Cystinuria in Dogs and Cats: What Do We Know after Almost 200 Years?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Etiopathogenesis of Cystinuria
2.1. COLA Transporter
2.2. Genetic Aspects
3. Cystine Urolithiasis
3.1. Solubility of Cystine in Urine
3.2. Prevalence of Cystine Urolithiasis
4. Diagnosis
4.1. Diagnosis of Cystine Urolithiasis
4.2. Diagnosis of Cystinuria
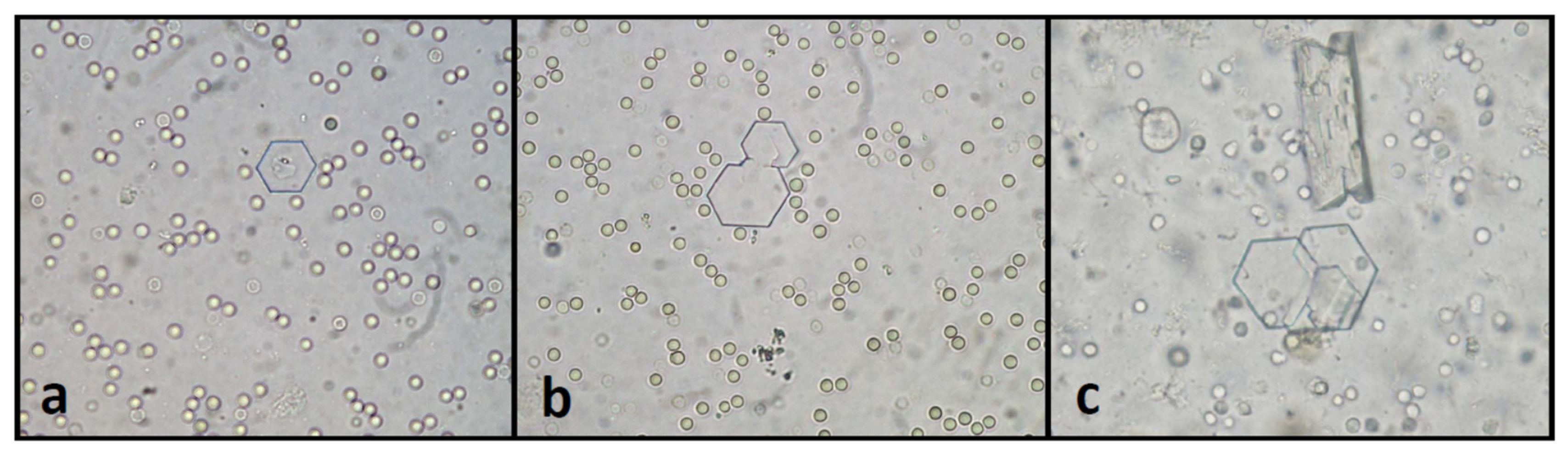
4.2.1. Urinalysis
4.2.2. Assessment of Aminoaciduria
4.2.3. Genetic Tests
5. Treatment and Prevention
5.1. Dietary Treatment
5.1.1. Protein
5.1.2. Sodium
5.1.3. Urinary pH
5.2. Medical Treatment
5.2.1. D-Penicillamine
5.2.2. 2-Merkaptopropionyl-glycine (Tiopronin)
5.2.3. Captopril
5.2.4. Bucillamine
5.3. Castration
5.4. Future Therapies
5.4.1. L-Cystine Dimethyl Ester and L-Cystine Methyl Ester (L-CDME and L-CME)
5.4.2. Alpha-Lipoic Acid
5.4.3. Selenium
5.4.4. Tolvaptan
6. Cystinuria in Cats
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattoo, A.; Goldfarb, D.S. Cystinuria. Semin. Nephrol. 2008, 28, 181–191. [Google Scholar] [CrossRef]
- Wollaston, W.H. On cystic oxide, a new species of urinary calculus. Phil. Trans. R. Soc. 1810, 100, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Garrod, A.E. The Croonian Lectures on inborn errors of metabolism. Lancet 1908, 172, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dent, C.E.; Rose, G.A. Aminoacid metabolism in cystinuria. Quart. J. Med. 1951, 20, 205–219. [Google Scholar] [PubMed]
- Lassaigne, J. Observation sur l’existence de l’oxide cystique dans un calcul vesical du chien, et essai analytique sur la composition élémentaire de cette substance particulière. Ann. Chim. Phys. 1823, 23, 328–334. [Google Scholar]
- Lulich, J.P.; Ulrich, L. Canine cystine urolithiasis: Causes, detection, dissolution and prevention. In Small Animal Clinical Nutrition, 5th ed.; Hand, M.S., Thatcher, C.D., Remillard, R.L., Roudebush, P., Novotny, B.J., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 881–890. [Google Scholar]
- Elliot, J.S.; Ribeiro, M.E.; Eusebio, E. Cystinuria in the blotched genet. Invest. Urol. 1968, 5, 568–571. [Google Scholar] [PubMed]
- Bush, M.; Bovee, K.C. Cystinuria in a maned wolf. J. Am. Vet. Med. Assoc. 1978, 173, 1159–1162. [Google Scholar]
- Bovee, K.C.; Bush, M.; Dietz, J.; Jezyk, P.; Segal, S. Cystinuria in the maned wolf of South America. Science 1981, 212, 919–920. [Google Scholar] [CrossRef]
- Mussart, N.B.; Coppo, J.A. Cystine nephrolithiasis in an endangered canid, Chrysocyon brachyurus (Carnivora: Canidae). Rev. Biol. Trop. 1999, 47, 623–625. [Google Scholar]
- Cannizzo, S.A.; Stinner, M.; Kennedy-Stoskopf, S. Prevalence of cystinuria in servals (Leptailurus serval) in the United States. J. Zoo Wildl. Med. 2017, 48, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.E. Cystine Urolithiasis in Ferrets. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 309–319. [Google Scholar] [CrossRef]
- Chillarón, J.; Font-Llitjós, M.; Fort, J.; Zorzano, A.; Goldfarb, D.S.; Nunes, V.; Palacín, M. Pathophysiology and treatment of cystinuria. Nat. Rev. Nephrol. 2010, 6, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Palacín, M.; Estévez, R.; Bertran, J.; Zorzano, A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev. 1998, 78, 969–1054. [Google Scholar] [CrossRef] [PubMed]
- Palacín, M.; Nunes, V.; Font-Llitjós, M.; Jiménez-Vidal, M.; Fort, J.; Gasol, E.; Pineda, M.; Feliubadaló, L.; Chillarón, J.; Zorzano, A. The genetics of heteromeric amino acid transporters. Physiology 2005, 20, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Sahota, A.; Tischfield, J.A.; Goldfarb, D.S.; Ward, M.D.; Hu, L. Cystinuria: Genetic aspects, mouse models, and a new approach to therapy. Urolithiasis 2019, 47, 57–66. [Google Scholar] [CrossRef]
- Fernández, E.; Carrascal, M.; Rousaud, F.; Abián, J.; Zorzano, A.; Palacín, M.; Chillarón, J. rBAT-b(0,+)AT heterodimer is the main apical reabsorption system for cystine in the kidney. Am. J. Physiol. Renal Physiol. 2002, 283, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treacher, R.J. Intestinal absorption of lysine in cystinuric dogs. J. Comp. Path. 1965, 75, 309–322. [Google Scholar] [CrossRef]
- Holtzapple, P.G.; Rea, C.; Bovee, K.; Segal, S. Characteristics of cystine and lysine transport in renal jejunal tissue from cystinuric dogs. Metab. Clin. Exp. 1971, 20, 1016–1022. [Google Scholar] [CrossRef]
- Tsan, M.F.; Wilson, T.H.; Jones, T.C. Canine cystinuria: Intestinal and renal amino acid transport. Am. J. Vet. Res. 1972, 33, 2463–2468. [Google Scholar]
- Bartges, J.W.; Callens, A.J. Urolithiasis. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 747–768. [Google Scholar] [CrossRef]
- Sanderson, S.L.; Osborne, C.A.; Lulich, J.P.; Bartges, J.W.; Pierdont, M.E.; Ogburn, P.N.; Koehler, L.A.; Swanson, L.L.; Bird, K.A.; Ulrich, L.K. Evaluation of urinary carnitine and taurine excretion in 5 cystinuric dogs with carnitine and taurine deficiency. J. Vet. Intern. Med. 2001, 15, 94–100. [Google Scholar] [CrossRef]
- Font-Llitjós, M.; Jiménez-Vidal, M.; Bisceglia, L.; Di Perna, M.; de Sanctis, L.; Rousaud, F.; Zelante, L.; Palacín, M.; Nunes, V. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J. Med. Genet. 2005, 42, 58–68. [Google Scholar] [CrossRef]
- Casal, M.L.; Giger, U.; Bovee, K.C.; Patterson, D.F. Inheritance of cystinuria and renal defect in Newfoundlands. J. Am. Vet. Med. Assoc. 1995, 207, 1585–1589. [Google Scholar]
- Henthorn, P.S.; Liu, J.L.; Gidalevich, T.; Fang, J.K.; Casal, M.L.; Patterson, D.F.; Giger, U. Canine cystinuria: Polymorphism in the canine SLC3A1 gene and identification of a nonsense mutation in cystinuric Newfoundland dogs. Hum. Genet. 2000, 107, 295–303. [Google Scholar] [CrossRef]
- Brons, A.K.; Henthorn, P.S.; Raj, K.; Fitzgerald, C.A.; Liu, J.; Sewell, A.C.; Giger, U. SLC3A1 and SLC7A9 Mutations in Autosomal Recessive or Dominant Canine Cystinuria: A New Classification System. J. Vet. Intern. Med. 2013, 27, 1400–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giger, U.; Brons, A.; Mizukami, K.; Slutsky, J.; Raj, K.; Fitzgerald, C.A.; Strickland, S.; Sewell, A.C.; Henthorn, P.S. Update on Fanconi syndrome and cystinuria. In Proceedings of the World Small Animal Veterinary Association Congress, Bangkok, Thailand, 15–18 May 2015. [Google Scholar]
- Harnevik, L.; Hoppe, A.; Soderkvist, P. SLC7A9 cDNA cloning and mutational analysis of SLC3A1 and SLC7A9 in canine cystinuria. Mamm. Genome 2006, 17, 769–776. [Google Scholar] [CrossRef]
- Brand, E.; Cahill, G.F.; Kassell, B. Canine cystinuria: V. Family history of two cystinuric Irish terriers and cystine determinations in dog urine. J. Biol. Chem. 1940, 133, 431–436. [Google Scholar] [CrossRef]
- Coe, F.L.; Parks, J.H.; Asplin, J.R. Medical progress—The pathogenesis and treatment of kidney stones. N. Engl. J. Med. 1992, 327, 1141–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartges, J.W.; Kirk, C.; Lane, I.F. Update: Management of calcium oxalate uroliths in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 969–987. [Google Scholar] [CrossRef]
- Knoll, T.; Zollner, A.; Wendt-Nordahl, G.; Michel, M.; Alken, P. Cystinuria in childhood and adolescence: Recommendations for diagnosis, treatment, and follow-up. Pediatr. Nephrol. 2005, 20, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Claes, D.J.; Jackson, E. Cystinuria: Mechanisms and management. Pediatr. Nephrol. 2012, 27, 2031–2038. [Google Scholar] [CrossRef]
- Krombach, P.; Wendt-Nordahl, G.; Knoll, T. Cystinuria and cystine stones. In Urinary Tract Stone Disease; Rao, P.N., Preminger, G.M., Kavanagh, J.P., Eds.; Springer: London, UK, 2011; pp. 207–215. [Google Scholar]
- Dent, C.E.; Senior, B. Studies on the treatment of cystinuria. Br. J. Urol. 1955, 27, 317–332. [Google Scholar] [CrossRef]
- Ling, G.V.; Ruby, A.L. Canine uroliths. Analysis of data derived from 813 specimens. Vet. Clin. N. Am. Small. Anim. Pract. 1986, 16, 303–316. [Google Scholar] [CrossRef]
- Case, L.C.; Ling, G.V.; Franti, C.E.; Ruby, A.L.; Stevens, F.; Johnson, D.L. Cystine-containing urinary calculi in dogs: 102 cases (1981–1989). J. Am. Vet. Med. Assoc. 1992, 201, 129–133. [Google Scholar] [PubMed]
- Osborne, C.A.; Lulich, J.P.; Polzin, D.J.; Sanderson, S.L.; Koehler, L.A.; Ulrich, L.K.; Bird, K.A.; Swanson, L.L.; Pederson, L.A.; Sudo, S.Z. Analysis of 77,000 canine uroliths. Perspectives from the Minnesota Urolith Center. Vet. Clin. N. Am. Small. Anim. Pract. 1999, 29, 17–38. [Google Scholar] [CrossRef]
- Osborne, C.A.; Lulich, J.P.; Kruger, J.M.; Ulrich, L.K.; Koehler, L.A. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: Perspectives from the Minnesota Urolith Center. Vet. Clin. N. Am. Small. Anim. Pract. 2009, 39, 183–197. [Google Scholar] [CrossRef]
- Low, W.W.; Uhl, J.M.; Kass, P.H.; Ruby, A.L.; Westropp, J.L. Evaluation of trends in urolith composition and characteristics of dogs with urolithiasis: 25,499 cases (1985–2006). JAVMA J. Am. Vet. Med. Assoc. 2010, 236, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Kopecny, L.; Palm, C.A.; Segev, G.; Westropp, J.L. Urolithiasis in dogs: Evaluation of trends in urolith composition and risk factors (2006–2018). J. Vet. Intern. Med. 2021, 35, 1406–1415. [Google Scholar] [CrossRef]
- Houston, D.M.; Moore, A.E.; Favrin, M.G.; Hoff, B. Canine urolithiasis: A look at over 16 000 urolith submissions to the Canadian Veterinary Urolith Centre from February 1998 to April 2003. Can. Vet. J. Rev. Vet. Can. 2004, 45, 225–230. [Google Scholar]
- Houston, D.M.; Moore, A.E.P. Canine and feline urolithiasis: Examination of over 50 000 urolith submissions to the Canadian Veterinary Urolith Centre from 1998 to 2008. Can. Vet. J. Rev. Vet. Can. 2009, 50, 1263–1268. [Google Scholar]
- Houston, D.M.; Weese, H.E.; Vanstone, N.P.; Moore, A.E.; Weese, J.S. Analysis of canine urolith submissions to the Canadian Veterinary Urolith Centre, 1998–2014. Can. Vet. J. Rev. Vet. Can. 2017, 58, 45–50. [Google Scholar]
- Del Angel-Caraza, J.; Diez-Prieto, I.; Pérez-García, C.C.; García-Rodríguez, M.B. Composition of lower urinary tract stones in canines in Mexico City. Urol. Res. 2010, 38, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Lopez, C.I.; Del-Angel-Caraza, J.; Ake-Chinas, M.A.; Quijano-Hernandez, I.A.; Barbosa-Mireles, M.A. Epidemiology of urolithiasis in dogs from Guadalajara City, Mexico. Vet. Mexico 2019, 6, 1–14. [Google Scholar] [CrossRef]
- White, E.G. Symposium on urolithiasis in the dog. I. Introduction and incidence. J. Small Anim. Pract. 1966, 7, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.D. Canine urolithiasis: Incidence, chemical composition and outcome of 100 cases. J. Small Anim. Pract. 1970, 11, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.T. The distribution of canine urinary calculi and their recurrence following treatment. J. Small Anim. Pract. 1974, 15, 437–444. [Google Scholar] [CrossRef]
- Allen, L.B.A.; Pratt, A.; Lulich, J.; Syme, H.M. Canine cystine urolithiasis: Investigation of cases identified in the United Kingdom. In Proceedings of the British Small Animal Veterinary Congress 2008, Birmingham, UK, 3–6 April 2008. [Google Scholar]
- Rogers, K.D.; Jones, B.; Roberts, L.; Rich, M.; Montalto, N.; Beckett, S. Composition of uroliths in small domestic animals in the United Kingdom. Vet. J. 2011, 188, 228–230. [Google Scholar] [CrossRef]
- Roe, K.; Pratt, A.; Lulich, J.; Osborne, C.; Syme, H.M. Analysis of 14,008 uroliths from dogs in the UK over a 10-year period. J. Small Anim. Pract. 2012, 53, 634–640. [Google Scholar] [CrossRef]
- Hesse, A. Canine urolithiasis: Epidemiology and analysis of urinary calculi. J. Small Anim. Pract. 1990, 31, 599–604. [Google Scholar] [CrossRef]
- Hesse, A.; Orzekowsky, H.; Neiger, R. Urolithiasis in dogs-15,494 cases (1979–2007). Kleintierpraxis 2012, 57, 633–639. [Google Scholar]
- Hesse, A.; Hoffmann, J.; Orzekowsky, H.; Neiger, R. Canine cystine urolithiasis: A review of 1760 submissions over 35 years (1979–2013). Can. Vet. J. Rev. Vet. Can. 2016, 57, 277–281. [Google Scholar]
- Breu, D.; Stieger, N.; Müller, E. Auftreten von Harnsteinen—Alters-, rasse- und geschlechtsspezifische Unterschiede bei Hunden aus Deutschland. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2021, 49, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Escolar, E.; Bellanato, J.; Medina, J.A. Structure and composition of canine urinary calculi. Res. Vet. Sci. 1990, 49, 327–333. [Google Scholar] [CrossRef]
- Riesgo, A.; Giménez, J.C.; Fermín Rodríguez, M.L.; García-Real, M.I.; Ruíz, M.J.; Daza, M.A. Retrospective study (2004–2017) of 137 uroliths diagnosed at the Complutense Veterinary Teaching Hospital. J. Vet. Intern. Med. 2019, 33, 1093. [Google Scholar]
- Vrabelova, D.; Silvestrini, P.; Ciudad, J.; Gimenez, J.C.; Ballesteros, M.; Puig, P.; Ruiz de Gopegui, R. Analysis of 2735 canine uroliths in Spain and Portugal. A retrospective study: 2004–2006. Res. Vet. Sci. 2011, 91, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Tomé, M.; Gonçalves, S.; Duarte Correia, J.H.; Pomba, C. Canine and feline urolithiasis in Portugal: A retrospective study 2004–2006. In Proceedings of the 17th ECVIM-CA Congress, Budapest, Hungary, 13–15 September 2007. [Google Scholar]
- Sosnar, M.; Bulkova, T.; Ruzicka, M. Epidemiology of canine urolithiasis in the Czech Republic from 1997 to 2002. J. Small Anim. Pract. 2005, 46, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kučera, J.; Kořistková, T. Dynamics of urolithiasis in dogs in the Czech Republic between 2003 and 2016. Veterinářství 2017, 67, 419–421. [Google Scholar]
- Mircean, M.; Giurgiu, G.; Mircean, V.; Katsaros, K. Epidemiologic, clinic and ethiopathogenic studies in canine urolithiasis. Bull. Univ. Agric. Sci. Vet. Med. Cluj. Napoca 2006, 63, 337–342. [Google Scholar]
- Blavier, A.; Sulter, A.; Bogey, A.; Novelli, K.; Billiemaz, B. Résultats des analyses par spectrométrie infrarouge de 1131 calculs urinaires canins prélevés de 2007 à 2010, en France. Prat. Méd. Chir. l’Anim. Cie. 2012, 47, 7–16. [Google Scholar] [CrossRef]
- Méric, T.; Sulter, A.; Bogey-Lambert, A.; Blavier, A.; Nelaton, C.; Canonne Guibert, M.; Manassero, M.; Benchekroun, G.; Maurey, C. Retrospective study of cystinic lithiasis in dogs in France. J. Vet. Intern. Med. 2020, 34, 3151. [Google Scholar]
- Bende, B.; Kovács, K.B.; Solymosi, N.; Németh, T. Characteristics of urolithiasis in the dog population of Hungary from 2001 to 2012. Acta Vet. Hung. 2015, 63, 323–336. [Google Scholar] [CrossRef] [Green Version]
- Brandenberger-Schenk, F.; Rothenanger, E.; Reusch, C.E.; Gerber, B. Uroliths of dogs in Switzerland from 2003 to 2009. Schweiz. Arch. Tierheilkd. 2015, 157, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Lund, H.S.; Thoresen, S.I. Increase in canine cystine urolithiasis in Norway. J. Vet. Intern. Med. 2020, 34, 3152. [Google Scholar]
- Burggraaf, N.D.; Westgeest, D.B.; Corbee, R.J. Analysis of 7866 feline and canine uroliths submitted between 2014 and 2020 in the The Netherlands. Res. Vet. Sci. 2021, 137, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.R.; Kirkman, J.H.; Hogan, J.; Holmes, S. Analysis of uroliths from cats and dogs in New Zealand, 1993–1996. N. Z. Vet. J. 1998, 46, 233–236. [Google Scholar] [CrossRef]
- Hunprasit, V.; Osborne, C.A.; Schreiner, P.J.; Bender, J.B.; Lulich, J.P. Epidemiologic evaluation of canine urolithiasis in Thailand from 2009 to 2015. Res. Vet. Sci. 2017, 115, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Urolith Center. 2020 Minnesota Urolith Center Global Data. Available online: https://www.vetmed.umn.edu/sites/vetmed.umn.edu/files/2020globaldata.pdf (accessed on 24 April 2021).
- Trevejo, R.; Yang, M.Y.; Lund, E.M. Epidemiology of surgical castration of dogs and cats in the United States. JAVMA J. Am. Vet. Med. Assoc. 2011, 238, 898–904. [Google Scholar] [CrossRef]
- Kubinyi, E.; Turcsan, B.; Miklosi, A. Dog and owner demographic characteristics and dog personality trait associations. Behav. Process. 2009, 81, 392–401. [Google Scholar] [CrossRef]
- O’Neill, D.G.; James, H.; Brodbelt, D.C.; Church, D.B.; Pergram, C. Prevalence of commonly diagnosed disorders in UK dogs under primary veterinary care: Results and applications. BMC Vet. Res. 2021, 17, 69. [Google Scholar] [CrossRef]
- Osborne, C.A.; Sanderson, S.L.; Lulich, J.P.; Bartges, J.W.; Ulrich, J.K.; Koehler, L.A.; Bird, K.A.; Swanson, L.L. Canine cystine urolithiasis. Cause, detection, treatment, and prevention. Vet. Clin. N. Am. Small. Anim. Pract. 1999, 29, 193–211. [Google Scholar] [CrossRef]
- Ling, G.V.; Franti, C.E.; Ruby, A.L.; Johnson, D.L. Urolithiasis in dogs II: Breed prevalence, and interrelations of breed, sex, age, and mineral composition. Am. J. Vet. Res. 1998, 59, 630–642. [Google Scholar] [PubMed]
- Kučera, J.; Kořistková, T. Epidemiologic characteristics of 442 cases of cystinuria in dogs. Veterinářství 2018, 68, 831–835. [Google Scholar]
- Ercolani, M.; Sahota, A.; Schuler, C.; Yang, M.; Evan, A.P.; Reimer, D.; Barone, J.G.; Tischfield, J.A.; Levin, R.M. Bladder outlet obstruction in male cystinuria mice. Int. Urol. Nephrol. 2010, 42, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Ling, G.V.; Franti, C.E.; Johnson, D.L.; Ruby, A.L. Urolithiasis in dogs III: Prevalence of urinary tract infection and interrelations of infection, age, sex, and mineral composition. Am. J. Vet. Res. 1998, 59, 643–649. [Google Scholar]
- Koehler, L.A.; Osborne, C.A.; Buettner, M.T.; Lulich, J.P.; Behnke, R. Canine uroliths: Frequently asked questions and their answers. Vet. Clin. N. Am. Small. Anim. Pract. 2009, 39, 161–181. [Google Scholar] [CrossRef]
- Bovee, K.C. Canine cystine urolithiasis. Vet. Clin. N. Am. Small Anim. Pract. 1986, 16, 211–215. [Google Scholar] [CrossRef]
- Weichselbaum, R.C.; Feeney, D.A.; Jessen, C.R.; Osborne, C.A.; Koehler, L.; Ulrich, L. Evaluation of the morphologic characteristics and prevalence of canine urocystoliths from a regional urolith center. Am. J. Vet. Res. 1998, 59, 379–387. [Google Scholar]
- Bovee, K.C.; McGuire, T. Qualitative and quantitative analysis of uroliths in dogs: Definitive determination of chemical type. J. Am. Vet. Med. Assoc. 1984, 185, 983–987. [Google Scholar] [PubMed]
- Brand, E.; Harris, M.M.; Biloon, S. Cystinuria: The excretion of a cystine complex which decomposes in the urine with the liberation of free cystine. J. Biol. Chem. 1930, 86, 315–331. [Google Scholar] [CrossRef]
- Osborne, C.A.; Lulich, J.P. Canine Cystine. Available online: https://vetmed.umn.edu/sites/vetmed.umn.edu/files/canine_cystine_uroliths.pdf (accessed on 5 May 2021).
- Hoppe, A.; Denneberg, T.; Jeppsson, J.O.; Kagedal, B. Urinary excretion of amino acids in normal and cystinuric dogs. Br. Vet. J. 1993, 149, 253–268. [Google Scholar] [CrossRef]
- Hoppe, A.; Denneberg, T. Cystinuria in the dog: Clinical studies during 14 years of medical treatment. J. Vet. Intern. Med. 2001, 15, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Denneberg, T.; Jeppsson, J.O.; Kagedal, B. Canine cystinuria: An extended study on the effects of 2-mercaptopropionylglycine on cystine urolithiasis and urinary cystine excretion. Br. Vet. J. 1993, 149, 235–251. [Google Scholar] [CrossRef]
- Lulich, J.P.; Berent, A.C.; Adams, L.G.; Westropp, J.L.; Bartges, J.W.; Osborne, C.A. ACVIM Small Animal Consensus Recommendations on the Treatment and Prevention of Uroliths in Dogs and Cats. J. Vet. Intern. Med. 2016, 30, 1564–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langston, C.; Gisselman, K.; Palma, D.; McCue, J. Methods of urolith removal. Compendium 2010, 32, 1–7. [Google Scholar]
- Queau, Y. Nutritional Management of Urolithiasis. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 175–186. [Google Scholar] [CrossRef]
- Stevenson, A.E.; Hynds, W.K.; Markwell, P.J. Effect of dietary moisture and sodium content on urine composition and calcium oxalate relative supersaturation in healthy miniature schnauzers and labrador retrievers. Res. Vet. Sci. 2003, 74, 145–151. [Google Scholar] [CrossRef]
- Norman, R.W.; Manette, W.A. Dietary restriction of sodium as a means of reducing urinary cystine. J. Urol. 1990, 143, 1193–1195. [Google Scholar] [CrossRef]
- Moussa, M.; Papatsoris, A.G.; Chakra, M.A.; Moussa, Y. Update on cystine stones: Current and future concepts in treatment. Intractable Rare Dis. Res. 2020, 9, 71–78. [Google Scholar] [CrossRef]
- Lotz, M.; Potts, J.T.; Holland, J.M.; Kiser, W.S.; Bartter, F.C. D-penicillamine therapy in cystinuria. J. Urol. 1966, 95, 257–263. [Google Scholar] [CrossRef]
- Langlois, D.K.; Lehner, A.F.; Buchweitz, J.P.; Ross, D.E.; Johnson, M.B.; Kruger, J.M.; Bailie, M.B.; Hauptman, J.G.; Schall, W.D. Pharmacokinetics and Relative Bioavailability of d-Penicillamine in Fasted and Nonfasted Dogs. J. Vet. Intern. Med. 2013, 27, 1071–1076. [Google Scholar] [CrossRef]
- Prot-Bertoye, C.; Lebbah, S.; Daudon, M.; Tostivint, I.; Jais, J.P.; Garcia, A.; Pontoizeau, C.; Cochat, P.; Bataille, P.; Bridoux, F.; et al. Adverse events associated with currently used medical treatments for cystinuria and treatment goals: Results from a series of 442 patients in France. BJU Int. 2019, 124, 849–861. [Google Scholar] [CrossRef]
- Osborne, C.A.; Lulich, J.P.; Unger, L.K. Canine and feline urolithiasis: Relationship of etiopathogenesis to treatment and prevention. In Canine and Feline Nephrology and Urology; Osborne, C.A., Finco, D., Eds.; Williams & Wilkins: Baltimore, MD, USA, 1995; Volume 1195, pp. 798–888. [Google Scholar]
- Kallistratos, G.; Fenner, O.; Berg, U. Cystinuria and L-cystine lithiasis in dogs. Experientia 1973, 29, 791. [Google Scholar] [CrossRef]
- Hoppe, A.; Denneberg, T.; Emanuelsson, B.M.; Kagedal, B.; Lindgren, S. Pharmacokinetics and bioavailability of 2-mercaptopropionylglycine administered intravenously and orally in dogs. J. Vet. Pharmacol. Ther. 1991, 14, 374–384. [Google Scholar] [CrossRef]
- Hoppe, A.; Denneberg, T.; Kagedal, B. Treatment of clinically normal and cystinuric dogs with 2-mercaptopropionylglycine. Am. J. Vet. Res. 1988, 49, 923–928. [Google Scholar]
- Osborne, C.A.; Polzin, D.J.; Lulich, J.P.; Kruger, J.M.; Johnston, G.R.; O’Brien, T.D.; Felice, L.J. Relationship of nutritional factors to the cause, dissolution, and prevention of canine uroliths. Vet. Clin. N. Am. Small. Anim. Pract. 1989, 19, 583–619. [Google Scholar] [CrossRef]
- Hoppe, A.; Denneberg, T.; Frank, A.; Kagedal, B.; Petersson, L.R. Urinary excretion of metals during treatment with D-penicillamine and 2-mercaptopropionylglycine in normal and cystinuric dogs. J. Vet. Pharmacol. Ther. 1993, 16, 93–102. [Google Scholar] [CrossRef]
- Giger, U.; Lee, J.W.; Fitzgerald, C.; Lin, J.; Erat, A.; Sewell, A.C.; Henthorn, P.S. Characterization of non-type I cystinuria in Irish Terriers. In Proceedings of the American College of Veterinary Internal Medicine Forum, Denver, CO, USA, 15–18 June 2011. [Google Scholar]
- Rimer, J.D.; An, Z.; Zhu, Z.; Lee, M.H.; Goldfarb, D.S.; Wesson, J.A.; Ward, M.D. Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science 2010, 330, 337–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Sahota, A.; Ward, M.D.; Goldfarb, D.S. Cystine growth inhibition through molecular mimicry: A new paradigm for the prevention of crystal diseases. Curr. Rheumatol. Rep. 2015, 17, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zee, T.; Bose, N.; Zee, J.; Beck, J.N.; Yang, S.; Parihar, J.; Yang, M.; Damodar, S.; Hall, D.; O’Leary, M.N.; et al. Alpha-Lipoic acid treatment prevents cystine urolithiasis in a mouse model of cystinuria. Nat. Med. 2017, 23, 288–290. [Google Scholar] [CrossRef]
- Mohammadi, M.; Shohani, A.; Khorami, H.; Mahdavi, K.N.; IzadPanahi, M.H.; Alizadeh, F.; Azizi, M. The effect of selenium supplementation on cystine crystal volume in patients with cystinuria. Biomedicine 2018, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Tang, Y.; Wang, J.; Wang, X.; Wang, Z.; Cao, D.; Han, P.; Wang, J. Tolvaptan treatment of cystine urolithiasis in a mouse model of cystinuria. World J. Urol. 2021, 39, 263–269. [Google Scholar] [CrossRef]
- Nelson, C.P.; Kurtz, M.P.; Venna, A.; Cilento, B.G., Jr.; Baum, M.A. Pharmacological dilutional therapy using the vasopressin antagonist tolvaptan for young patients with cystinuria: A pilot investigation. Urology 2020, 144, 65–70. [Google Scholar] [CrossRef]
- Osborne, C.A.; Clinton, C.W.; Brunkow, H.C.; Frost, A.P.; Johnston, G.R. Epidemiology of naturally occurring feline uroliths and urethral plugs. Vet. Clin. N. Am. Small. Anim. Pract. 1984, 14, 481–489. [Google Scholar] [CrossRef]
- Osborne, C.A.; Lulich, J.P.; Thumchai, R.; Ulrich, L.K.; Koehler, L.A.; Bird, K.A.; Bartges, J.W. Feline urolithiasis. Etiology and pathophysiology. Vet. Clin. N. Am. Small. Anim. Pract. 1996, 26, 217–232. [Google Scholar]
- Cannon, A.B.; Westropp, J.L.; Ruby, A.L.; Kass, P.H. Evaluation of trends in urolith composition in cats: 5,230 cases (1985-2004). JAVMA J. Am. Vet. Med. Assoc. 2007, 231, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Houston, D.M.; Moore, A.E.; Favrin, M.G.; Hoff, B. Feline urethral plugs and bladder uroliths: A review of 5484 submissions 1998–2003. Can. Vet. J. Rev. Vet. Can. 2003, 44, 974–977. [Google Scholar]
- Houston, D.M.; Vanstone, N.P.; Moore, A.E.; Wesse, H.E.; Weese, J.S. Evaluation of 21426 feline bladder urolith submissions to the Canadian Veterinary Urolith Centre (1998–2014). Can. Vet. J. 2016, 57, 196–201. [Google Scholar]
- Schenk, F.; Rothenanger, E.; Reusch, C.; Gerber, B. Analysis of 855 feline and 468 canine uroliths in Switzerland between 2002 and 2009. In Proceedings of the 20th ECVIM-CA Congress, Toulouse, France, 9–11 September 2010. [Google Scholar]
- Hunprasit, V.; Pusoonthornthum, P.; Koehler, L.; Lulich, J.P. Epidemiologic evaluation of feline urolithiasis in Thailand from 2010 to 2017. Thai J. Vet. Med. 2019, 49, 101–105. [Google Scholar]
- Dibartola, S.P.; Chew, D.J.; Horton, M.L. Cystinuria in a cat. J. Am. Vet. Med. Assoc. 1991, 198, 102–104. [Google Scholar]
- Osborne, C.A.; Lulich, J.; Sanderson, S.; Rogers, Q.; Giger, U. Feline cystine urolithiasis—18 cases. Feline Pr. 1999, 27, 31–32. [Google Scholar]
- Mizukami, K.; Raj, K.; Osborne, C.; Giger, U. Cystinuria Associated with Different SLC7A9 Gene Variants in the Cat. PLoS ONE 2016, 11, e0159247. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, K.; Raj, K.; Giger, U. Feline Cystinuria Caused by a Missense Mutation in the SLC3A1 Gene. J. Vet. Intern. Med. 2015, 29, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Hilton, S.; Mizukami, K.; Giger, U. Cystinuria caused by a SLC7A9 missense mutation in Siamese-crossbred littermates in Germany. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2017, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.A.; Lulich, J.P. Feline Cystine. Available online: https://vetmed.umn.edu/sites/vetmed.umn.edu/files/feline_cystine_uroliths.pdf (accessed on 5 May 2021).

| Phenotype | Type I-A | Type II-A | Type II-B | Type III | |
|---|---|---|---|---|---|
| Mode of inheritance | Autosomal recessive | Autosomal dominant | Autosomal dominant | Sex limited | |
| Gene | SLC3A1 | SLC3A1 | SLC7A9 | Undetermined | |
| Sex | Males and females | Males and females | Males and females | Intact adult males | |
| Androgen dependence | No | No | No | Yes | |
| COLA μmol/g creatinine * | homozygous | ≥8000 | ≥8000 | ≤4000 | |
| heterozygous | ≤500 | ≥3000 | ≥700 | ||
| Breeds | Newfoundland Landseer Labrador | Australian Cattle Dog | Miniature Pincher | Mastiff and related breeds Scottish Deerhound Irish Terrier | |
| Location | Author | Method of Analysis | Years | Total Number | Cystine Uroliths | Sex | Age | Breeds |
|---|---|---|---|---|---|---|---|---|
| America | ||||||||
| United States | Ling and Ruby (1986) [36] | quantitative | 1981–1984 | 813 | 21 (2.6%) | 20 males 1 female | ||
| United States | Case et al. (1992) [37] | crystallography | 1981–1989 | 5375 | 107 (2.0%) | 106 males 1 female | mean 4.5 years | Australian Cattle Dog, Mastiff, English Bulldog |
| United States | Osborne et al. (1999) [38] | quantitative including infrared spectroscopy | 1981–1997 | 77,191 | 760 (1%) a | |||
| United States | Osborne et al. (2009) [39] | quantitative including infrared spectroscopy | 1981–2007 | 451,891 | 3 402 (0.8%) | |||
| United States | Low et al. (2010) [40] | crystallography | 1985–2006 | 25,499 | 320 (1.3%) | 313 males 7 females | English Bulldog (OR 44.2), Newfoundland (OR 12.6), Dachshund (OR 7.6), Chihuahua (OR 5.6), Miniature Pinscher (OR 9.3), Welsh Corgi (OR 5.0) b | |
| United States | Kopecny et al. (2021) [41] | quantitative | 2006–2018 | 10,444 | 279 (2.7%) | 273 males (192 intact males) 5 females | Mastiff (OR 52.7), Australian Cattle Dog (OR 30.8), Pitbull Terrier (OR 12.9), Rottweiler (OR 11.9), English Bulldog (OR 10.1), Bulldog (OR 9.1) c Females: Pitbull Terrier, crossbreed, Newfoundland | |
| Canada | Houston et al. (2004) [42] | crystallography (+another quantitative methods) d | 1998–2003 | 16,647 | 59 (0.4%) | 58 males 1 female | mean in males 4.3 years Female–4 years old | English Bulldog, Newfoundland, Chihuahua, Rottweiler, Scottish Deerhound |
| Canada | Houston and Moore (2009) [43] | crystallography (+ another quantitative methods) d | 1998–2008 | 40,637 | 115 (0.3%) e | |||
| Canada | Houston et al. (2017) [44] | crystallography (+ another quantitative methods) d | 1998–2014 | 79,965 | 480 (0.6%) f | significantly more frequent in males | Scottish Deerhound, Whippet, Newfoundland | |
| Mexico | Del Angel-Caraza et al. (2010) [45] | quantitative | 105 | 1 (1%) | male | 4–6 years | ||
| Mexico | Mendoza-Lopez et al. (2019) [46] | quantitative | 195 | 0 | ||||
| Europe | ||||||||
| UK | White (1966) [47] | chemical methods | 1st series 1944 | 103 | 18 (18%) | males | Corgi, Dachshund | |
| 2nd series 1961–1966 | 737 | 114 (15.5%) | males | |||||
| UK (Scotland) | Weaver (1970) [48] | chemical methods | 1961–1968 | 100 | 20 (20%) | males | mean 5.3 years | Basset Hound, Irish Terrier |
| UK | Clark (1974) [49] | X-ray diffraction | 110 | 24 (22%) | males | 4.9 ± 2.03 years | ||
| UK | Allen et al. (2008) [50] | quantitative g | 2002–2006 | 11,027 | 348 (3.2%) | 347 males 1 female | mean 73 months | Staffordshire Bull Terrier |
| UK | Rogers et al. (2011) [51] | 2002–2010 | 5591 | 180 (3.2%) | males | |||
| UK | Roe et al. (2012) [52] | quantitative g | 1997–2006 | 14,008 | 424 (3%) | more common in males | majority at the age 36–72 months | English bulldog (OR 60.88), Staffordshire Bull Terrier (OR 8.71), Rottweiler (OR 6.99), Jack Russel Terrier (OR 2.32) h |
| Germany | Hesse (1990) [53] | 1731 | 387 (22.4%) | Dachshund, Munsterlander, Irish Terrier | ||||
| Germany | Hesse et al. (2012) [54] | 1979–2007 | 15,494 | 1491 (9.9%) i | 1476 males 15 females | 6.0 ± 2.5 years | Dachshund, Dobermann Pinscher, Poodle, Cocker spaniel, Schnauzer, Yorkshire Terrier, Pekingese, Shih-tzu, Dalmatian | |
| Germany | Hesse et al. (2016) [55] | 1979–2013 | 20,316 | 1760 (8.7%) | 1741 males 19 females | 5.9 ± 2.5 | ||
| Germany | Breu et al. (2021) [56] | 2017–2019 | 2772 | 421 (15.2%) | Males: 324 intact, 61 castrated Females: 6 intact 4 castrated j | median 5 years | French Bulldogs, Bulldogs, Chihuahua, Dachshund | |
| Spain | Escolar et al. (1990) [57] | 171 | 44 (26%) | males | ||||
| Spain | Riesgo et al. (2018) [58] | quantitative g | 2004–2017 | 116 | 9 (7.8%) | males | 2–12 years | Basset Hound |
| Spain and Portugal | Vrabelova et al. (2011) [59] | quantitative g | 2004–2006 | 2765 | 87 (3%) | 86 males 1 female | Bulldogs | |
| Portugal | Tomé et al. (2007) [60] | quantitative g | 2004–2006 | 299 | 20 (6.7%) | |||
| Czech Republic | Sosnar et al. (2005) [61] | infrared spectroscopy | 1997–2002 | 1366 | 77 (5.6%) | 45 males k | Dachshund Basset Hound | |
| Czech Republic | Kučera and Kořistková (2017) [62] | infrared spectroscopy | 2003–2016 | 803 | 41 (5.1%) | |||
| Romania | Mircean et al. (2006) [63] | infrared spectroscopy | 2005–2006 | 20 | 2 (10%) | males | ||
| France | Blavier et al. (2012) [64] | infrared spectroscopy | 2007–2010 | 1131 | 42 (3.7%) | |||
| France | Méric et al. (2020) [65] | 2054 | 183 (8.9%) | 182 males1 female | English Bulldog, American Staffordshire Terrier, French Bulldog, Staffordshire Bull Terriers, Dachshunds | |||
| Hungary | Bende et al. (2015) [66] | infrared spectroscopy | 2001–2012 | 2543 | 108 (4.2%) | 96 males l | 58 ± 31.3 months | Basset Hound (OR 40.2), Bulldog (OR 18.6), Rottweiler (OR 13.9), Min. Pinscher (OR 12.7), Wirehaired Dachshund (OR 7.6), Dachshund (OR 6.5), Chihuahua (OR 4.8) m |
| Switzerland | Brandenberger-Schenk et al. (2015) [67] | quantitative g | 2003–2009 | 490 | 17 (3%) | males | median 3.9 years (range 0.6–10.1) | English Bulldog |
| Norway | Lund and Thoresen (2020) [68] | 2010–2019 | 684 | 97 (14.2%) n | Males: 91 intact, 2 castrated Females: 3 intact 1 castrated | |||
| The Netherlands | Burggraaf et al. (2021) [69] | quantitative | 2014–2020 | 4369 | 601 (13.8%) | 593 males (455 intact, 138 neutered) 8 females (2 intact, 6 neutered) | American Staffordshire Terrier, Basset Hound, Chihuahua, English Bulldog, French Bulldog, Miniature Pinscher, Rottweiler, Dachshund, Yorkshire Terrier | |
| Asia and Oceania | ||||||||
| New Zealand | Jones et al. (1998) [70] | X-ray diffraction | 1993–1996 | 316 | 24 (7.6%) | |||
| Thailand | Hunprasit et al. (2017) [71] | quantitative g | 2009–2015 | 8560 | 136 (1.6%) | 126 males 2 females o | 4.8 ± 2.4 | Chihuahua, French Bulldog, Shih-tzu, Miniature Pinscher |
| Canine Breeds | ||
|---|---|---|
| Afghan | French Bulldog | Pug |
| Akita Inu | German Braque | Puli |
| Alaskan Malamute | Golden Retriever | Rat Terrier |
| American Staffordshire Terrier | Great Dane | Rottweiler |
| Australian Cattle Dog | Husky | Rough Collie |
| Australian Shepherd Dog | Chihuahua | Saluki |
| Australian Terrier | Irish Terrier | Samoyed |
| Basenji | Jack Russel Terrier | Scottish Deerhound |
| Basset Hound | Kromfohrländer | Scottish Terrier |
| Bichon Frise | Labrador Retriever | Setter |
| Border Collie | Landseer | Shetland Collie |
| Borzoi | Lhasa Apso | Shetland Sheepdog |
| Boxer | Maltese | Shih Tzu |
| Brussels Griffon | Mastiff | Schnauzer |
| Bull Mastiff | Miniature Pinscher | Silky Terrier |
| Cairn Terrier | Miniature Poodle | Staffordshire Bull Terrier |
| Cavalier King Charles Spaniel | Miniature Schnauzer | Staffordshire Terrier |
| Cocker Spaniel | Munsterlander | Swedish Lapphund |
| Dachshund | Newfoundland | Tibetian Spaniel |
| Dalmatian | Old English Sheepdog | Welsh Corgi |
| Dobermann | Pekingese | West Highland White Terrier |
| Drever | Pitbull Terrier | Whippet |
| English Bulldog | Pointer | Yorkshire Terrier |
| Fox Terrier | Poodle | |
| Location | Author | Years | Total Number | Cystine Uroliths | Sex | Age | Breeds |
|---|---|---|---|---|---|---|---|
| America | |||||||
| United States | Osborne et al. (1984) [112] | 328 | 0 | ||||
| United States | Osborne et al. (1996) [113] | 9481 | 26 (0.3%) | 17 males 6 females | 3.2 years (range 4 months–11 years) | DSH, DLH, Siamese, Korat | |
| United States | Cannon et al. (2007) [114] | 1985–2004 | 5230 | 7 (0.1%) | 3 males 4 females | 4× DSH | |
| United States | Osborne et al. (2009) [39] | 1981–2007 | 94,776 | 92 (0.1%) | |||
| United States | Kopecny et al. (2021) [41] | 2005–2018 | 3940 | 2 (0.05%) | |||
| Canada | Houston et al. (2003) [115] | 1998–2003 | 4866 uroliths 618 urethral plugs | 5 uroliths (0.1%) 1 plug (0.2%) | |||
| Canada | Houston et al. (2009) [43] | 1998–2008 | 11,353 | 11 (0.1%) | |||
| Canada | Houston et al. (2016) [116] | 1998–2014 | 21,426 | 20 (0.1%) | |||
| Europe | |||||||
| Spain | Escolar et al. (2003) [57] | 34 | 0 | ||||
| Portugal | Tomé et al. (2007) [60] | 2004–2006 | 65 | 0 | |||
| Switzerland | Schenk et al. (2010) [117] | 2002–2009 | 855 | 2 (0.2%) | |||
| Spain | Riesgo et al. (2018) [58] | 2004–2017 | 21 | 0 | |||
| The Netherlands | Burggraaf et al. (2021) [69] | 2014–2020 | 3497 | 4 (0.1%) | 3 males 1 female | ||
| Asia and Oceania | |||||||
| New Zealand | Jones et al. (1998) [70] | 1993–1996 | 53 | 0 | |||
| Thailand | Hunprasit et al. (2019) [118] | 2010–2017 | 923 | 7 (0.8%) | 4 males 3 females | 6× DSH 1× Persian | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovaříková, S.; Maršálek, P.; Vrbová, K. Cystinuria in Dogs and Cats: What Do We Know after Almost 200 Years? Animals 2021, 11, 2437. https://doi.org/10.3390/ani11082437
Kovaříková S, Maršálek P, Vrbová K. Cystinuria in Dogs and Cats: What Do We Know after Almost 200 Years? Animals. 2021; 11(8):2437. https://doi.org/10.3390/ani11082437
Chicago/Turabian StyleKovaříková, Simona, Petr Maršálek, and Kateřina Vrbová. 2021. "Cystinuria in Dogs and Cats: What Do We Know after Almost 200 Years?" Animals 11, no. 8: 2437. https://doi.org/10.3390/ani11082437
APA StyleKovaříková, S., Maršálek, P., & Vrbová, K. (2021). Cystinuria in Dogs and Cats: What Do We Know after Almost 200 Years? Animals, 11(8), 2437. https://doi.org/10.3390/ani11082437







