Noninvasive Thermographic Photographing as an Assessment of the State of Discomfort in a Dog Receiving Radiation Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
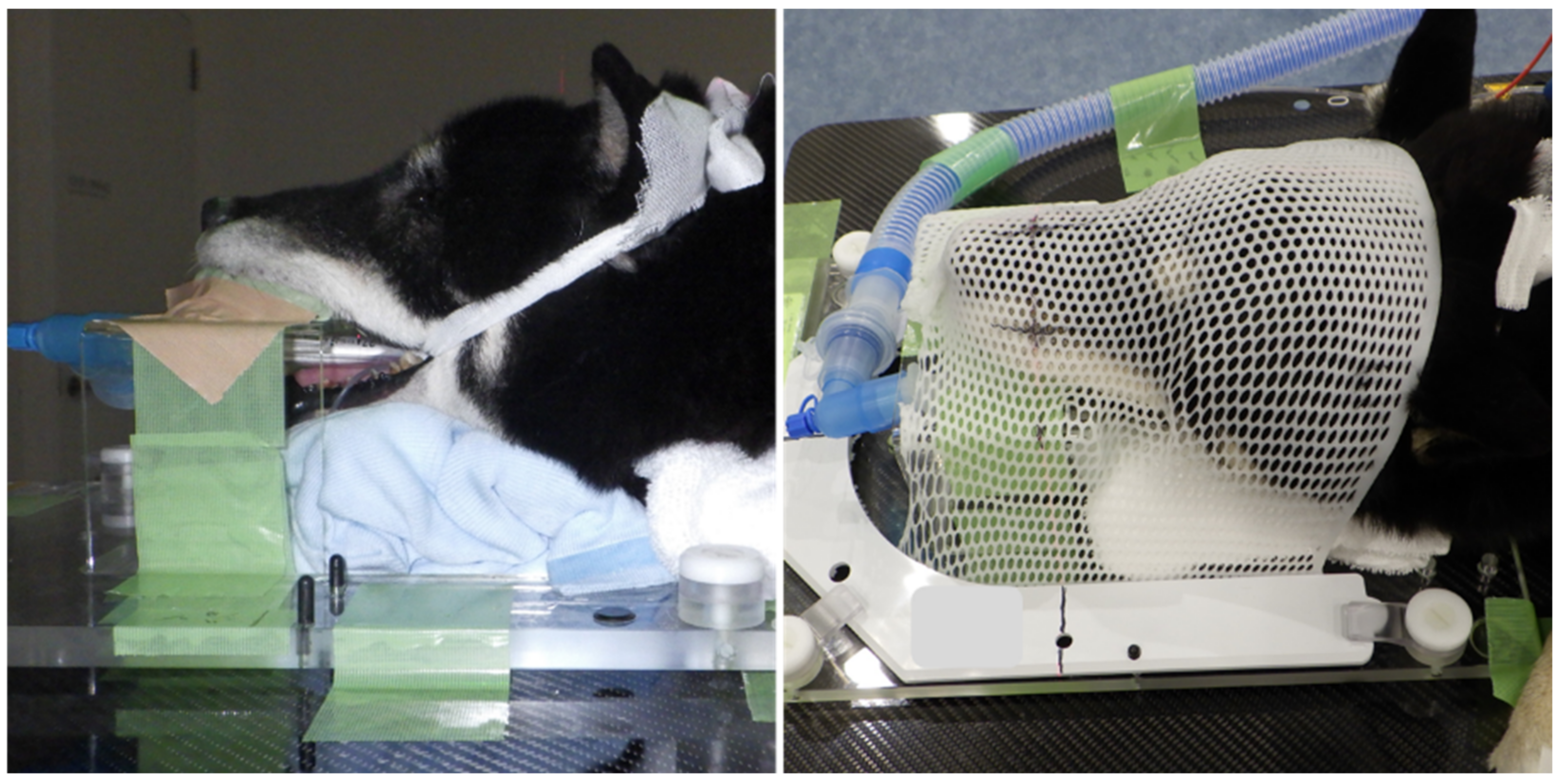
2.1. Case Presentation
2.2. Thermography and Behavioral Analysis
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casas-Alvarado, A.; Mota-Rojas, D.; Hernández-Ávalos, I.; Mora-Medina, P.; Olmos-Hernández, A.; Verduzco-Mendoza, A.; Reyes-Sotelo, B.; Martínez-Burnes, J. Advances in infrared thermography: Surgical aspects, vascular changes, and pain monitoring in veterinary medicine. J. Therm. Biol. 2020, 92, 102664. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-invasive measurement of stress in dairy cows using infrared thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Autio, E.; Neste, R.; Airaksinen, S.; Heiskanen, M.-L. Measuring the Heat Loss in Horses in Different Seasons by Infrared Thermography. J. Appl. Anim. Welf. Sci. 2006, 9, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Redaelli, V.; Ludwig, N.; Nanni Costa, L.; Crosta, L.; Riva, J.; Luzi, F. Potential application of thermography (IRT) in animal production and for animal welfare. A case report of working dogs. Ann. Ist. Super. Sanita 2014, 50, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.A.; Forrest, L.J. Intensity-modulated radiation therapy and helical tomotherapy: Its origin, benefits, and potential applications in veterinary medicine. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Time, dose, and fractionation in radiotherapy. In Radiobiology for the Radiologist; Online Access with Subscription: LWW Classic Book Collection; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 378–397. ISBN 9780781741514. [Google Scholar]
- Hill, R.P.; Bristow, R.G. The scientific basis of radiotherapy. In The Basic Science of Oncology; Ian, T., Richard, P.H., Robert, G.B., Robert, G., Bristow, P.D., Eds.; McGraw Hill Professional: New York, NY, USA, 2005; pp. 289–321. ISBN 9780071387743. [Google Scholar]
- Vainionpää, M.; Raekallio, M.; Tuhkalainen, E.; Hänninen, H.; Alhopuro, N.; Savolainen, M.; Junnila, J.; Hielm-Björkman, A.; Snellman, M.; Vainio, O. Comparison of three thermal cameras with canine hip area thermographic images. J. Vet. Med. Sci. 2012, 74, 1539–1544. [Google Scholar] [CrossRef] [Green Version]
- Varjú, G.; Pieper, C.F.; Renner, J.B.; Kraus, V.B. Assessment of hand osteoarthritis: Correlation between thermographic and radiographic methods. Rheumatology 2004, 43, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Vianna, D.M.L.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [Green Version]
- Maillot, O.; Leduc, N.; Atallah, V.; Escarmant, P.; Petit, A.; Belhomme, S.; Sargos, P.; Vinh-Hung, V. Evaluation of acute skin toxicity of breast radiotherapy using thermography: Results of a prospective single-centre trial. Cancer/Radiothérapie 2018, 22, 205–210. [Google Scholar] [CrossRef]
- Elliot, K.M.; Mayer, M.N. Radiation therapy for tumors of the nasal cavity and paranasal sinuses in dogs. Can. Vet. J. 2009, 50, 309–312. [Google Scholar]
- Wilson, D.W.; Dungworth, D.L. Tumors of the Respiratory Tract. Tumors Domest. Anim. 2002, 4, 365–399. [Google Scholar]
- Withrow, S.J.; Vail, D.M. Withrow and MacEwen’s Small Animal Clinical Oncology; Elsevier Health Sciences: New York, NY, USA, 2006; ISBN 9781437702019. [Google Scholar]
- Ogilvie, G.K.; Moore, A.S. Managing the Canine Cancer Patient: A Practical Guide to Compassionate Care; Veterinary Learning Systems: Yardley, PA, USA, 2006; ISBN1 188425456X. ISBN2 9781884254567. [Google Scholar]
- Cohn, L.A. Canine Nasal Disease: An Update. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Plickert, H.D.; Tichy, A.; Hirt, R.A. Characteristics of canine nasal discharge related to intranasal diseases: A retrospective study of 105 cases. J. Small Anim. Pract. 2014, 55, 145–152. [Google Scholar] [CrossRef]
- Théon, A.P.; Madewell, B.R.; Harb, M.F.; Dungworth, D.L. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J. Am. Vet. Med. Assoc. 1993, 202, 1469–1475. [Google Scholar] [PubMed]
- Lana, S.E.; Dernell, W.S.; LaRue, S.M.; Lafferty, M.J.; Douple, E.B.; Brekke, J.H.; Withrow, S.J. Slow release cisplatin combined with radiation for the treatment of canine nasal tumors. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 1997, 38, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Lana, S.E.; Dernell, W.S.; Lafferty, M.H.; Withrow, S.J.; LaRue, S.M. Use of radiation and a slow-release cisplatin formulation for treatment of canine nasal tumors. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2004, 45, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Arfuso, F.; Alberghina, D.; Giudice, E.; Gianesella, M.; Piccione, G. Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodology. J. Therm. Biol. 2017, 69, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mathews, K.; Kronen, P.W.; Lascelles, D.; Nolan, A.; Robertson, S.; Steagall, P.V.M.; Wright, B.; Yamashita, K. Guidelines for Recognition, Assessment and Treatment of Pain. J. Small Anim. Pract. 2014, 55, E10–E68. [Google Scholar] [CrossRef] [PubMed]
- Cugmas, B.; Šušterič, P.; Gorenjec, N.R.; Plavec, T. Comparison between rectal and body surface temperature in dogs by the calibrated infrared thermometer. Vet. Anim. Sci. 2020, 9, 100120. [Google Scholar] [CrossRef]
- Repac, J.; Alvarez, L.X.; Lamb, K.; Gillette, R.L. Evaluation of Thermographic Imaging in Canine Hindlimb Muscles after 6 Min of Walking—A Pilot Study. Front. Vet. Sci. 2020, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Valentini, S.; Bruno, E.; Nanni, C.; Musella, V.; Antonucci, M.; Spinella, G. Superficial Heating Evaluation by Thermographic Imaging before and after Tecar Therapy in Six Dogs Submitted to a Rehabilitation Protocol: A Pilot Study. Animals 2021, 11, 249. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.; Heinzl, E.; Bellucci, D.; Prato-Previde, E.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. Clin. Appl. Res. 2014, 10, 17–23. [Google Scholar] [CrossRef]
- Denham, H.D.C.; Bradshaw, J.W.S.; Rooney, N.J. Repetitive behaviour in kennelled domestic dog: Stereotypical or not? Physiol. Behav. 2014, 128, 288–294. [Google Scholar] [CrossRef]
- Part, C.E.; Kiddie, J.L.; Hayes, W.A.; Mills, D.S.; Neville, R.F.; Morton, D.B.; Collins, L.M. Physiological, physical and behavioural changes in dogs (Canis familiaris) when kennelled: Testing the validity of stress parameters. Physiol. Behav. 2014, 133, 260–271. [Google Scholar] [CrossRef]
- Otte, J. Hyperthermia in cancer therapy. Eur. J. Pediatr. 1988, 147, 560–569. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, M.; Russo, U.; Papapietro, V.R.; Ceccarelli, F.; Pogliacomi, F.; Vaienti, E.; Piccolo, C.; Capasso, R.; Sica, A.; Cioce, F.; et al. Radiofrequency ablation of osteoid osteoma. Acta Biomed. 2018, 89, 175–185. [Google Scholar] [CrossRef]
- Salyer, S.A.; Wavreille, V.A.; Fenger, J.M.; Jennings, R.N.; Selmic, L.E. Evaluation of microwave ablation for local treatment of dogs with distal radial osteosarcoma: A pilot study. Vet. Surg. 2020, 49, 1396–1405. [Google Scholar] [CrossRef] [PubMed]




| Behavior | Operational Discription |
|---|---|
| Sniffing | Muzzle/nose oriented in a clearly observable direction |
| Licking | Licking nose or lip |
| Yawning | Opening mouth widely and inhaling |
| Panting | Tongue exposed and observable breathing |
| Mouth breathing | Observable breathing through the mouth |
| Stretching | Extending body and one or more front and/or hind legs while remaining stationary |
| Whining | A cyclic vocalization |
| Nausea | Abnormal heaving or chewing |
| Head down | Lying down with head placed on front paws |
| Head up | Lying down with head away from front paws |
| Standing | Supported upright with all four legs |
| Sitting | Supported by two extended front legs and two flexed back legs |
| Back posture | Showing back against the camera |
| Before RT [Median (Range)] | After RT [Median (Range)] | p-Value | |
|---|---|---|---|
| Head up | 27.8 (0–49.4) | 20.4 (2.8–40.6) | 0.53 |
| Mouth breathing | 3.4 (0–13.4) | 2.6 (0–18.3) | 0.98 |
| Head down | 14.3 (0–36.8) | 29.3 (0–46.9) | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeki, K.; Kutara, K.; Iwata, E.; Miyabe, M.; Shimizu, Y.; Wada, Y.; Ohnishi, A.; Matsuda, A.; Miyama, T.S.; Asanuma, T. Noninvasive Thermographic Photographing as an Assessment of the State of Discomfort in a Dog Receiving Radiation Therapy. Animals 2021, 11, 2496. https://doi.org/10.3390/ani11092496
Saeki K, Kutara K, Iwata E, Miyabe M, Shimizu Y, Wada Y, Ohnishi A, Matsuda A, Miyama TS, Asanuma T. Noninvasive Thermographic Photographing as an Assessment of the State of Discomfort in a Dog Receiving Radiation Therapy. Animals. 2021; 11(9):2496. https://doi.org/10.3390/ani11092496
Chicago/Turabian StyleSaeki, Kaori, Kenji Kutara, Eri Iwata, Masahiro Miyabe, Yuki Shimizu, Yuko Wada, Akihiro Ohnishi, Akira Matsuda, Takako Shimokawa Miyama, and Taketoshi Asanuma. 2021. "Noninvasive Thermographic Photographing as an Assessment of the State of Discomfort in a Dog Receiving Radiation Therapy" Animals 11, no. 9: 2496. https://doi.org/10.3390/ani11092496






