Parasite Fauna of the Dusky Grouper (Epinephelus marginatus, Lowe 1834) from the Central Mediterranean Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
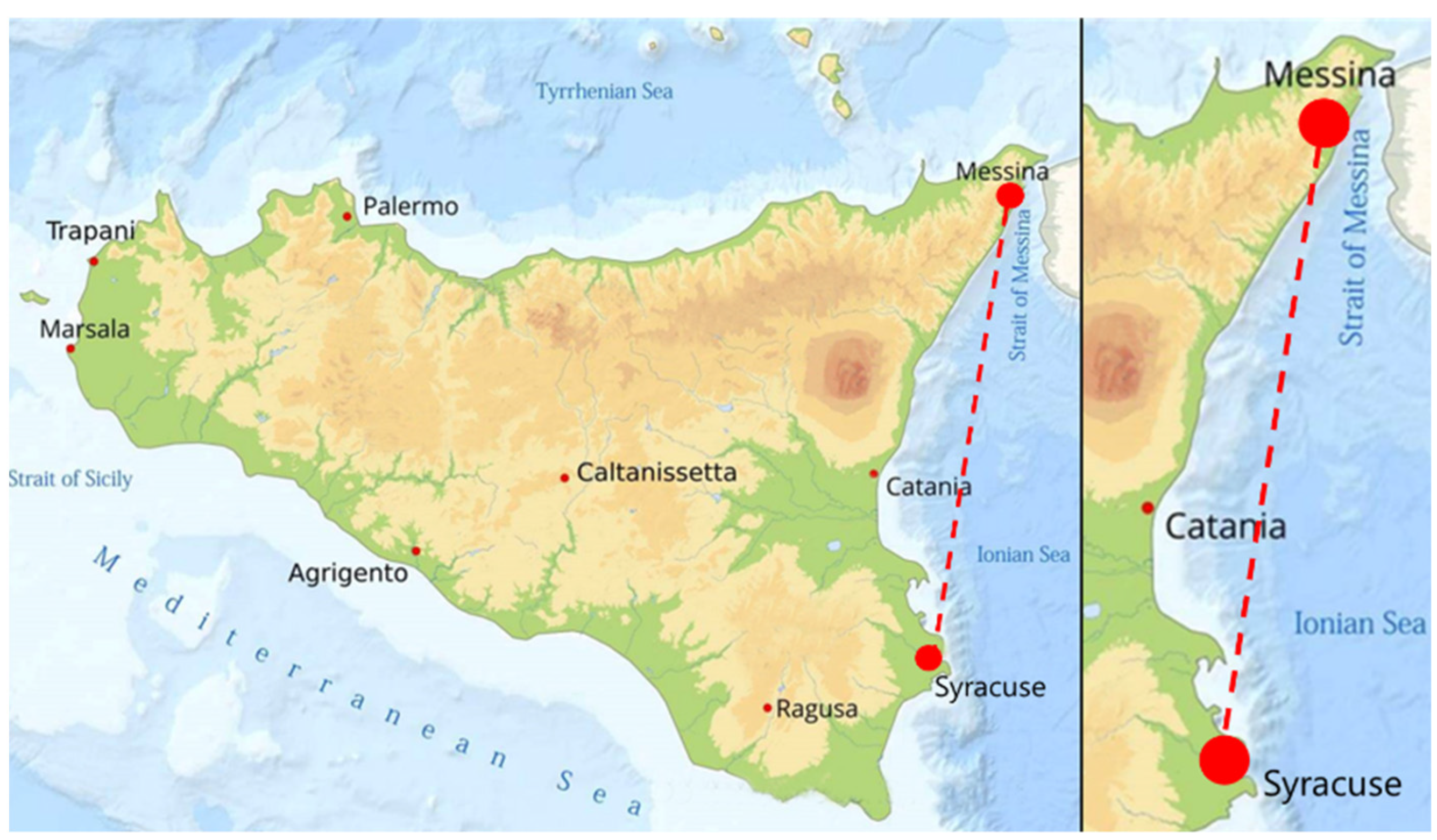
2.1. Fish Sampling
2.2. Anatomopathological and Parasitological Examination
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Linde, M.; Grau, A.M.; Riera, F.; Massutí-Pascual, E. Analysis of trophic ontogeny in Epinephelus marginatus (Serranidae). Cybium 2004, 28, 27–35. [Google Scholar]
- Reñones, O.; Polunin, N.V.C.; Goni, R. Size related shifts of Epinephelus marginatus in a western Mediterranean littoral ecosystem: An isotope and stomach content analysis. J. Fish. Biol. 2002, 61, 122–137. [Google Scholar] [CrossRef]
- Begossi, A.; Silvano, R.A.M. Ecology and ethnoecology of dusky grouper [garoupa, Epinephelus marginatus (Lowe, 1834)] along the coast of Brazil. J. Ethnob. Ethnom. 2008, 4, 20. [Google Scholar] [CrossRef]
- Tupper, M.; Sheriffn, N. Capture-based aquaculture of groupers. In Capture-Based Aquaculture; Global Overview; FAO Fisheries Technical Paper; Lovatelli, A., Holthus, P.F., Eds.; FAO: Rome, Italy, 2008; pp. 217–253. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2015.4. 2015. Available online: http://www.iucnredlist.org (accessed on 5 June 2018).
- Mahmoud, N.E.; Alhindy, M.K.; Fahmy, M.M. Trypanorhynch cestodes infecting Mediterranean Sea fishes, Egypt: Callitetrarhynchus gracilis larvae (Pintner, 1931) as a bio-indicator of heavy metals pollution. J. Oceanogr. Mar. Sci. 2015, 3, 133. [Google Scholar]
- Merella, P.; Renones, O.; Garippa, G. Reinstatement of Philometra jordanoi (Lopez-Neyra, 1951) (Nematoda, Philometridae): A parasite of the Mediterranean dusky grouper Epinephelus marginatus (Lowe) (Osteichthyes, Serranidae). Syst. Parasitol. 2005, 61, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Moravec, F.; Glamuzina, B.; Marino, G.; Merella, P.; Di Cave, D. Occurrence of Philometra lateolabracis (Nematoda: Philometridae) in the gonads of marine perciform fishes in the Mediterranean region. Dis. Aquat. Organ. 2003, 53, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Roumbedakis, K.; Marchiori, N.C.; Paseto, A.; Gonçalves, E.; Luque, J.; Cepeda, P.B.; Sanches, E.; Martins, M. Parasite fauna of wild and cultured dusky-grouper Epinephelus marginatus (Lowe, 1834) from Ubatuba, Southeastern Brazil. Braz. J. Biol. 2013, 73, 871–878. [Google Scholar] [CrossRef]
- Roumbedakis, K.; Marchiori, N.C.; Garcia, P.; Pereira, J.; Castro, L.A.S.; Martins, M.L. Helicometrina nimia Linton, 1910 (Digenea: Opecoelidae) in dusky grouper Epinephelus marginatus (Lowe, 1834) (Teleostei: Serranidae) from southeastern Brazil. Braz. J. Biol. 2014, 74, 472–479. [Google Scholar] [CrossRef][Green Version]
- Santos, C.P.; Buchmann, K.; Gibson, D.I. Pseudorhabdosynochus spp. (Monogenea: Diplectanidae) from the gills of Epinephelus spp. in Brazilian water. Syst. Par. 2000, 45, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Genc, E. Infestation status of gnathiid isopod juveniles parasitic on dusky grouper (Epinephelus marginatus) from the northeast Mediterranean Sea. Parasitol. Res. 2007, 101, 761. [Google Scholar] [CrossRef]
- Marino, F.; Giannetto, S.; Paradiso, M.L.; Bottari, T.; De Vico, G.; Macrì, B. Tissue damage and haematophagia due to praniza larvae (Isopoda: Gnathiidae) in some aquarium seawater teleosts. Dis Aquat. Organ. 2004, 59, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Rizgalla, J.; Bron, J.E.; Shinn, A.P.; Herath, T.K.; Paladini, G.; Ferguson, H.W. Ulcerative dermatitis in wild dusky grouper Epinephelus marginatus (Lowe) from Libyan waters. J. Fish. Dis. 2016, 39, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Canestri-Trotti, G.; Fioravanti, M.L.; Patarnello, P.P.; Restani, R. Capsule branchiali da trematodi Didymozoidae in cernie (Epinephelus guaza) (Perciformes: Serranidae) dell’Adriatico meridionale. Parassitologia 1994, 36, 26. [Google Scholar]
- Gijόn-Botella, H.; Lόpez-Romàn, R. Branquial parasites of Epinephelus guaza (L.) in the Canary Archipelago. Parasitol. Int. 1987, 47, 620. [Google Scholar]
- Moravec, F.; Chaabane, A.; Neifar, L.; Gey, D.; Justine, J.L. Descriptions of Philometra aenei n. sp. and P. tunisiensis. n sp. (Nematoda: Philometridae) from Epinephelus spp. off Tunisia confirm a high degree of host specificity of gonad-infecting species of Philometra Costa, 1845 in groupers (Serranidae). Syst. Parasitol. 2016, 93, 115–128. [Google Scholar] [CrossRef]
- Polinas, M.; Mele, S.; Padrós, F.; Merella, P.; Antuofermo, E.; Gouraguine, A.; Reñones, O. Ecological and histopathological aspects of Didymodiclinus sp. (Trematoda: Didymozoidae) parasite of the dusky grouper, Epinephelus marginatus (Osteichthyes: Serranidae), from the western Mediterranean Sea. J. Fish. Dis. 2018, 41, 1385–1393. [Google Scholar] [CrossRef]
- Yamaguti, S. Digenetic Trematodes of Hawaiian Fishes; Interscience Publishers: New York, NY, USA, 1970. [Google Scholar]
- Bartoli, P.; Bray, R.A.; Gibson, D.I. Opecoelidae (Digenea) from western Mediterranean fishes: Three rare species. Syst. Parasitol. 2003, 55, 81–95. [Google Scholar] [CrossRef]
- Moravec, F.; Chaabane, A.; Justine, J.L.; Neifar, L. Two gonad-infecting species of Philometra (Nematoda: Philometridae) from groupers (Serranidae) off Tunisia, with a key to Philometra species infecting serranid gonads. Parasite 2016, 23, 8. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission; CABI Publish: Wallingford, Oxon, UK, 2000. [Google Scholar]
- Berland, B. Nematodes from some Norwegian marine fishes. Sarsia 1961, 2, 1–50. [Google Scholar] [CrossRef]
- Petter, A.J.; Maillard, C. Larves d’ascarides parasites de poissons en Méditerranée occidentale. Bull. Mus. Nam. Hist. Nat. Paris 4th Sér. Sect. A 1988, 10, 347–369. [Google Scholar]
- Cable, R.M. An. Illustrated Laboratory Manual of Parasitology; Burgess: Minniapolis, MN, USA, 1961. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Par. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Bray, R.A.; Justine, J.L. Bucephalidae (Digenea) from epinephelines (Serranidae: Perciformes) from the waters off New Caledonia, including Neidhartia lochepintade n. sp. Parasite 2013, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Fischthal, J.H.; Thomas, J.D. Digenetic trematodes of marine fishes from Ghana: Families Acanthocolpidae, Bucephalidae, Didymozoidae. Proc. Helminthol. Soc. Wash. 1968, 35, 237–247. [Google Scholar]
- Marino, F.; Busalacchi, B.; Bottari, T.; Rinelli, P.; Gaglio, G. Occurrence and prevalence of Philometra filiformis (Stossich, 1896) on Pagellus erythrinus (Linnaeus, 1758) in the southern Tyrrhenian Sea. J. Appl. Ichthyol. 2016, 32, 687–692. [Google Scholar] [CrossRef]
- Lòpez-Neyra, C.R. Sanguinofilaria jordanoi n. sp. (Nematoda: Filaroidea). Boletìn de la Universidad de Granada. Farmacia 1951, 1, 291–293. [Google Scholar]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef]




| P (%) | MA | MI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parasites with Indirect Life Cycle | Total | Warm Months | Cold Months | Total | Warm Months | Cold Months | Total | Warm Months | Cold |
| Infection Site | Months | ||||||||
| Prosorhynchus caudovatus (S, I) | 42.9 | 30.4 A | 48.9 | 5.32 | 4.39 | 5.77 | 7.90 | 7.77 | 8.47 |
| Podocotyle temensis (S, I) | 28.6 | 17.4 A | 34.0 | 1.14 | 0.43 | 1.49 | 1.70 | 0.77 | 2.19 |
| Hemipera sp. (I) | 1.4 | 0 | 2.1 | 0.01 | 0 | 0.02 | 0.02 | 0 | 0.03 |
| Didymodiclinus sp. (G) | 18.6 | 7.4 | 19.1 | 1.10 | 0.95 | 1.17 | 1.63 | 1.69 | 1.72 |
| Philometra jordanoi (GO) | 5.7 | 13.0 | 2.1 | 0.40 | 0.34 | 0.02 | 0.19 | 0.61 | 0.03 |
| Anisakis sp. type II (S, I) | 5.7 | 8.7 | 4.3 | 0.21 | 0.48 | 0.06 | 0.31 | 0.84 | 0.09 |
| Capillaria sp. (S) | 1.4 | 0 | 2.1 | 0.01 | 0 | 0.02 | 0.02 | 0 | 0.03 |
| Contracaecum sp. (S) | 1.4 | 4.3 | 0 | 0.10 | 0.04 | 0 | 0.02 | 0.08 | 0 |
| P (%) | MA | MI | |||||||
| Parasites with Direct Life Cycle | Total | Warm Months | Cold Months | Total | Warm Months | Cold Months | Total | Warm Months | Cold Months |
| Infection Site | |||||||||
| Pseudempleurosoma sp. (G) | 1.4 | 4.3 | 0 | 0.01 | 0.04 | 0 | 0.02 | 0.08 | 0 |
| Megalocotyle hexacantha (G) | 1.4 | 4.3 | 0 | 0.01 | 0.04 | 0 | 0.02 | 0.07 | 0 |
| Biometric Data of Epinephelus marginatus | ||
| Parasite Species | Weight (g) | Total Length (cm) |
| Pseudempleurosoma sp. | r = 0.07, p = 0.56 | r = 0.13, p = 0.27 |
| Megalocotyde hexacantha | r = −0.02, p = 0.86 | r = 0.03, p = 0.82 |
| Prosorhynchus caudovatus | r = 0.08, p = 0.52 | r = 0.20, p = 0.10 |
| Didymodiclinus sp. | r = 0.42, p = 0.0003 | r = 0.37, p = 0.001 |
| Philometra jordanoi | r = 0.53, p < 0.0001 | r = 0.14, p = 0.001 |
| Anisakis sp. type II | r = 0.06, p = 0.65 | r = 0.12, p = 0.32 |
| Podocotyle sp. | r = 0.02, p = 0.88 | r = 0.12, p = 0.32 |
| Hemipera sp. | r = 0.01, p = 0.93 | r = 0.06, p = 0.65 |
| Capillaria sp. | r = −0.07, p = 0.54 | r = −0.10, p = 0.40 |
| Contracaecum sp. | r = 0.11, p = 0.37 | r = 0.19, p = 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Benedetto, G.; Arfuso, F.; Ferrara, M.C.; Brianti, E.; Gaglio, G. Parasite Fauna of the Dusky Grouper (Epinephelus marginatus, Lowe 1834) from the Central Mediterranean Sea. Animals 2021, 11, 2523. https://doi.org/10.3390/ani11092523
De Benedetto G, Arfuso F, Ferrara MC, Brianti E, Gaglio G. Parasite Fauna of the Dusky Grouper (Epinephelus marginatus, Lowe 1834) from the Central Mediterranean Sea. Animals. 2021; 11(9):2523. https://doi.org/10.3390/ani11092523
Chicago/Turabian StyleDe Benedetto, Giovanni, Francesca Arfuso, Maria Catena Ferrara, Emanuele Brianti, and Gabriella Gaglio. 2021. "Parasite Fauna of the Dusky Grouper (Epinephelus marginatus, Lowe 1834) from the Central Mediterranean Sea" Animals 11, no. 9: 2523. https://doi.org/10.3390/ani11092523
APA StyleDe Benedetto, G., Arfuso, F., Ferrara, M. C., Brianti, E., & Gaglio, G. (2021). Parasite Fauna of the Dusky Grouper (Epinephelus marginatus, Lowe 1834) from the Central Mediterranean Sea. Animals, 11(9), 2523. https://doi.org/10.3390/ani11092523







