Description of an Ultrasound-Guided Transverse Approach to the Transversus Thoracis Plane Block and Evaluation of Injectate Spread in Canine Cadavers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
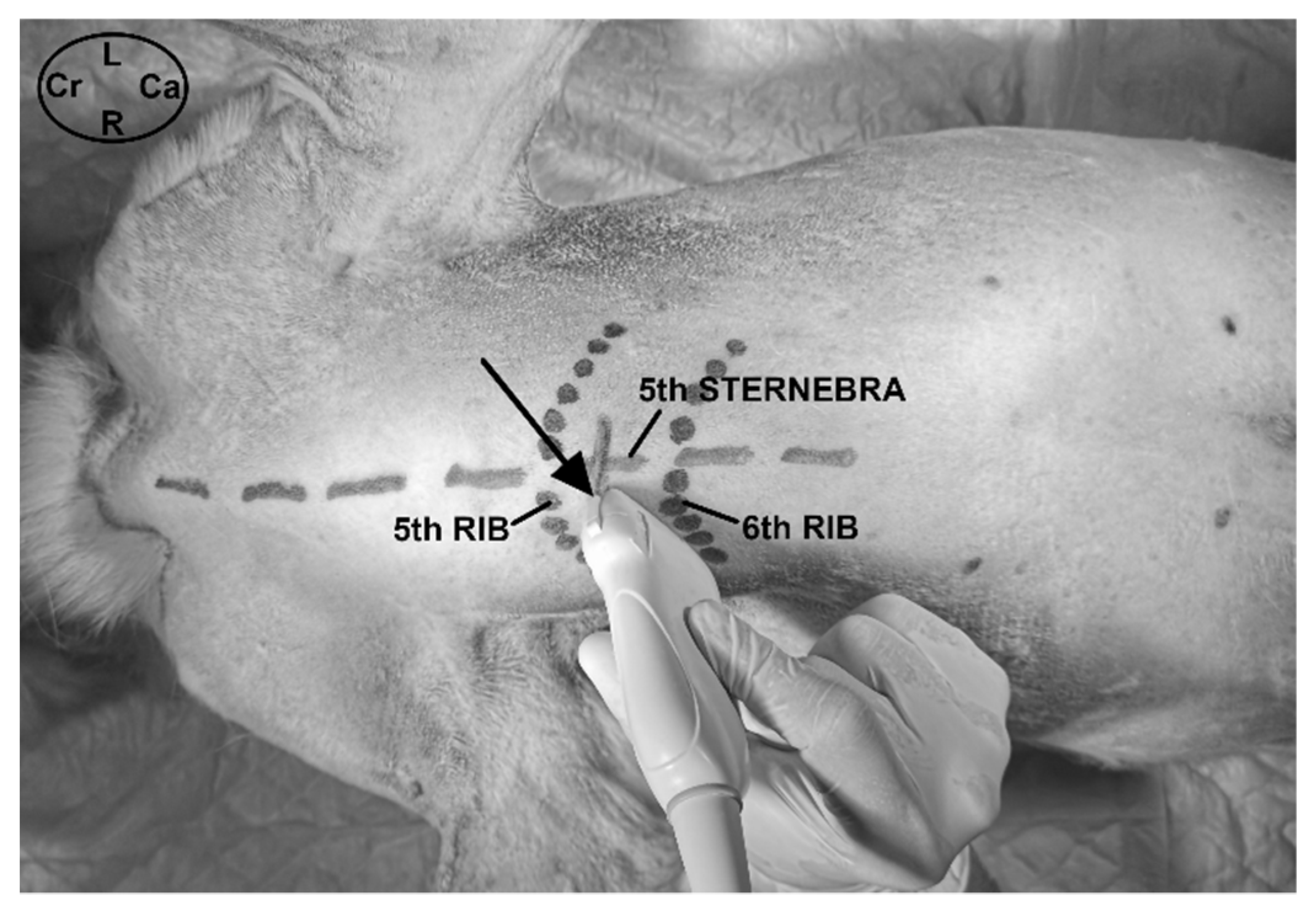
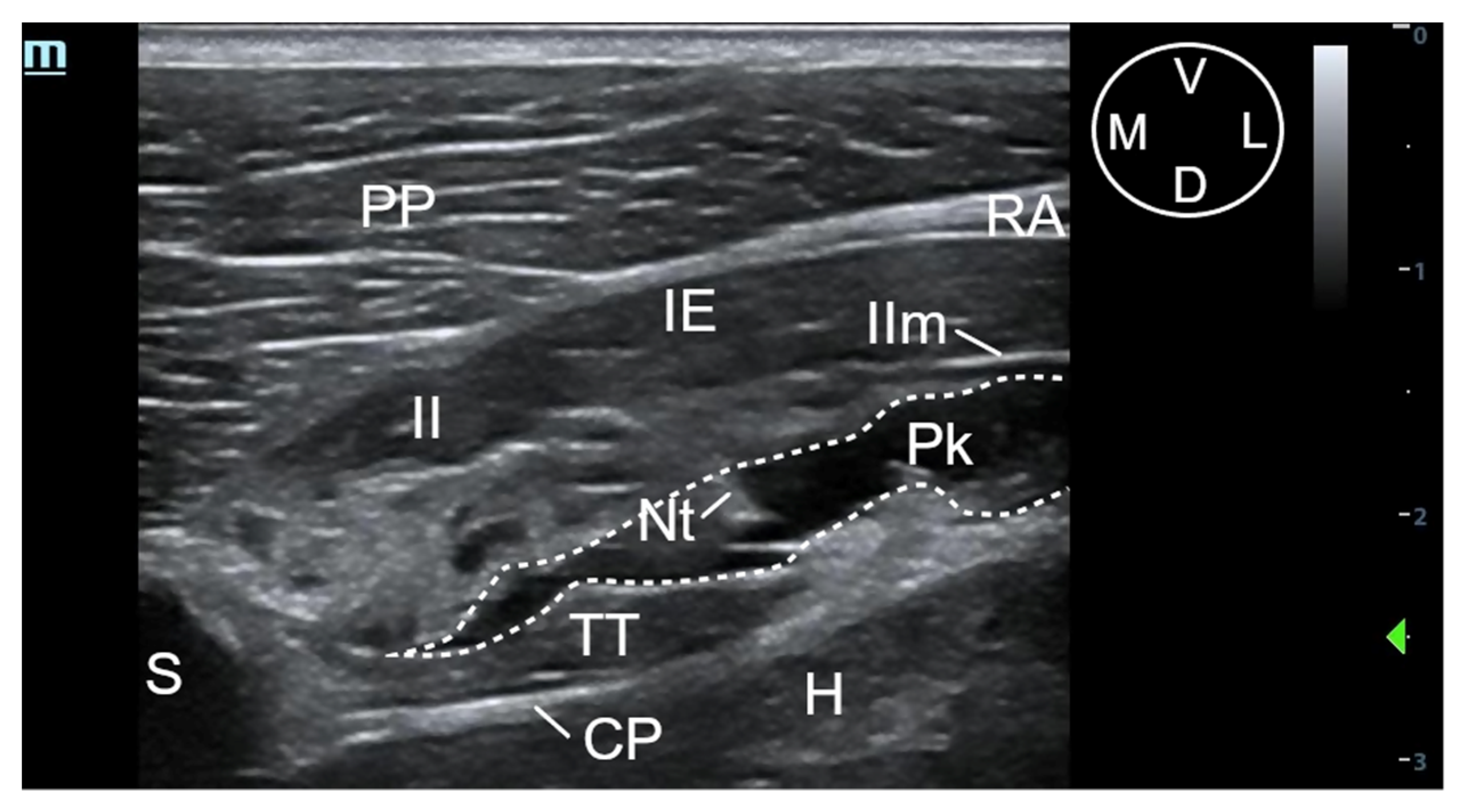
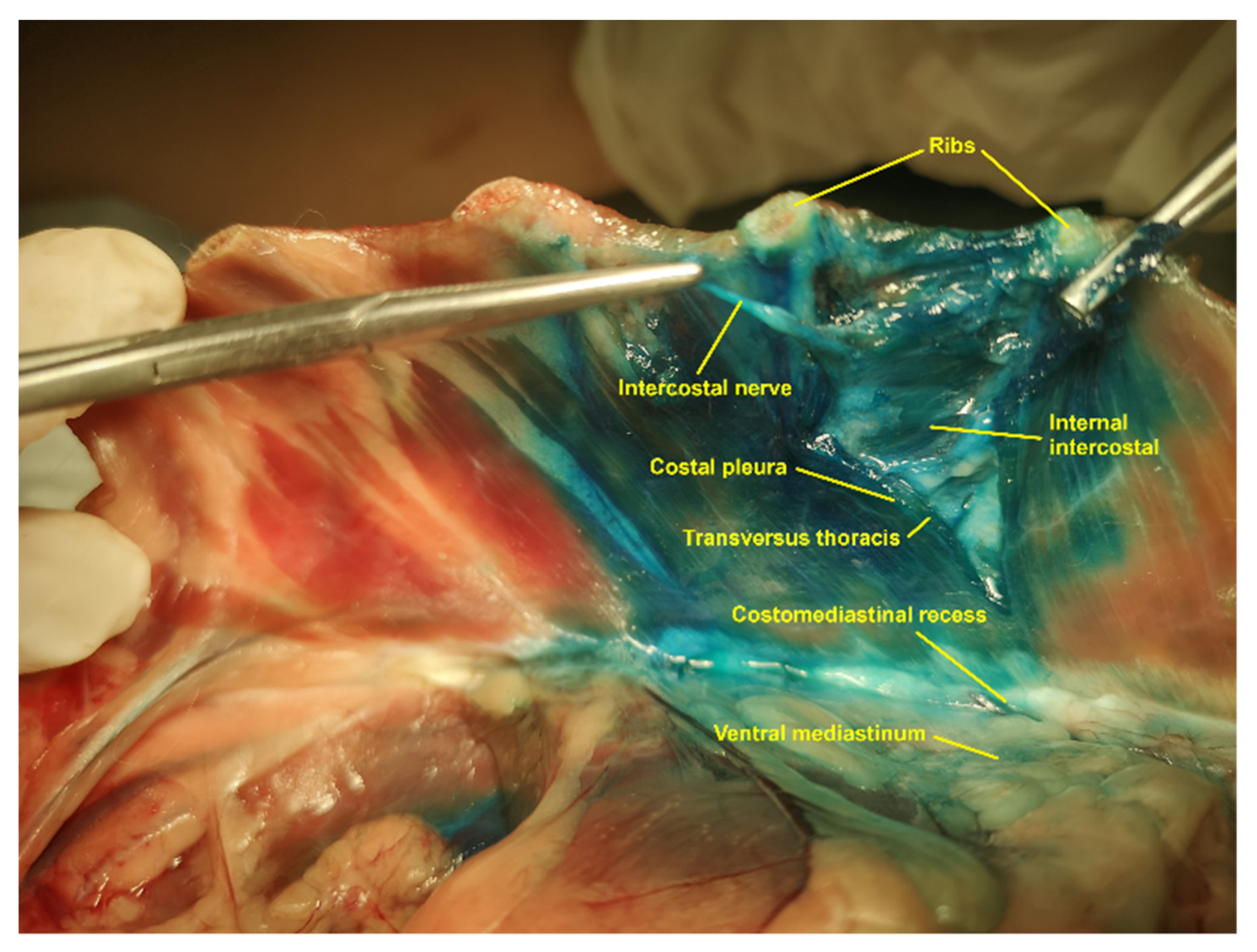
2.2. Phase I: Anatomical and Ultrasound Study
2.3. Phase II: Evaluation of the Injectable Solution Spread
2.3.1. Ultrasound-Guided t-TTP Injection
2.3.2. Evaluation of Methylene Blue-Lidocaine Solution
2.4. Statistical Analysis
3. Results
3.1. Phase I: Anatomical and Ultrasound Study
3.1.1. Gross Anatomical Description
3.1.2. Description of the Sonoanatomy
3.2. Phase II: Evaluation of the Injectable Solution Spread
3.2.1. Ultrasound-Guided t-TTP Injection
3.2.2. Evaluation of Methylene Blue-Lidocaine Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Grade | Description |
|---|---|
| Good | The shaft and the tip of the needle can be visualized completely. |
| Poor | The needle tip is only visualized after gentle in- and out-movements or jiggling of the needle. |
| Absent | The needle tip cannot be recognized. |
References
- Mazzeffi, M.; Khelemsky, Y. Poststernotomy pain: A clinical review. J. Cardiothorac. Vasc. Anest. 2011, 25, 1163–1178. [Google Scholar] [CrossRef]
- Gregory, A.J.; Grant, M.C.; Manning, M.W.; Cheung, A.T.; Ender, J.; Sander, M.; Zarbock, A.; Stoppe, C.; Meineri, M.; Grocott, H.P.; et al. Enhanced recovery after cardiac surgery (ERAS Cardiac) recommendations: An important first step—But there is much work to be done. J. Cardiothorac. Vasc. Anesth. 2020, 34, 39–47. [Google Scholar] [CrossRef]
- Raj, N. Regional anesthesia for sternotomy and bypass-Beyond the epidural. Paediatr. Anesth. 2019, 29, 519–529. [Google Scholar] [CrossRef]
- Thompson, S.; Johnson, J.M. Analgesia in dogs after intercostal thoracotomy: Comparison of morphine, selective intercostal nerve block, and interpleural regional analgesia with bupivacaine. Vet. Surg. 1991, 20, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P.A.; Kirk, A.J.; Liles, J.H.; Hayes, P.H.; Dark, J.H. Post-operative analgesia following thoracotomy in the dog: An evaluation of the effects of bupivacaine intercostal nerve block and nalbuphine on respiratory function. Lab. Anim. 1991, 25, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascoe, P.J.; Dyson, D.H. Analgesia after lateral thoracotomy in dogs. Epidural morphine vs. intercostal bupivacaine. Vet. Surg. 1993, 22, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Portela, D.A.; Campoy, L.; Otero, P.E.; Martin-Flores, M.; Gleed, R.D. Ultrasound-guided thoracic paravertebral injection in dogs: A cadaveric study. Vet. Anaesth. Analg. 2017, 44, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Portela, D.A.; Fuensalida, S.; Viscasillas, J.; Verdier, N.; Otero, P.E. Peripheral nerve blocks of the thorax and abdomen. In Manual of Small Animal Regional Anesthesia: Illustrated Anatomy for Nerve Stimulation and Ultrasound-Guided Nerve Blocks, 2nd ed.; Otero, P.E., Portela, D.A., Eds.; Editorial Inter-Médica: Buenos Aires, Argentina, 2019; pp. 219–272. [Google Scholar]
- Asorey, I.; Sambugaro, B.; Bhalla, R.J.; Drozdzynska, M. Ultrasound-guided serratus plane block as an effective adjunct to systemic analgesia in four dogs undergoing thoracotomy. Open Vet. J. 2021, 10, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H.; Takeda, Y.; Ishikawa, S.; Otake, H. Ultrasound-guided transversus thoracic muscle plane block: A cadaveric study of the spread of injectate. J. Clin. Anesth. 2015, 27, 696. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Vissa, D.; Ganapathy, S.; Johnson, M.; Zhou, J. Transversus thoracic muscle plane block on a cadaver with history of coronary artery bypass grafting. Reg. Anesth. Pain Med. 2017, 42, 535–537. [Google Scholar] [CrossRef]
- Fujii, S.; Bairagi, R.; Roche, M.; Zhou, J.R. Transversus thoracis muscle plane block. BioMed. Res. Int. 2019, 2019, 1716365. [Google Scholar] [CrossRef]
- Abdelbaser, I.I.; Mageed, N.A. Analgesic efficacy of ultrasound guided bilateral transversus thoracis muscle plane block in pediatric cardiac surgery: A randomized, double-blind, controlled study. J. Clin. Anesth. 2020, 67, 110002. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Gong, H.; Zhang, B. Efficacy of bilateral transversus thoracis muscle plane block in pediatric patients undergoing open cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2430–2434. [Google Scholar] [CrossRef]
- Fujii, S.; Roche, M.; Jones, P.M.; Vissa, D.; Bainbridge, D.; Zhou, J.R. Transversus thoracis muscle plane block in cardiac surgery: A pilot feasibility study. Reg. Anesth. Pain Med. 2019, 44, 556–560. [Google Scholar] [CrossRef]
- Piraccini, E.; Biondi, G.; Byrne, H.; Calli, M.; Bellantonio, D.; Musetti, G.; Maitan, S. Ultrasound guided transversus thoracic plane block, parasternal block and fascial planes hydrodissection for internal mammary post thoracotomy pain syndrome. Eur. J. Pain 2018, 22, 1673–1677. [Google Scholar] [CrossRef]
- Aydin, M.E.; Celik, M.; Celik, E.C.; Ahiskalioglu, E.O.; Selvitopi, K. Transversus thoracic muscle plane block for persistent parasternal pain: The Tietze syndrome. J. Clin. Anesth. 2020, 63, 109755. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H.; Kimura, S.; Otake, H. Bilateral breast cancer resection performed under the bilateral transversus thoracic muscle plane block. J. Clin. Anesth. 2016, 33, 413. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H.; Otake, H. A combination of an erector spinae plane block and a transversus thoracic muscle plane block for partial mastectomy. J. Clin. Anesth. 2019, 54, 1. [Google Scholar] [CrossRef]
- Ueshima, H.; Otake, H. Continuous transversus thoracic muscle plane block is effective for the median sternotomy. J. Clin. Anesth. 2017, 37, 174. [Google Scholar] [CrossRef]
- Ueshima, H.; Otake, H. Comparison of spread of transversus thoracic plane block by sagittal and transverse approach in a clinical setting. J. Clin. Anesth. 2017, 43, 4–5. [Google Scholar] [CrossRef]
- Zublena, F.; Briganti, A.; De Gennaro, C.; Corletto, F. Ultrasound-guided parasternal injection in dogs: A cadaver study. Vet. Anaesth. Analg. 2021, 48, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.E.; de Lahunta, A. Spinal nerves. In Miller’s Anatomy of the Dog, 4th ed.; Carioto, L., Ed.; Elsevier: St. Louis, MO, USA, 1993; pp. 224–228. [Google Scholar]
- Dam, M.; Moriggl, B.; Hansen, C.K.; Hoerman, R.; Bendtsen, T.F.; Børglum, J. The pathway of injectate spread with the transmuscular quadratus lumborum block: A cadaver study. Anesth. Analg. 2017, 125, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H.; Otake, H. Where is an appropriate injection point for an ultrasound-guided transversus thoracic muscle plane block? J. Clin. Anesth. 2016, 33, 190–191. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.B.; Jacobsohn, E.; Kopacz, D.J.; Desphande, S.; Helman, J.D.; Salinas, F.; Hall, R.A. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: The effect on postoperative pain, pulmonary function, and tracheal extubation times. Anest. Analg. 2005, 100, 25–32. [Google Scholar] [CrossRef]
- Thomson, A.C.S.; Portela, D.A.; Romano, M.; Otero, P.E. Evaluation of the effect of ultrasound guidance on the accuracy of intercostal nerve injection: A canine cadaveric study. Vet. Anaesth. Analg. 2021, 48, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ueshima, H.; Otake, H. Ultrasound-guided transversus thoracic muscle plane block: Complication in 299 consecutive cases. J. Clin. Anesth. 2017, 41, 60. [Google Scholar] [CrossRef] [PubMed]
- Kull, K.; Baer, G.A.; Samarütel, J.; Sand, J.; Rosenberg, P.H. Distribution of local anesthetic solution in retromediastinal block. Preliminary experimental results. Reg. Anesth. 1997, 22, 308–312. [Google Scholar] [CrossRef]
- Campoy, L.; Martin-Flores, M.; Looney, A.L.; Hollis, N.E.; Ludders, J.W.; Stewart, J.; Gleed, R.D.; Asakawa, M. Distribution of a lidocaine-methylene blue solution staining in brachial plexus, lumbar plexus and sciatic nerve blocks in the dog. Vet. Anaesth. Analg. 2008, 35, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Portela, D.A.; Otero, P.E.; Briganti, A.; Romano, M.; Corletto, F.; Breghi, G. Femoral nerve block: A novel psoas compartment lateral pre-iliac approach in dog. Vet. Anaesth. Analg. 2013, 40, 194–204. [Google Scholar] [CrossRef]


| Intercostal Nerves | LV | HV | p-Value * |
|---|---|---|---|
| T2 | 1 (12.5%) | 2 (25.0%) | 1 |
| T3 | 5 (62.5%) | 6 (75.0%) | 1 |
| T4 | 7 (87.5%) | 7 (87.5%) | 1 |
| T5 | 7 (87.5%) | 7 (87.5%) | 1 |
| T6 | 4 (50.0%) | 7 (87.5%) | 0.282 |
| T7 | 2 (25.0%) | 3 (37.5%) | 1 |
| TTP Segment | LV | HV | p-Value * |
|---|---|---|---|
| TTP (2) | 3 (37.5%) | 6 (75.0%) | 0.315 |
| TTP (3) | 6 (75.0%) | 7 (87.5%) | 1 |
| TTP (4) | 7 (87.5%) | 8 (100.0%) | 1 |
| TTP (5) | 8 (100.0%) | 8 (100.0%) | NA |
| TTP (6) | 5 (62.5%) | 7 (87.5%) | 0.569 |
| TTP (7) | 2 (25.0%) | 4 (50.0%) | 0.608 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaman, M.; González-Marrón, A.; Lorente, C.; Bonastre, C.; Laborda, A. Description of an Ultrasound-Guided Transverse Approach to the Transversus Thoracis Plane Block and Evaluation of Injectate Spread in Canine Cadavers. Animals 2021, 11, 2657. https://doi.org/10.3390/ani11092657
Alaman M, González-Marrón A, Lorente C, Bonastre C, Laborda A. Description of an Ultrasound-Guided Transverse Approach to the Transversus Thoracis Plane Block and Evaluation of Injectate Spread in Canine Cadavers. Animals. 2021; 11(9):2657. https://doi.org/10.3390/ani11092657
Chicago/Turabian StyleAlaman, Manuel, Adrián González-Marrón, Cristina Lorente, Cristina Bonastre, and Alicia Laborda. 2021. "Description of an Ultrasound-Guided Transverse Approach to the Transversus Thoracis Plane Block and Evaluation of Injectate Spread in Canine Cadavers" Animals 11, no. 9: 2657. https://doi.org/10.3390/ani11092657
APA StyleAlaman, M., González-Marrón, A., Lorente, C., Bonastre, C., & Laborda, A. (2021). Description of an Ultrasound-Guided Transverse Approach to the Transversus Thoracis Plane Block and Evaluation of Injectate Spread in Canine Cadavers. Animals, 11(9), 2657. https://doi.org/10.3390/ani11092657







