Feeding Ecology of Wild Brown-Nosed Coatis and Garbage Exploration: A Study in Two Ecological Parks
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Parks Studied
2.2. Collection and Processing of Samples
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kiper, T. Role of ecotourism in sustainable development. In Advances in Landscape Architecture; IntechOpen: London, UK, 2013; pp. 773–802. [Google Scholar]
- Piñeiro, A.; Barja, I.; Silván, G.; Illera, J.C. Effects of tourist pressure and reproduction on physiological stress response in wildcats: Management implications for species conservation. Wildl. Res. 2012, 39, 532–539. [Google Scholar] [CrossRef]
- Boyle, S.A.; Samson, F.B. Effects of nonconsumptive recreation on wildlife: A review. Wildl. Soc. Bull. 1985, 1, 110–116. [Google Scholar]
- Duffus, D.A.; Dearden, P. Non-consumptive wildlife-oriented recreation: A conceptual framework. Biol. Conserv. 1990, 53, 213–231. [Google Scholar] [CrossRef]
- Lott, D.F.; McCoy, M. Asian rhinos Rhinoceros unicornis on the run? Impact of tourist visits on one population. Biol. Conserv. 1995, 73, 23–26. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Effects of ecotourists on bird behaviour at Loxahatchee National Wildlife Refuge, Florida. Environ. Conserv. 1998, 25, 13–21. [Google Scholar] [CrossRef]
- Budowski, G. Tourism and environmental conservation: Conflict, coexistence, or symbiosis? Environ. Conserv. 1976, 3, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Jim, C.Y. Visitor management in recreation areas. Environ. Conserv. 1989, 16, 19–32. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Daszak, P.; Cunnigham, A.A.; Hyatt, A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001, 78, 103–116. [Google Scholar] [CrossRef]
- Alves-Costa, C.P.; Fonseca, G.A.B.; Christófaro, C. Variation in the diet of the brown-nosed coati (Nasua nasua) in southeastern Brazil. J. Mammal. 2004, 85, 478–482. [Google Scholar] [CrossRef]
- dos Santos, V.A.; Beisiegel, B.M. A dieta de Nasua nasua (Linnaeus, 1766) no Parque Ecológico do Tietê, SP. Rev. Bras. Zoociências 2006, 8, 199–203. [Google Scholar]
- Ferreira, G.A.; Nakano-Oliveira, E.; Genaro, G.; Lacerda-Chaves, A.K. Diet of the coati Nasua nasua (Carnivora: Procyonidae) in an area of woodland inserted in an urban environment in Brazil. Rev. Chil. Hist. Nat. 2013, 86, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Gabrey, S.W. Bird and small mammal abundance at four types of waste-management facilities in northeast Ohio. Landsc. Urban Plant. 1997, 37, 223–233. [Google Scholar] [CrossRef]
- Ross, G.A. Ibis in urban Sydney: A gift from Ra or a pharaoh’s curse? In Urban. Wildlife: More than Meets the Eye; Lunney, D., Burgin, S., Eds.; Royal Zoological Society of New South Wales: Sydney, Australia, 2004; pp. 148–152. [Google Scholar]
- MMA Ministério do Meio Ambiente. Plano de Manejo–Parque Nacional do Caparaó. Available online: https://www.icmbio.gov.br/parnacaparao/plano-de-manejo (accessed on 21 January 2020).
- Projeto Quatis. Available online: https://sites.google.com/site/projetoquatis/parque-das-mangabeiras (accessed on 22 January 2020).
- Zalewski, A. Does size dimorphism reduce competition between sexes? The diet of male and female pine martens at local and wider geographical scales. Acta Theriol. 2007, 52, 237–250. [Google Scholar] [CrossRef]
- Maehr, D.S.; Brady, J.R. Food habits of bobcats in Florida. J. Mammal. 1986, 67, 133–138. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team 202. R 4.0.0. Available online: https://www.r-project.org/ (accessed on 12 April 2020).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Newbury Park, CA, USA, 2018. [Google Scholar]
- Olifiers, N.; Bianchi, R.C.; D’Andrea, P.S.; Mourão, G.; Gompper, M.E. Estimating age of carnivores from the Pantanal region of Brazil. Wildl. Biol. 2010, 16, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.D.; Minchin, P.; Minchin, R.; O’Hara, G.; Simpson, P.; Solymos, M.; et al. Vegan: Community Ecology Package (R package Version 2.5-5) 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 22 January 2020).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Prosser, C.L. Comparative Animal Physiology, Environmental and Metabolic Animal Physiology, 4th ed.; Wiley-Liss: Hoboken, NJ, USA, 1991; pp. 1–12. ISBN 978-0-471-85767-9. [Google Scholar]
- Louzada, M.L.C.; Bortoletto, A.P.; Canella, D.S.; Baraldi, L.G.; Levy, R.B.; Claro, R.M.; Moubarac, J.-C.; Cannon, G.; Monteiro, C.A. Alimentos ultraprocessados e perfil nutricional da dieta no Brasil. Rev. Saúde Pública 2015, 49, 38–49. [Google Scholar]
- Gompper, M.E. Sociality and asociality in white-nosed coatis (Nasua narica): Foraging costs and benefits. Behav. Ecol. 1996, 7, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Clutton-Brock, T.H.; Iason, G.R.; Guiness, F.E. Sexualsegregation and density related changes in habitat use in female andmale red deer (Cervus elaphus L.). J. Zool. 1987, 211, 275–289. [Google Scholar] [CrossRef]
- Kamilar, J.M.; Pokempner, A.A. Does body mass dimorphism increase male–female dietary niche separation? A comparative study of primates. Behaviour 2008, 145, 1211–1234. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Coogan, S.C.P.; Raubenheimer, D. Do wild carnivores forage for prey or nutrients? BioEssays 2015, 37, 701–709. [Google Scholar] [CrossRef]
- McNab, B.K. The Physiological Ecology of Vertebrates: A View from Energetics. Ithaca and London; Comstock Publishing Associates: New York, NY, USA, 2002; p. 576. [Google Scholar]
- Hayward, A.; Gillooly, J.F. The cost of sex: Quantifying energetic investment in gamete production by males and females. PLoS ONE 2011, 6, e16557. [Google Scholar] [CrossRef]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Koike, S.; Morimoto, H.; Goto, Y.; Kozakai, C.; Yamazaki, K. Insectivory by five sympatric carnivores in cool-temperate deciduous forests. Mammal. Study 2012, 37, 73–83. [Google Scholar] [CrossRef]
- Aronson, L.R.; Brockman, D.J.; Brown, D.C. Gastrointestinal Emergencies. Vet. Clin. N. Am. 2000, 30, 555–579. [Google Scholar] [CrossRef]
- Aguiar, L.M.; Moro-Rios, R.F.; Silvestre, T.; Silva-Pereira, J.E.; Bilski, D.R.; Passos, F.C.; Sekiama, M.L.; Rocha, V.J. Diet of brown-nosed coatis and crab-eating raccoons from a mosaic landscape with exotic plantations in southern Brazil. Stud. Neotrop. Fauna Environ. 2011, 46, 153–161. [Google Scholar] [CrossRef]
- Beisiegel, B.M.; Mantovani, W.H. Habitat use, home range and foraging preferences of the coati Nasua nasua in pluvial tropical Atlantic Forest area. J. Zool. 2006, 269, 77–87. [Google Scholar] [CrossRef]
- Hirsch, B.T. Seasonal variation in the diet of ring-tailed coatis (Nasua nasua) in Iguazu, Argentina. J. Mammal. 2009, 90, 136–143. [Google Scholar] [CrossRef] [Green Version]


| PMMF_PMMM | Av. Dissim | SD | Average Female | Average Male | Cumulative | Cumulative % | Contrib. % |
| Invertebrates | 0.30 | 0.26 | 258.67 | 95.20 | 0.43 | 42.79 | 42.79 |
| Vegetal | 0.21 | 0.21 | 57.00 | 145.40 | 0.73 | 73.48 | 30.69 |
| Garbage | 0.11 | 0.19 | 17.50 | 48.00 | 0.89 | 88.84 | 15.36 |
| Vertebrates | 0.08 | 0.10 | 37.92 | 29.60 | 1.00 | 100.00 | 11.16 |
| NI | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 100.00 | 0.00 |
| PMMF_PNCF | Av. Dissim | SD | Average Female | Average Male | Cumulative | Cumulative % | Contrib. % |
| Invertebrates | 0.35 | 0.22 | 258.67 | 374.11 | 0.51 | 50.78 | 50.78 |
| Vegetal | 0.21 | 0.18 | 57.00 | 314.83 | 0.82 | 81.96 | 31.18 |
| Vertebrates | 0.10 | 0.11 | 37.92 | 95.61 | 0.97 | 97.26 | 15.29 |
| Garbage | 0.02 | 0.03 | 17.50 | 5.56 | 1.00 | 99.99 | 2.74 |
| NI | 0.00 | 0.00 | 0.00 | 0.11 | 1.00 | 100.00 | 0.01 |
| PMMF_PNCM | Av. Dissim | SD | Average Female | Average Male | Cumulative | Cumulative % | Contrib. % |
| Vegetal | 0.35 | 0.28 | 57.00 | 437.75 | 0.50 | 49.96 | 49.96 |
| Invertebrates | 0.25 | 0.27 | 258.67 | 108.00 | 0.86 | 86.10 | 36.14 |
| Vertebrates | 0.07 | 0.09 | 37.92 | 50.50 | 0.96 | 96.29 | 10.19 |
| Garbage | 0.03 | 0.03 | 17.50 | 14.00 | 1.00 | 100.00 | 3.71 |
| NI | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 100.00 | 0.00 |
| PMMM_PNCF | Av. Dissim | SD | Average Female | Average Male | Cumulative | Cumulative % | Contrib. % |
| Invertebrates | 0.29 | 0.17 | 95.20 | 374.11 | 0.45 | 45.02 | 45.02 |
| Vegetal | 0.22 | 0.17 | 145.40 | 314.83 | 0.79 | 78.85 | 33.83 |
| Vertebrates | 0.08 | 0.08 | 29.60 | 95.61 | 0.91 | 90.98 | 12.12 |
| Garbage | 0.06 | 0.12 | 48.00 | 5.56 | 1.00 | 99.99 | 9.01 |
| NI | 0.00 | 0.00 | 0.00 | 0.11 | 1.00 | 100.00 | 0.01 |
| PMMM_PNCM | Av. Dissim | SD | Average Female | Average Male | Cumulative | Cumulative % | Contrib. % |
| Vegetal | 0.37 | 0.23 | 145.40 | 437.75 | 0.54 | 54.22 | 54.22 |
| Invertebrates | 0.19 | 0.19 | 95.20 | 108.00 | 0.82 | 81.50 | 27.29 |
| Garbage | 0.07 | 0.16 | 48.00 | 14.00 | 0.92 | 92.47 | 10.97 |
| Vertebrates | 0.05 | 0.04 | 29.60 | 50.50 | 1.00 | 100.00 | 7.53 |
| NI | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 100.00 | 0.00 |
| PNCF_PNCM | Av. Dissim | SD | Average Female | Average Male | Cumulative | Cumulative % | Contrib. % |
| Vegetal | 0.30 | 0.22 | 314.83 | 437.75 | 0.49 | 49.21 | 49.21 |
| Invertebrates | 0.23 | 0.20 | 374.11 | 108.00 | 0.87 | 86.92 | 37.70 |
| Vertebrates | 0.07 | 0.08 | 95.61 | 50.50 | 0.98 | 98.25 | 11.33 |
| Garbage | 0.01 | 0.01 | 5.56 | 14.00 | 1.00 | 99.99 | 1.75 |
| NI | 0.00 | 0.00 | 0.11 | 0.00 | 1.00 | 100.00 | 0.01 |
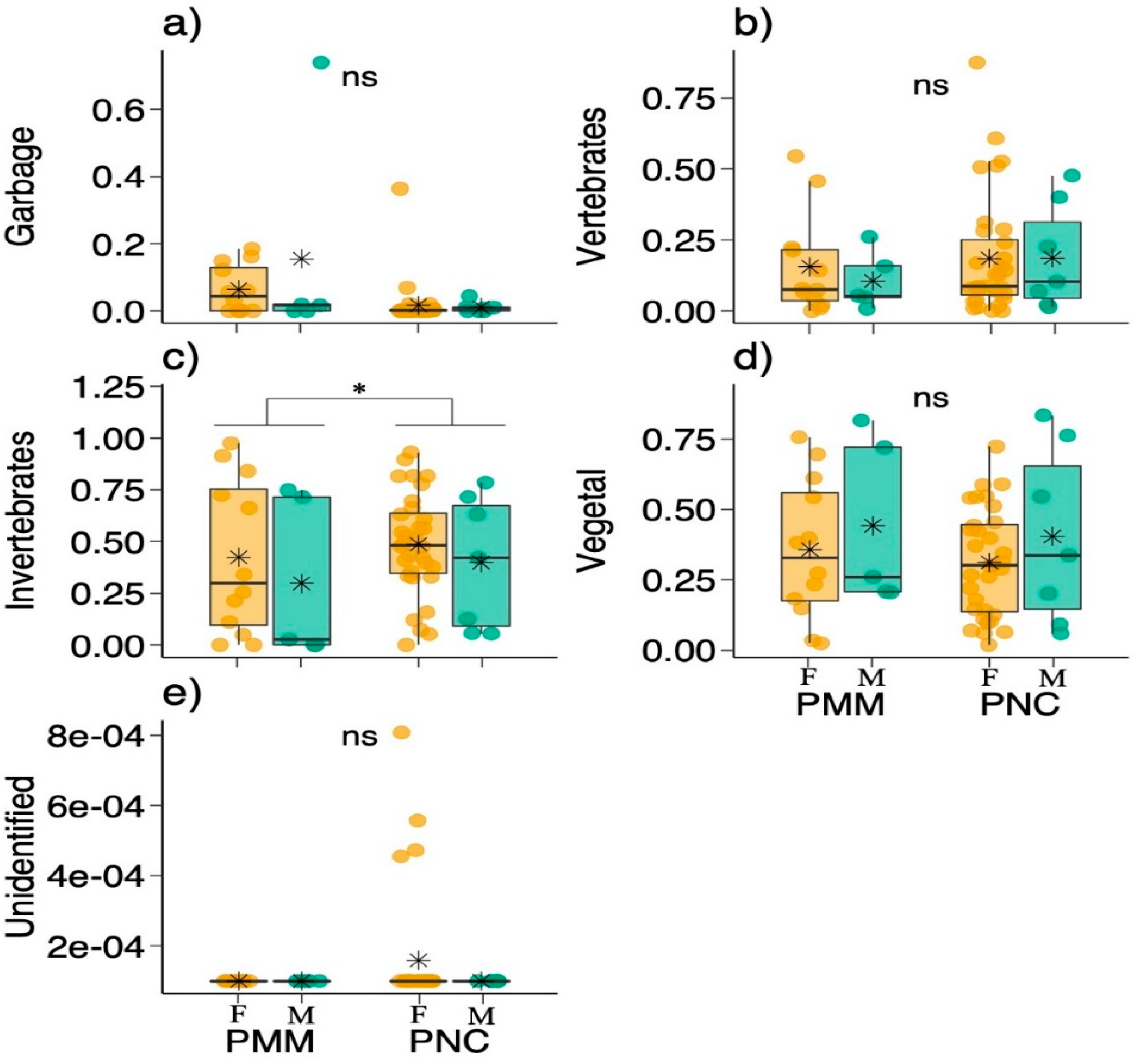
| Response | LRT | Df | p-Value |
|---|---|---|---|
| Garbage | |||
| Sex | 0.065 | 1 | 0.798 |
| Place | 1.724 | 1 | 0.189 |
| Sex:Place | 0.023 | 1 | 0.879 |
| Vertebrates | |||
| Sex | 0.043 | 1 | 0.836 |
| Place | 0.686 | 1 | 0.407 |
| Sex:Place | 0.012 | 1 | 0.913 |
| Invertebrates | |||
| Sex | 1.717 | 1 | 0.190 |
| Place | 4.635 | 1 | 0.031 * |
| Sex:Place | 0.757 | 1 | 0.384 |
| Vegetation | |||
| Sex | 1.497 | 1 | 0.221 |
| Place | 0.235 | 1 | 0.628 |
| Sex:Place | 0.069 | 1 | 0.792 |
| Unidentified | |||
| Sex | 0.426 | 1 | 0.514 |
| Place | 0.794 | 1 | 0.373 |
| Sex:Place | 0.255 | 1 | 0.614 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, D.H.; Calixto, E.; Cesario, C.S.; Repoles, R.B.; de Paula Lopes, W.; Oliveira, V.S.; Brinati, A.; Hemetrio, N.S.; Silva, I.O.; Boere, V. Feeding Ecology of Wild Brown-Nosed Coatis and Garbage Exploration: A Study in Two Ecological Parks. Animals 2021, 11, 2412. https://doi.org/10.3390/ani11082412
Rodrigues DH, Calixto E, Cesario CS, Repoles RB, de Paula Lopes W, Oliveira VS, Brinati A, Hemetrio NS, Silva IO, Boere V. Feeding Ecology of Wild Brown-Nosed Coatis and Garbage Exploration: A Study in Two Ecological Parks. Animals. 2021; 11(8):2412. https://doi.org/10.3390/ani11082412
Chicago/Turabian StyleRodrigues, Delma Henriques, Eduardo Calixto, Clarice Silva Cesario, Renata Barcelos Repoles, Waldomiro de Paula Lopes, Viviane Silva Oliveira, Alessandro Brinati, Nadja Simbera Hemetrio, Ita Oliveira Silva, and Vanner Boere. 2021. "Feeding Ecology of Wild Brown-Nosed Coatis and Garbage Exploration: A Study in Two Ecological Parks" Animals 11, no. 8: 2412. https://doi.org/10.3390/ani11082412








