Sexual Differentiation and Primordial Germ Cell Distribution in the Early Horse Fetus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Micro-Computed Tomography (microCT) Scanning
2.3. Immunohistochemistry
2.4. Multiplex Immunofluorescence
2.5. Molecular Analyses
2.6. Statistical Analysis
3. Results
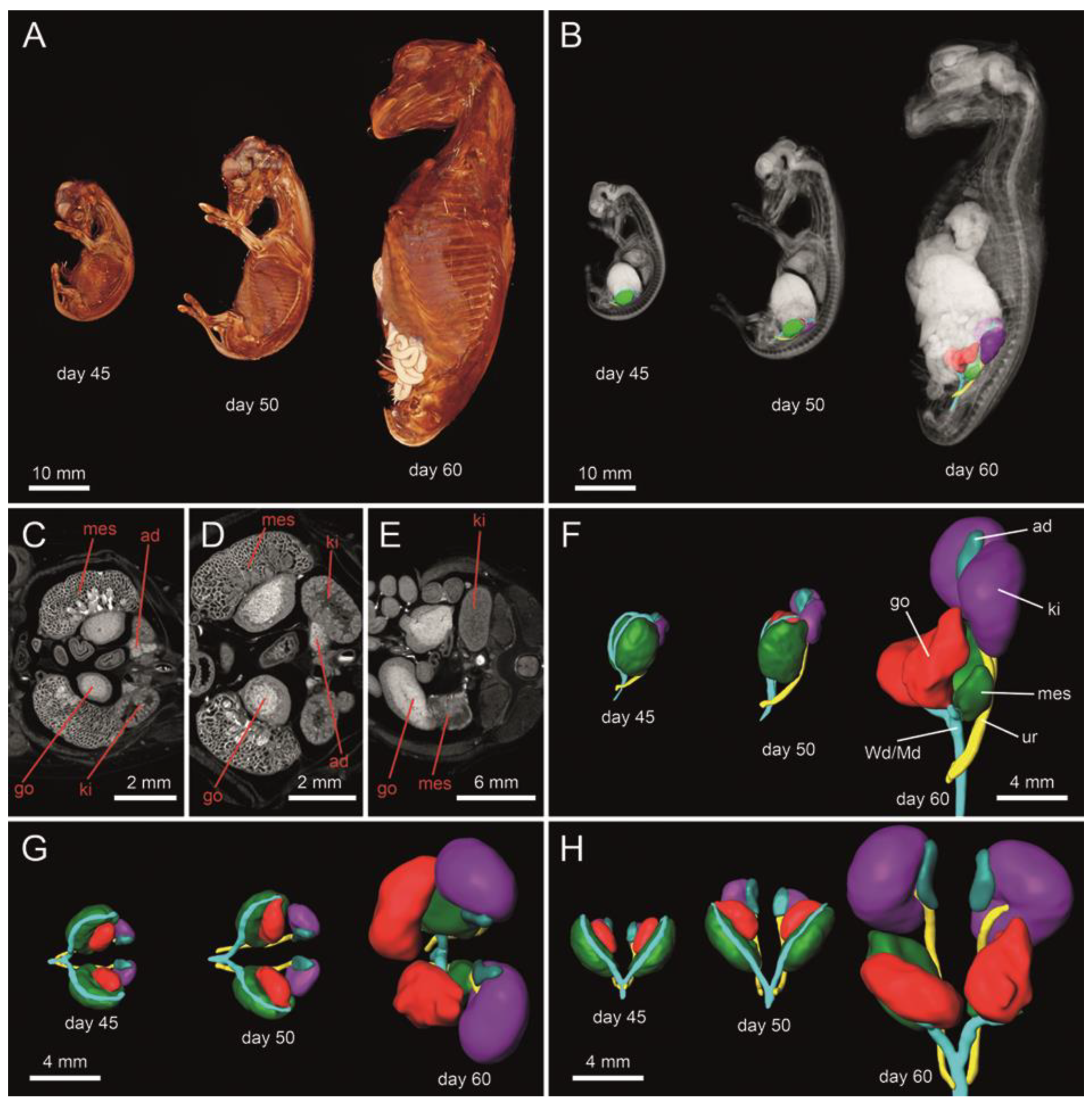
3.1. Micro-Computed Tomography
3.2. Descriptive Histological Evaluations
3.3. Immunohistochemistry
3.4. Immunofluorescence
3.5. Molecular Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, D.; Palmer, S.; Koopman, P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007, 87, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Ungewitter, E.K.; Yao, H.H. How to make a gonad: Cellular mechanisms governing formation of the testes and ovaries. Sex Dev. 2013, 7, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Gier, H.T.; Marion, G.B. Development of Mammalian Testes and Genital Ducts1. Biol. Reprod. 1969, 1, 1–23. [Google Scholar] [CrossRef]
- Kerr, C.L.; Hill, C.M.; Blumenthal, P.D.; Gearhart, J.D. Expression of pluripotent stem cell markers in the human fetal ovary. Hum. Reprod. 2008, 23, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Mamsen, L.S.; Ernst, E.H.; Borup, R.; Larsen, A.; Olesen, R.H.; Ernst, E.; Anderson, R.A.; Kristensen, S.G.; Andersen, C.Y. Temporal expression pattern of genes during the period of sex differentiation in human embryonic gonads. Sci. Rep. 2017, 7, 15961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoard, S.A.; Wise, T.H.; Fahrenkrug, S.C.; Ford, J.J. Temporal and spatial localization patterns of Gata4 during porcine gonadogenesis. Biol. Reprod. 2001, 65, 366–374. [Google Scholar] [CrossRef]
- Allen, W.R. Fetomaternal interactions and influences during equine pregnancy. Reproduction 2001, 121, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Canisso, I.F.; Ball, B.A.; Esteller-Vico, A.; Williams, N.M.; Squires, E.L.; Troedsson, M.H. Changes in maternal androgens and oestrogens in mares with experimentally-induced ascending placentitis. Equine. Vet. J. 2017, 49, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Legacki, E.L.; Ball, B.A.; Corbin, C.J.; Loux, S.C.; Scoggin, K.E.; Stanley, S.D.; Conley, A.J. Equine fetal adrenal, gonadal and placental steroidogenesis. Reproduction 2017, 154, 445. [Google Scholar] [CrossRef] [PubMed]
- Pashen, R.L.; Sheldrick, E.L.; Allen, W.R.; Flint, A.P. Dehydroepiandrosterone synthesis by the fetal foal and its importance as an oestrogen precursor. J. Reprod. Fertil. Suppl. 1982, 32, 389–397. [Google Scholar] [PubMed]
- Walt, M.L.; Stabenfeldt, G.H.; Hughes, J.P.; Neely, D.P.; Bradbury, R. Development of the equine ovary and ovulation fossa. J. Reprod. Fertil. Suppl. 1979, 471–477. [Google Scholar]
- Barreto, R.S.N.; Romagnolli, P.; Mess, A.M.; Rigoglio, N.N.; Sasahara, T.H.C.; Simoes, L.S.; Fratini, P.; Matias, G.S.S.; Jacob, J.C.F.; Gastal, E.L.; et al. Reproductive system development in male and female horse embryos and fetuses: Gonadal hyperplasia revisited. Theriogenology 2018, 108, 118–126. [Google Scholar] [CrossRef]
- Aurich, C.; Schneider, J. Sex determination in horses—current status and future perspectives. Anim. Reprod. Sci. 2014, 146, 34–41. [Google Scholar] [CrossRef]
- Kenngott, R.A.M.; Vermehren, M.; Ebach, K.; Sinowatz, F. The role of ovarian surface epithelium in folliculogenesis during fetal development of the bovine ovary: A histological and immunohistochemical study. Sex. Dev. Genet. Mol. Biol. Evol. Endocrinol. Embryol. Pathol. Sex Determ. Differ. 2013, 7, 180–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gall, L.; De Smedt, V.; Ruffini, S. Co-Expression of Cytokeratins and Vimentin in Sheep Cumulus-Oocyte Complexes. Alteration of Intermediate Filament Distribution by Acrylamide. Dev. Growth Differ. 1992, 34, 579–587. [Google Scholar] [CrossRef]
- Kenngott, R.A.-M.; Sauer, U.; Vermehren, M.; Sinowatz, F. Expression of Intermediate Filaments and Germ Cell Markers in the Developing Bovine Ovary: An Immunohistochemical and Laser-Assisted Microdissection Study. Cells Tissues Organs. 2014, 200, 153–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinovic, V.; Vukusic Pusic, T.; Restovic, I.; Bocina, I.; Filipovic, N.; Saraga-Babic, M.; Vukojevic, K. Expression of Epithelial and Mesenchymal Differentiation Markers in the Early Human Gonadal Development. Anat. Rec. Hoboken N. J. 2007 2017, 300, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Fröjdman, K.; Ekblom, P.; Sorokin, L.; Yagi, A.; Pelliniemi, L.J. Differential distribution of laminin chains in the development and sex differentiation of mouse internal genitalia. Int. J. Dev. Biol. 1995, 39, 335–344. [Google Scholar]
- Fröjdman, K.; Miner, J.H.; Sanes, J.R.; Pelliniemi, L.J.; Virtanen, I. Sex-specific localization of laminin alpha 5 chain in the differentiating rat testis and ovary. Differ. Res. Biol. Divers. 1999, 64, 151–159. [Google Scholar] [CrossRef]
- Terauchi, K.J.; Shigeta, Y.; Iguchi, T.; Sato, T. Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res. 2016, 365, 197–208. [Google Scholar] [CrossRef]
- Inomata, T.; Inoue, S.; Sugawara, H.; Kajihara, H.; Shinomiya, T.; Wagai, I.; Ninomiya, H.; Oshida, T.; Shirai, M.; Hashimoto, Y. Developmental changes in paramesonephric and mesonephric ducts and the external genitalia in swine fetuses during sexual differentiation. J. Vet. Med. Sci. 1993, 55, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruenwald, P. The relation of the growing müllerian duct to the wolffian duct and its importance for the genesis of malformations. Anat. Rec. 1941, 81, 1–19. [Google Scholar] [CrossRef]
- Kenngott, R.A.; Sinowatz, F. Expression and distribution of intermediate-filament proteins and laminin during the development of the bovine Mullerian duct. Anat. Histol. Embryol. 2008, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.D.; Behringer, R.R. Molecular Genetics of Müllerian Duct Formation, Regression and Differentiation. Sex. Dev. 2014, 8, 281–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orvis, G.D.; Behringer, R.R. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 2007, 306, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, A.J.; Cowan, G.; Kinnell, H.L.; Anderson, R.A.; Saunders, P.T. Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad. PLoS ONE 2011, 6, e20249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houmard, B.; Small, C.; Yang, L.; Naluai-Cecchini, T.; Cheng, E.; Hassold, T.; Griswold, M. Global gene expression in the human fetal testis and ovary. Biol. Reprod. 2009, 81, 438–443. [Google Scholar] [CrossRef] [Green Version]
- Childs, A.J.; Kinnell, H.L.; He, J.; Anderson, R.A. LIN28 is selectively expressed by primordial and pre-meiotic germ cells in the human fetal ovary. Stem. Cells Dev. 2012, 21, 2343–2349. [Google Scholar] [CrossRef] [Green Version]
- Gillis, A.J.; Stoop, H.; Biermann, K.; van Gurp, R.J.; Swartzman, E.; Cribbes, S.; Ferlinz, A.; Shannon, M.; Oosterhuis, J.W.; Looijenga, L.H. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int. J. Androl. 2011, 34. [Google Scholar] [CrossRef]
- West, J.A.; Viswanathan, S.R.; Yabuuchi, A.; Cunniff, K.; Takeuchi, A.; Park, I.H.; Sero, J.E.; Zhu, H.; Perez-Atayde, A.; Frazier, A.L.; et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 2009, 460, 909–913. [Google Scholar] [CrossRef] [Green Version]
- Enriquez, V.A.; Cleys, E.R.; Da Silveira, J.C.; Spillman, M.A.; Winger, Q.A.; Bouma, G.J. High LIN28A Expressing Ovarian Cancer Cells Secrete Exosomes That Induce Invasion and Migration in HEK293 Cells. Biomed. Res. Int. 2015, 2015, 701390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeckerle, N.; Eildermann, K.; Drummer, C.; Ehmcke, J.; Schweyer, S.; Lerchl, A.; Bergmann, M.; Kliesch, S.; Gromoll, J.; Schlatt, S.; et al. The pluripotency factor LIN28 in monkey and human testes: A marker for spermatogonial stem cells? Mol. Hum. Reprod. 2012, 18, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, S.; Urven, L.; Ginther, O.J. Distribution of putative primordial germ cells in equine embryos. Equine. Vet. J. Suppl. 1997, 72–76. [Google Scholar] [CrossRef]
- Lee, G.; Jung, H.; Yoon, M. The Lin28 expression in stallion testes. PLoS ONE 2016, 11, e0165011. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Song, H.; Yoon, M. The KIT is a putative marker for differentiating spermatogonia in stallions. Anim. Reprod. Sci. 2015, 152, 39–46. [Google Scholar] [CrossRef]
- Podico, G.; Canisso, I.F.; Ellerbrock, R.E.; Dias, N.W.; Mercadante, V.R.G.; Lima, F.S. Assessment of peripheral markers and ultrasonographic parameters in pregnant mares receiving intramuscular or intrauterine cloprostenol. Theriogenology 2020, 142, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.T.C.; Kaps, M.; Scarlet, D.; Handschuh, S.; Gautier, C.; Melchert, M.; Aurich, J.; Aurich, C. Low plasma progestin concentration during the early postovulatory phase impairs equine conceptus development in the late preimplantation phase. Reprod. Fertil. Dev. 2020, 32, 1156–1167. [Google Scholar] [CrossRef]
- Ball, B.A.; Conley, A.J.; MacLaughlin, D.T.; Grundy, S.A.; Sabeur, K.; Liu, I.K. Expression of anti-Mullerian hormone (AMH) in equine granulosa-cell tumors and in normal equine ovaries. Theriogenology 2008, 70, 968–977. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Anaya, G.; Molina, A.; Valera, M.; Moreno-Millán, M.; Azor, P.; Peral-García, P.; Demyda-Peyrás, S. Sex chromosomal abnormalities associated with equine infertility: Validation of a simple molecular screening tool in the Purebred Spanish Horse. Anim. Genet. 2017, 48, 412–419. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.; Takahashi, S.; Capel, B. Two distinct origins for Leydig cell progenitors in the fetal testis. Dev. Biol. 2011, 352, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Tilmann, C.; Capel, B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development 1999, 126, 2883–2890. [Google Scholar] [CrossRef] [PubMed]
- Inomata, T.; Eguchi, Y.; Nakamura, T. Origin of müllerian duct and its later developmental changes in relation to wolffian duct in bovine fetuses. J. Vet. Med. Ser. A 1989, 36, 166–174. [Google Scholar] [CrossRef]
- Jacob, M.; Christ, B.; Jacob, H.J.; Poelmann, R.E. The role of fibronectin and laminin in development and migration of the avian Wolffian duct with reference to somitogenesis. Anat. Embryol. 1991, 183, 385–395. [Google Scholar] [CrossRef]
- Kobayashi, A.; Stewart, C.A.; Wang, Y.; Fujioka, K.; Thomas, N.C.; Jamin, S.P.; Behringer, R.R. β-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development 2011, 138, 1967–1975. [Google Scholar] [CrossRef] [Green Version]
- Esteves, C.L.; Sharma, R.; Dawson, L.; Taylor, S.E.; Pearson, G.; Keen, J.A.; McDonald, K.; Aurich, C.; Donadeu, F.X. Expression of putative markers of pluripotency in equine embryonic and adult tissues. Vet. J. 2014, 202, 533–535. [Google Scholar] [CrossRef]






| Antibody | Clone | Host | Raised against (Species) | Manufacturer | Catalog # | Dilution | Target |
|---|---|---|---|---|---|---|---|
| AMH | polyclonal | Goat | human | Santa Cruz, Dallas, TX, USA | Sc-6886 | 1:200 | Sertoli cells |
| LIN28 | polyclonal | Rabbit | human | Abcam, Cambridge, UK | Ab46020 | 1:15.000 | PGCs |
| Ki67 | 8D5 | Mouse | human | Cell Signaling, Danvers, MA, USA | 9449 | 1:400 | Proliferating cells |
| CD117 | polyclonal | Rabbit | human | Dako, Santa Clara, CA, USA | A4502 | 1:600 | Pluripotent cells |
| Vimentin | V9 | Mouse | pig | Dako | M0725 | 1:500 | Mesenchyme–endothelium |
| Laminin | polyclonal | Rabbit | rat | Dako | Z0097 | 1:10.000 | Basal lamina |
| Pan Cytokeratin | AE1 + AE3 | Mouse | human | Cell Marque, Rocklin, CA, USA | 313M-16 | 1:500 | Epithelium–cytoskeleton |
| ß-catenin | 9G2 | Mouse | human | Acris, Herford, Deutschland | AM00020PU-N | 1:400 | Cell–cell adhesion |
| Tyramide Triple Immunofluorescence | |||||||
| LIN28 | polyclonal | Rabbit | human | Abcam | Ab46020 | 1:1000 | PGCs |
| CD117 | polyclonal | Rabbit | human | Dako | A4502 | 1:100 | Pluripotent cells |
| Ki67 | 8D5 | Mouse | human | Cell Signaling | 9449 | 1:200 | Proliferating cells |
| Ki67 | CD117 | LIN28 | Ki67 of LIN28 | CD117 of LIN28 | |
|---|---|---|---|---|---|
| Male (n = 4) | 6.2 ± 1.2 | 5.9 ± 1.0 | 4.5 ± 0.3 a | 3.2 ± 1.2 | 84.6 ± 4.7 |
| Female (n = 8) | 5.6 ± 0.9 | 3.6 ± 0.9 | 3.0 ± 0.4 b | 7.5 ± 1.7 | 86.8 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarlet, D.; Handschuh, S.; Reichart, U.; Podico, G.; Ellerbrock, R.E.; Demyda-Peyrás, S.; Canisso, I.F.; Walter, I.; Aurich, C. Sexual Differentiation and Primordial Germ Cell Distribution in the Early Horse Fetus. Animals 2021, 11, 2422. https://doi.org/10.3390/ani11082422
Scarlet D, Handschuh S, Reichart U, Podico G, Ellerbrock RE, Demyda-Peyrás S, Canisso IF, Walter I, Aurich C. Sexual Differentiation and Primordial Germ Cell Distribution in the Early Horse Fetus. Animals. 2021; 11(8):2422. https://doi.org/10.3390/ani11082422
Chicago/Turabian StyleScarlet, Dragos, Stephan Handschuh, Ursula Reichart, Giorgia Podico, Robyn E. Ellerbrock, Sebastián Demyda-Peyrás, Igor F. Canisso, Ingrid Walter, and Christine Aurich. 2021. "Sexual Differentiation and Primordial Germ Cell Distribution in the Early Horse Fetus" Animals 11, no. 8: 2422. https://doi.org/10.3390/ani11082422







