, AL, USA, 1998; pp. 141–146. [Google Scholar]
- Min, B.R.; Pinchak, W.E.; Fulford, J.D.; Puchala, R. Wheat pasture bloat dynamics, in vitro ruminal gas production, and potential bloat mitigation with condensed tannins. J. Anim. Sci. 2005, 83, 1322–1331. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Pinchak, W.E.; Fulford, J.D. In vitro and in vivo rumen fermentation and gas production: Influence of corn and mineral oils and their bloat potential. Anim. Feed Sci. Technol. 2007, 133, 192–205. [Google Scholar] [CrossRef]
- Muyzer, G.; De Waal, E.C.; Litterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase-chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffield, V.C.; Cox, D.R.; Lerman, L.S.; Myers, R.M. Attachment of a 40-base-pair G+C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection by single-base changes. Proc. Natl. Acad. Sci. USA 1989, 86, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Reysenbach, A.L.; Giver, L.J.; Wickham, G.C.; Pace, N.R. Differential amplification of rRNA genes by polymerase chain reaction. Appl. Environ. Microbiol. 1992, 58, 3417–3418. [Google Scholar] [CrossRef] [Green Version]
- Don, R.H.; Cox, P.T.; Wainwright, M.J.; Baker, K.; Mattick, J.S. Touch down PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991, 19, 4008. [Google Scholar] [CrossRef] [Green Version]
- Wawer, C.; Muyzer, G. Genetic diversity of Desulfovibrio spp. in environmental samples analyzed by denaturing gradient gel electrophoresis of [NiFe] hydrogenase gene fragments. Appl. Environ. Microbiol. 1995, 61, 2203–2210. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein digestibility in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Woodward, S.L.; Waghorn, G.C.; Lassey, K.R.; Laboyrie, P.G. Does feeding sulla (Hedysarum coronarium) reduces methane emissions from dairy cows? Proc. N. Z. Soc. Anim. Prod. 2002, 62, 227–230. [Google Scholar]
- Puchala, R.; Min, B.R.; Goetsch, A.L.; Sahlu, T. The effect of a condensed tannin containing forage on methane emission by goats. J. Anim. Sci. 2005, 83, 182–191. [Google Scholar] [CrossRef]
- Krueger, W.K.; Gutierrez-Bañuelos, H.; Carstens, G.E.; Min, B.R.; Pinchak, W.E.; Gomez, R.R.; Anderson, R.C.; Krueger, N.A.; Forbes, T.D.A. Effects of dietary tannin source on performance, feed efficiency, ruminal fermentation, and carcass and non-carcass traits in steers fed a high-grain diet. Anim. Feed Sci. Technol. 2010, 159, 1–9. [Google Scholar] [CrossRef]
- Turner, S.A.; Waghorn, G.C.; Woodwards, S.L.; Thomson, N.A. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) affect the detailed composition of milk from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2005, 65, 283–289. [Google Scholar]
- Min, B.R.; McNabb, W.C.; Barry, T.N.; Kemp, P.D.; Waghorn, G.C.; McDonald, M.F. The effect of condensed tannins in Lotus corniculatus upon reproductive efficiency and wool production in sheep during late summer and autumn. J. Agric. Sci. 1999, 132, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Fulford, J.D.; Puchala, R. Effects of feed additives on in vitro and in vivo rumen characteristics and frothy bloat dynamics in steers grazing wheat pasture. Anim. Feed Sci. Technol. 2005, 123–124, 615–629. [Google Scholar] [CrossRef]
- Min, B.R.; Hart, S.P. Tannins for suppression of internal parasites. J. Anim. Sci. 2003, 81, E102–E109. [Google Scholar]
- Koenig, K.M.; Beauchemin, K.A. Effect of feeding condensed tannins in high protein finishing diets containing corn distiller’s grains on ruminal fermentation, nutrient digestibility, and route of nitrogen excretion in beef cattle. J. Anim. Sci. 2018, 96, 4398–4413. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Fulford, J.D.; Puchala, R. Effect of condensed tannins supplementation level on weight gain and in vitro and in vivo bloat precursors in steers grazing winter wheat. J. Anim. Sci. 2006, 84, 2546–2554. [Google Scholar] [CrossRef] [Green Version]
- Hervas, G.; Frutos, P.; Giraldez, F.J.; Mantecon, A.R.; Del Pino, M.C.A. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- Waghorn, G.C. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Waghorn, G.C. Condensed tannins and nutrient absorption from the small intestine. In Proceedings of the 1996 Canadian Society of Animal Science Annual Meeting; Rode, L.M., Ed.; Animal Science Research Development, Ministry of Supply & Services: Lethbridge, AB, Canada, 1996; pp. 175–189. [Google Scholar]
- Molan, A.L.; Attwood, G.T.; Min, B.R.; McNabb, W.C. The effect of condensed tannins from Lotus pedunculatus and Lotus corniculatus on the growth of proteolytic rumen bacteria in vitro and their possible mode of action. Can. J. Microbiol. 2001, 47, 626–633. [Google Scholar] [CrossRef]
- McNeill, D.M.; Osborne, N.; Komolong, M.K.; Nankervis, D. Condensed tannins in the genus Leucaena and their nutritional significance for ruminants. In Proceedings of the Workshop on Leucaena Adaptation, Quality and Farming Systems, Hanoi, Vietnam, 9–14 February 1998; Shelton, H.M., Gutteridge, R.C., Mullen, B.F., Bray, R.A., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 1998; Volume 86, pp. 205–214. [Google Scholar]
- Edwards, J.; McEwan, N.R.; Travis, A.J.; Wallace, R.J. 16S rDNA library-based analysis of ruminal bacterial diversity. Antonie Leeuwenhoek 2004, 86, 263–281. [Google Scholar] [CrossRef]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.; Ganda, E.K.; De Oliveira Filho, J.C.; Lorenzo, G.; Trojacanec, P.; Bicalho, R.C. Prepartum and postpartum rumen fluid microbiomes; characterization and correlation with production traits in dairy cows. Appl. Environ. Microbiol. 2015, 81, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; et al. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Micro. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.M.D.; Cabral, C.; Redondo, L.M.; Pin Viso, N.D.; Colombatto, D.; Farber, M.D.; Miyakawa, M.E.F. Impact of chestnut and quebracho tannins on rumen microbiota of bovines. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myer, P.R.; Smith, T.P.L.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen microbiome from steers differing in feed efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rum. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Min, B.R.; Attwood, G.T.; Reilly, K.; Sun, W.; Peters, J.S.; Barry, T.N.; McNabb, W.C. Lotus corniculatus condensed tannins decrease in vivo populations of proteolytic bacteria and affect nitrogen metabolism in the rumen of sheep. Can. J. Microbiol. 2002, 48, 911–921. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.; Cheng, K.J. Effects of sainfoin (Onobrychis viciifolia scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef] [Green Version]
- Min, B.R.; Attwood, G.T.; McNabb, W.C.; Molan, A.L.; Barry, T.N. The effect of condensed tannins from Lotus corniculatus on the proteolytic activities and growth of rumen bacteria. Anim. Feed Sci. Technol. 2005, 121, 45–58. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Hernandez, K.; Hernandez, C.; Hume, M.E.; Valencia, E.; Fulford, J.D. Effects of plant tannin supplementation on animal responses and in vivo ruminal bacterial populations associated with bloat in heifers grazing wheat forage. Prof. Anim. Sci. 2012, 28, 464–472. [Google Scholar] [CrossRef]
- Nelson, K.E.; Pell, A.N.; Doane, P.H.; Giner-Chavez, B.I.; Schofield, P. Chemical and biological assays to evaluate bacterial inhibition by tannins. J. Chem. Ecol. 1997, 23, 1175–1194. [Google Scholar] [CrossRef]
- Brooker, J.D.; O’Donovan, L.A.; Skene, I.; Clarke, K.; Blackall, L.; Muslera, P. Streptococcus caprinus sp. Nov., a tannin-resistant ruminal bacterium from feral goats. Lett. Appl. Microbiol. 1994, 18, 313–318. [Google Scholar] [CrossRef]
- Plumb, J.J.; Blackall, L.L.; Klieve, A.V. Rumen bacterial diversity with and without mulga (Acasia anuera) tannins. In Tannins in Livestock and Human Nutrition; Brooker, J.D., Ed.; Australian Centre for International Agricultural Research (ACIAR): Adelaide, Australia, 2000; pp. 156–160. [Google Scholar]
- Min, B.R.; Perkins, D.; Wright, C.; Dawod, A.; Min, B.R.; Terrill, T.H.; Eun, J.S.; Shange, R.; Yang, S.Y.; Gurung, N. Effects of feeding two different tannin-containing diets on ruminal fermentation profiles and microbial community changes in meat goats. Agric. Food Anal. Bacteriol. 2015, 5, 153–165. [Google Scholar]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochem. Biophys. Acta Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Nelson, K.E.; Thonney, M.L.; Woolston, T.K.; Zinder, S.H.; Pell, A.N. Phenotypic and phylogenetic characterization of ruminal tannin-tolerant bacteria. Appl. Environ. Microbiol. 1998, 64, 3824–3830. [Google Scholar] [CrossRef] [Green Version]
- Whitford, M.F.; Foster, R.J.; Beard, C.E.; Gong, J.; Teather, R.M. Phylogenetic analysis of rumen bacteria by comparative sequence analysis of cloned 16S rRNA genes. Anaerobe 1998, 4, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kocherginskaya, S.A.; Aminov, R.A.; White, B.A. Analysis of the rumen bacterial diversity under two different diet conditions using denaturing gradient gel electrophoresis, random sequencing, and statistical ecology approaches. Anaerobe 2001, 7, 119–134. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; Auldist, M.J.; Beauchemin, K.A.; McGinn, S.M.; Waghorn, G.C.; Eckard, R.J. Potential use of Acacia mearnsii condensed tannins to reduce methane emissions and nitrogen excretion from grazing dairy cows. Can. J. Anim. Sci. 2009, 89, 241–251. [Google Scholar] [CrossRef] [Green Version]
- McMahon, L.R.; Majak, W.; McAllister, T.A.; Hall, J.W.; Jones, G.A.; Popp, J.D.; Cheng, K.J. Effect of sainfoin on in vitro digestion of fresh alfalfa and bloat in steers. Can. J. Anim. Sci. 1999, 79, 203–212. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S. Methane mitigation from ruminants using tannins and saponins, a status review. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Jayanegara, A.; Leiber, F.; Kreuzer, M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J. Anim. Phys. Anim. Nut. 2012, 96, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. The divergence between purified hydrolyzable and condensed tannin effects on methane emission, rumen fermentation, and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saminathan, M.; Sieo, C.C.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W. Effects of condensed tannin fractions of different molecular weights on population and diversity of bovine rumen methanogenic archaea in vitro, as determined by high-throughput sequencing. Anim. Feed Sci. Technol. 2016, 216, 146–160. [Google Scholar] [CrossRef]
- Naumann, H.; Sepela, R.; Rezaire, A.; Masih, S.E.; Zeller, W.E.; Reinhardt, L.A.; Robe, J.T.; Sullivan, M.L.; Hagerman, A.E. Relationships between structures of condensed tannins from Texas legumes and methane production during in vitro rumen digestion. Molecules 2018, 23, 2123. [Google Scholar] [CrossRef] [Green Version]

| Item | Winter Wheat Forage (DM Basis) | |||
|---|---|---|---|---|
| 7 March | 17 April | SEM | p-Value | |
| Dry matter | 92.2 | 93.5 | 1.52 | 0.15 |
| Crude protein | 29.5 a | 13.7 b | 1.26 | 0.001 |
| Neutral detergent fiber | 42.2 b | 47.9 a | 0.80 | 0.01 |
| Acid detergent fiber | 26.8 | 27.5 | 3.36 | 0.11 |
| IVDMD, % | 90.1 a | 85.4 b | 1.32 | 0.001 |
| Item | Percentage of CT/kg BW | SEM | p-Value | ||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.07 | |||
| Number of steers | 6 | 6 | 6 | - | - |
| Animal performance | |||||
| Day 0, before co-variate | 170.3 | 152.3 | 179.6 | 7.10 | 0.21 |
| Day 0, after co-variate 1 | 181.7 | 181.7 | 181.7 | - | - |
| Day 10 | 192.9 | 192.6 | 197.1 | 2.37 | 0.28 |
| Day 20 | 199.6 | 200.7 | 201.3 | 2.13 | 0.34 |
| Day 42 | 215.1 | 217.6 | 226.2 | 7.04 | 0.32 |
| Day 50 | 226.8 | 227.7 | 232.3 | 2.03 | 0.10 |
| Day 60, final BW | 232.3 b | 238.4 a,b | 246.0 a | 2.50 | 0.04 |
| ADG, kg/d | 0.84 b | 0.94 a,b | 1.00 a | 0.045 | 0.04 |
| p-value | |||||
| CT | - | - | - | - | 0.17 |
| Time | - | - | - | - | 0.01 |
| CT by time interaction | - | - | - | - | 0.65 |
| Item | Percentage of CT/kg BW | SEM | p-Value | ||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.07 | |||
| Number of steers | 6 | 6 | 6 | - | - |
| Ruminal DNA concentration (ng/µL) | |||||
| Day 0 | 27.5 | 24.9 | 24.7 | 3.85 | 0.87 |
| Day 20 | 65.4 | 75.2 | 80.7 | 12.51 | 0.20 |
| Day 60 | 100.4 | 112.5 | 129.3 | 12.61 | 0.06 |
| Average | 64.4 b | 70.8 a,b | 78.2 a | 9.65 | 0.04 |
| p-value | |||||
| CT | - | - | - | - | 0.12 |
| Time | - | - | - | - | 0.001 |
| CT by time of grazing interaction | - | - | - | - | 0.84 |
| Item 1 | Percent of CT/kg BW | ||||
|---|---|---|---|---|---|
| 0 | 0.05 | 0.07 | SEM | p-Value | |
| In vitro gas production | |||||
| Total gas, mL/12 h | 58.3 a | 41.5 b | 36.0 b | 3.28 | 0.01 |
| Total gas, mL/g DM | 33.7 a | 23.9 b | 20.9 b | 1.52 | 0.001 |
| Gas production parameters 1 | |||||
| Gas potential (a + b) | 72.3 a | 52.9 b | 49.4 b | 5.11 | 0.01 |
| Gas rate (c) 2 | 0.133 | 0.107 | 0.110 | 0.020 | 0.39 |
| CH4 production | |||||
| CH4 (mL/g DM) | 0.91 a | 0.73 a,b | 0.26 b | 0.09 | 0.001 |
| CH4 (mg/g DM) | 0.65 a | 0.52 a,b | 0.19 b | 0.07 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, B.R.; Pinchak, W.E.; Hume, M.E.; Anderson, R.C. Effects of Condensed Tannins Supplementation on Animal Performance, Phylogenetic Microbial Changes, and In Vitro Methane Emissions in Steers Grazing Winter Wheat. Animals 2021, 11, 2391. https://doi.org/10.3390/ani11082391
Min BR, Pinchak WE, Hume ME, Anderson RC. Effects of Condensed Tannins Supplementation on Animal Performance, Phylogenetic Microbial Changes, and In Vitro Methane Emissions in Steers Grazing Winter Wheat. Animals. 2021; 11(8):2391. https://doi.org/10.3390/ani11082391
Chicago/Turabian StyleMin, Byeng R., William E. Pinchak, Michael E. Hume, and Robin C. Anderson. 2021. "Effects of Condensed Tannins Supplementation on Animal Performance, Phylogenetic Microbial Changes, and In Vitro Methane Emissions in Steers Grazing Winter Wheat" Animals 11, no. 8: 2391. https://doi.org/10.3390/ani11082391






