Osmoregulatory Plasticity of Juvenile Greater Amberjack (Seriola dumerili) to Environmental Salinity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sampling Protocol
2.3. Plasma and Tissue Parameters
2.4. Na+/K+-ATPase Activity (NKA)
2.5. Ussing Chamber Experiments
2.6. RNA Isolation and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Plasma and Tissue Parameters
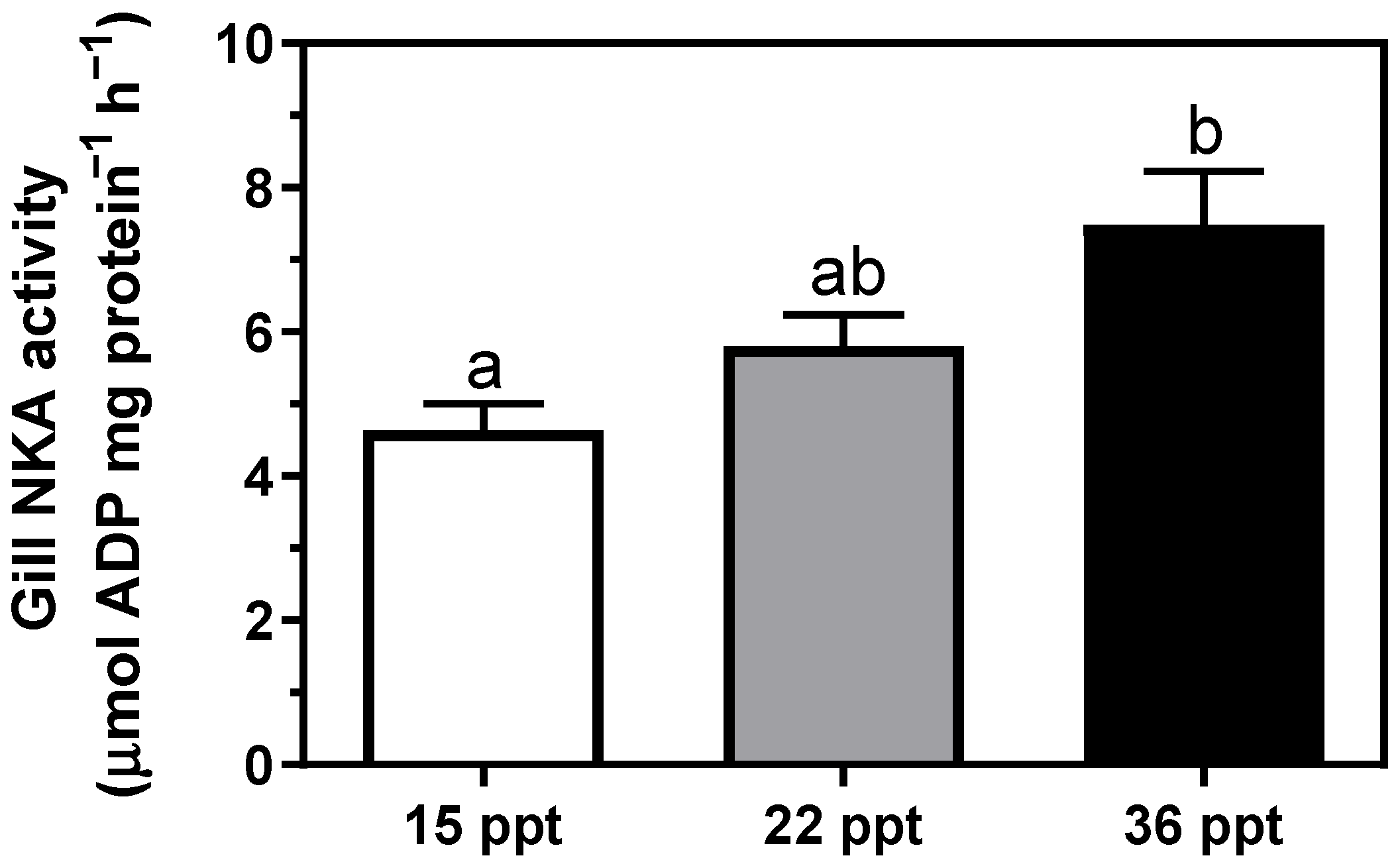
3.2. Na+/K+-ATPase Activity (NKA)
3.3. Ussing Chamber Experiments
3.4. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cultured Aquatic Species Information Programme. Seriola dumerili. Text by Jerez Herrera, S. and Vassallo Agius, R. In: FAO Fisheries Division. 2021. Available online: http://www.fao.org/fishery/culturedspecies/Seriola_dumerili/en (accessed on 10 June 2021).
- Mazzola, A.; Favaloro, E.; Sarà, G. Cultivation of the Mediterranean amberjack, Seriola dumerili (Risso, 1810), in submerged cages in the Western Mediterranean Sea. Aquaculture 2000, 181, 257–268. [Google Scholar] [CrossRef]
- Yamamoto, T.; Teruya, K.; Hara, T.; Hokazono, H.; Hashimoto, H.; Suzuki, N.; Iwashita, Y.; Matsunari, H. Nutritional evaluation of live food organisms and commercial dry feeds used for seed production of amberjack Seriola dumerili. Fish. Sci. 2008, 74, 1096–1108. [Google Scholar] [CrossRef]
- Fernández-Montero, A.; Caballero, M.J.; Torrecillas, S.; Tuset, V.M.; Lombarte, A.; Ginés, R.R.; Izquierdo, M.; Robaina, L.; Montero, D. Effect of temperature on growth performance of greater amberjack (Seriola dumerili, Risso 1810) Juveniles. Aquac. Res. 2018, 49, 908–918. [Google Scholar] [CrossRef]
- Barton, B.A.; Ribas, L.; Acerete, L.; Tort, L. Effects of chronic confinement on physiological responses of juvenile gilthead sea bream, Sparus aurata L., to acute handling. Aquac. Res. 2005, 36, 172–179. [Google Scholar] [CrossRef]
- Wang, N.; Xu, X.; Kestemont, P. Effect of temperature and feeding frequency on growth performances, feed efficiency and body composition of pikeperch juveniles (Sander lucioperca). Aquaculture 2009, 289, 70–73. [Google Scholar] [CrossRef]
- El-Araby, D.A.; Amer, S.A.; Khalil, A.A. Effect of different feeding regimes on the growth performance, antioxidant activity, and health of Nile tilapia, Oreochromis niloticus. Aquaculture 2020, 528, 735572. [Google Scholar] [CrossRef]
- Boeuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Blanco Garcia, A.; Partridge, G.J.; Flik, G.; Roques, J.A.C.; Abbink, W. Ambient salinity and osmoregulation, energy metabolism and growth in juvenile yellowtail kingfish (Seriola lalandi, Valenciennes 1833) in a recirculating aquaculture system. Aquac. Res. 2015, 46, 2789–2797. [Google Scholar] [CrossRef]
- Alameda, M.; Barany, A.; González-Manzano, G.; Saavedra, M.; Roo, J.; Muñoz, J.L.; Mancera, J.M. Sistema de recirculación y cultivo de seriola (S. dumerili): Influencia de la salinidad ambiental. In Libro de Actas XVII Congreso Nacional de Acuicultura (CNA): Cartagena, Spain. 2019, 437–438. Available online: https://drive.google.com/file/d/1RFcMPWmFJj99-R95hG2qQu0xP_iPMn6t/view (accessed on 10 June 2021).
- Grosell, M. The Role of the Gastrointestinal Tract in Salt and Water Balance, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 30. [Google Scholar]
- Takei, Y.; Loretz, C.A. 7—The gastrointestinal tract as an endocrine/neuroendocrine/paracrine organ: Organization, chemical messengers and physiological targets. In The Multifunctional Gut of Fish; Grosell, M., Farrell, A.P., Brauner, C.J., Eds.; Fish Physiology; Academic Press: Cambridge, MA, USA, 2010; Volume 30, pp. 261–317. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.S.; Grosell, M. Ion Osmoregulation, and Acid—Base Balance. In Physiology of Fishes; Evans, D.H., Claiborne, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 177–230. [Google Scholar]
- Alves, A.; Gregório, S.F.; Egger, R.C.; Fuentes, J. Molecular and functional regionalization of bicarbonate secretion cascade in the intestine of the European sea bass (Dicentrarchus labrax). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 233, 53–64. [Google Scholar] [CrossRef]
- Gregorio, S.F.; Carvalho, E.S.M.; Encarnacao, S.; Wilson, J.M.; Power, D.M.; Canario, A.V.M.; Fuentes, J. Adaptation to different salinities exposes functional specialization in the intestine of the sea bream (Sparus aurata L.). J. Exp. Biol. 2012, 470–479. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- McCormick, S.D. Smolt Physiology and Endocrinology. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2012; Volume 32, ISBN 9780123969514. [Google Scholar]
- Sangiao-Alvarellos, S.; Arjona, F.J.; Martín Del Río, M.P.; Míguez, J.M.; Mancera, J.M.; Soengas, J.L. Time course of osmoregulatory and metabolic changes during osmotic acclimation in Sparus auratus. J. Exp. Biol. 2005, 208, 4291–4304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martos-Sitcha, J.A.; Mancera, J.M.; Calduch-Giner, J.A.; Yúfera, M.; Martínez-Rodríguez, G.; Pérez-Sánchez, J. Unraveling the Tissue-Specific Gene Signatures of Gilthead Sea Bream (Sparus aurata L.) after Hyper- and Hypo-Osmotic Challenges. PLoS ONE 2016, 11, e0148113. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, B.; De Raedemaecker, F.; McGrath, D.; Brophy, D. An experimental investigation of salinity effects on growth, development and condition in the European flounder (Platichthys flesus L.). J. Exp. Mar. Biol. Ecol. 2011, 410, 39–44. [Google Scholar] [CrossRef]
- Mancera, J.; McCormick, S. Role of Prolactin, Growth Hormone, Insulin-like Growth Factor I and Cortisol in Teleost Osmoregulation. Fish Osmoregul. 2007, 497–515. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine control of osmoregulation in teleost fish. Am. Zool. 2001, 41, 781–794. [Google Scholar] [CrossRef]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.; Butler, A. The Somatomedin Hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef]
- Mancera, J.M.; Carrion, R.L.; del Rio, M.D.M. Osmoregulatory action of PRL, GH, and cortisol in the gilthead seabream (Sparus aurata L.). Gen. Comp. Endocrinol. 2002, 129, 95–103. [Google Scholar] [CrossRef]
- Fukamachi, S.; Sugimoto, M.; Mitani, H.; Shima, A. Somatolactin selectively regulates proliferation and morphogenesis of neural-crest derived pigment cells in medaka. Proc. Natl. Acad. Sci. USA 2004, 101, 10661–10666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, T. Cell biology of somatolactin. Int. Rev. Cytol. 1996, 169, 1–24. [Google Scholar] [CrossRef]
- Gorissen, M.; Flik, G. The Endocrinology of the Stress Response in Fish: An Adaptation-Physiological View. In Fish Physiology; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 35, pp. 75–111. [Google Scholar]
- Slominski, A.T. Proopiomelanocortin signaling system is operating in mast cells. J. Investig. Dermatol. 2006, 126, 1934–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grau, A.; Crespo, S.; Sarasquete, M.C.; de Canales, M.L.G. The digestive tract of the amberjack Seriola dumerili, Risso: A light and scanning electron microscope study. J. Fish Biol. 1992, 41, 287–303. [Google Scholar] [CrossRef]
- Keppler, D.; Decker, K. Glycogen. Determination with Amyloglucosidase. Methods Enzym. Anal. 1974, 3, 1127–1131. [Google Scholar]
- McCormick, S.D. Methods for Nonlethal Gill Biopsy and Measurement of Na+, K+ -ATPase Activity. Can. J. Fish. Aquat. Sci. 1993, 50, 656–658. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soengas, J.L.; Sangiao-Alvarellos, S.; Laiz-Carrión, R.; Mancera, J.M. Energy metabolism and osmotic acclimation in teleost fish. In Fish Osmoregulation; CRC Press: Boca Raton, FL, USA, 2019; pp. 277–307. ISBN 0429063903. [Google Scholar]
- McCormick, S.D.; Farrell, A.P.; Brauner, C.J. Fish physiology: Euryhaline Fishes; Academic Press: Cambridge, MA, USA, 2013; ISBN 0123972329. [Google Scholar]
- Grosell, M.; Farrell, A.P. The Multifunctional Gut of Fish; Grosell, M., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 30, ISBN 9780123749826. [Google Scholar]
- Tresguerres, M.; Levin, L.R.; Buck, J.; Grosell, M. Modulation of NaCl absorption by [HCO32-] in the marine teleost intestine is mediated by soluble adenylyl cyclase. AJP Regul. Integr. Comp. Physiol. 2010, 299, R62–R71. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Jarabo, I.; Barany, A.; Jerez-Cepa, I.; Mancera, J.M.; Fuentes, J. Intestinal response to salinity challenge in the Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 204, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Barany, A.; Shaughnessy, C.A.; Fuentes, J.; Mancera, J.M.; McCormick, S.D. Osmoregulatory role of the intestine in the sea lamprey (Petromyzon marinus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R410–R417. [Google Scholar] [CrossRef]
- Shaughnessy, C.A.; McCormick, S.D. Functional characterization and osmoregulatory role of the Na+-K+-2Cl- cotransporter in the gill of sea lamprey (Petromyzon marinus), a basal vertebrate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R17–R29. [Google Scholar] [CrossRef]
- Martos-Sitcha, J.A.; Gregório, S.F.; Carvalho, E.S.M.; Canario, A.V.M.; Power, D.M.; Mancera, J.M.; Martínez-Rodríguez, G.; Fuentes, J. AVT is involved in the regulation of ion transport in the intestine of the sea bream (Sparus aurata). Gen. Comp. Endocrinol. 2013, 193, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundell, K.; Sundh, H. Intestinal fluid absorption in anadromous salmonids: Importance of tight junctions and aquaporins. Front. Physiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, C.P.; Cramb, G. Differential expression of absorptive cation-chloride-cotransporters in the intestinal and renal tissues of the European eel (Anguilla anguilla). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 149, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.; Kusakabe, M.; Pipil, S.; Iwasaki, W.; Suzuki, Y.; Wong, M.K.-S.; Ozaki, H. Molecular mechanisms underlying active desalination and low water permeability in the esophagus of eels acclimated to seawater. Am. J. Physiol. Integr. Comp. Physiol. 2016, 312, R231–R244. [Google Scholar] [CrossRef] [Green Version]
- McCormick, S.D.; Regish, A.M.; Christensen, A.K.; Bjornsson, B.T. Differential regulation of sodium-potassium pump isoforms during smolt development and seawater exposure of Atlantic salmon. J. Exp. Biol. 2013, 216, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Poenar, D.P.; Yang, G.; Wan, W.K.; Feng, S. Low-Cost Method and Biochip for Measuring the Trans-Epithelial Electrical Resistance (TEER) of Esophageal Epithelium. Materials 2020, 13, 2354. [Google Scholar] [CrossRef]
- Barany, A.; Shaughnessy, C.A.; Pelis, R.M.; Fuentes, J.; Mancera, J.M.; McCormick, S.D. Tissue- and salinity-specific Na–Cl cotransporter (NCC) orthologues involved in the sea lamprey (Petromyzon marinus) adaptive osmoregulation. Sci. Rep. 2021, in press. [Google Scholar]
- Sundell, K.; Jutfelt, F.; Olsen, R.; Sandblom, E.; Hansen, T. Intestinal transport mechanisms and plasma cortisol levels during normal and out-of-season parr–smolt transformation of Atlantic salmon, Salmo salar. Aquaculture 2003, 222, 265–285. [Google Scholar] [CrossRef]
- Brauner, C.J.; Seidelin, M.; Madsen, S.S.; Jensen, F.B. Effects of freshwater hyperoxia and hypercapnia and their influences on subsequent seawater transfer in Atlantic salmon (Salmo salar) smolts. Can. J. Fish. Aquat. Sci. 2000, 57, 2054–2064. [Google Scholar] [CrossRef]
- Brett, J.R.; Groves, T.D.D. Physiological energetics. Fish Physiol. 1979, 8, 280–352. [Google Scholar]
- Jobling, M. Fish bioenergetics. Oceanogr. Lit. Rev. 1995, 9, 785. [Google Scholar]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Gonçalves, O.; Páscoa, I.; Martín del Río, M.P.; Mancera, J.M. Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 2009, 287, 419–426. [Google Scholar] [CrossRef]
- Picha, M.E.; Turano, M.J.; Beckman, B.R.; Borski, R.J. Endocrine Biomarkers of Growth and Applications to Aquaculture: A Minireview of Growth Hormone, Insulin-Like Growth Factor (IGF)-I, and IGF-Binding Proteins as Potential Growth Indicators in Fish. N. Am. J. Aquac. 2008, 70, 196–211. [Google Scholar] [CrossRef]
- Vega-Rubín de Celis, S.; Rojas, P.; Gómez-Requeni, P.; Albalat, A.; Gutiérrez, J.; Médale, F.; Kaushik, S.J.; Navarro, I.; Pérez-Sánchez, J. Nutritional assessment of somatolactin function in gilthead sea bream (Sparus aurata): Concurrent changes in somatotropic axis and pancreatic hormones. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 533–542. [Google Scholar] [CrossRef]
- Fernández-Montero, Á.; Torrecillas, S.; Acosta, F.; Kalinowski, T.; Bravo, J.; Sweetman, J.; Roo, J.; Makol, A.; Docando, J.; Carvalho, M.; et al. Improving greater amberjack (Seriola dumerili) defenses against monogenean parasite Neobenedenia girellae infection through functional dietary additives. Aquaculture 2021, 534, 736317. [Google Scholar] [CrossRef]
- Brazenor, A.K.; Hutson, K.S. Effects of temperature and salinity on the life cycle of Neobenedenia sp. (Monogenea: Capsalidae) infecting farmed barramundi (Lates calcarifer). Parasitol. Res. 2015, 114, 1875–1886. [Google Scholar] [CrossRef]




| Gene | Sequences (5′ → 3′) | Amplicon Size (bp) | GenBank Acc. No |
|---|---|---|---|
| actb | FW: CTCTTCCAGCCTTCCTTCCT | 110 | MW311085.1 XM_022757055.1 |
| RV: GTGTTGGCGTACAGGTCCTT | |||
| eef1a | FW: GGAAGTTCGAGACCAGCAAG | 144 | MW311086.1 XM_022744048.1 |
| RV: CAGCCTCAAACTCACCAACA | |||
| gh | FW: GACCCTGAACCAGAACCTGA | 128 | MW311089.1 XM_022769709.1 |
| RV: AGCGATGGAGAACAGATGCT | |||
| pomca | FW: TGCATCCAGCTCTGTCACTC | 136 | MW311092.1 XM_022770028.1 |
| RV: TAGCCTGAGGTGAGGAGGA | |||
| pomcb | FW: CTGGCCGGTCAGTTGGAGGG | 107 | MW311093.1 XM_022757428 |
| RV: ATACACACTGCCCTGTCTCT | |||
| prl | FW: CAGAGGCAGACCTGTTGTCA | 87 | MW311094.1 XM_022747913.1 |
| RV: GCGTGTTAGCAGAGGAGGAC | |||
| sl | FW: TATTTGCGTCGAGCTGTC | 97 | MW311095.1 XM_022746319.1 |
| RV: AAGAGGCAGCGAGGAATACA |
| Parameters | 15 Psu | 22 Psu | 36 Psu |
|---|---|---|---|
| Glucose (mM) | 12.81 ± 0.89 a | 7.36 ± 0.77 b | 10.44 ± 0.39 a |
| Lactate (mM) | 5.20 ± 0.50 a | 4.81 ± 0.30 ab | 3.74 ± 0.23 b |
| Triglycerides (mM) | 2.01 ± 0.16 | 1.81 ± 0.20 | 1.87 ± 0.10 |
| Glucose (mmol g−1 w.w.) | 70.78 ± 4.58 a | 93.69 ± 4.52 ab | 103.30 ± 10.56 b |
| Glycogen (mmol g−1 w.w.) | 36.67 ± 4.75 a | 54.63 ± 4.18 ab | 61.98 ± 8.23 b |
| Triglycerides (mmol g−1 w.w.) | 48.96 ± 5.38 a | 67.49 ± 2.65 b | 51.05 ± 5.33 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barany, A.; Gilannejad, N.; Alameda-López, M.; Rodríguez-Velásquez, L.; Astola, A.; Martínez-Rodríguez, G.; Roo, J.; Muñoz, J.L.; Mancera, J.M. Osmoregulatory Plasticity of Juvenile Greater Amberjack (Seriola dumerili) to Environmental Salinity. Animals 2021, 11, 2607. https://doi.org/10.3390/ani11092607
Barany A, Gilannejad N, Alameda-López M, Rodríguez-Velásquez L, Astola A, Martínez-Rodríguez G, Roo J, Muñoz JL, Mancera JM. Osmoregulatory Plasticity of Juvenile Greater Amberjack (Seriola dumerili) to Environmental Salinity. Animals. 2021; 11(9):2607. https://doi.org/10.3390/ani11092607
Chicago/Turabian StyleBarany, Andre, Neda Gilannejad, María Alameda-López, Liliana Rodríguez-Velásquez, Antonio Astola, Gonzalo Martínez-Rodríguez, Javier Roo, Jose Luis Muñoz, and Juan Miguel Mancera. 2021. "Osmoregulatory Plasticity of Juvenile Greater Amberjack (Seriola dumerili) to Environmental Salinity" Animals 11, no. 9: 2607. https://doi.org/10.3390/ani11092607






