Fascioliasis in Llama, Lama glama, in Andean Endemic Areas: Experimental Transmission Capacity by the High Altitude Snail Vector Galba truncatula and Epidemiological Analysis of Its Reservoir Role
Abstract
:Simple Summary
Abstract
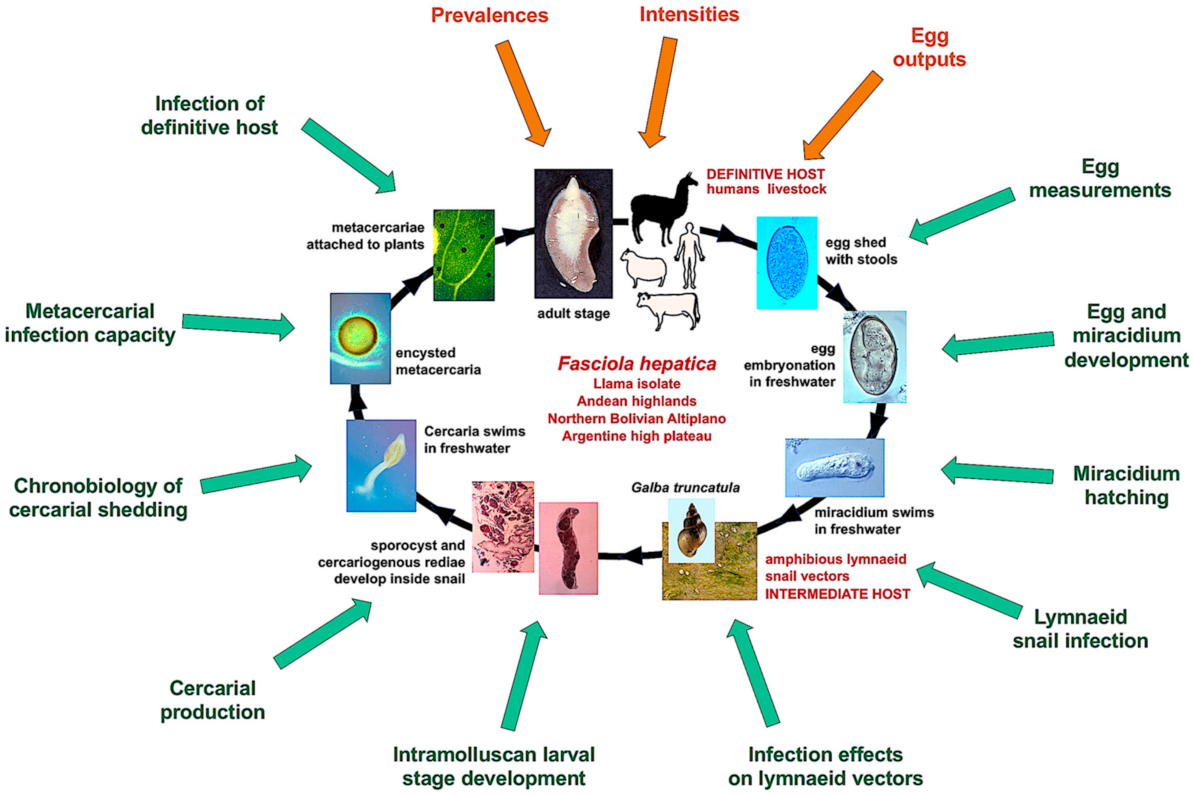
1. Introduction
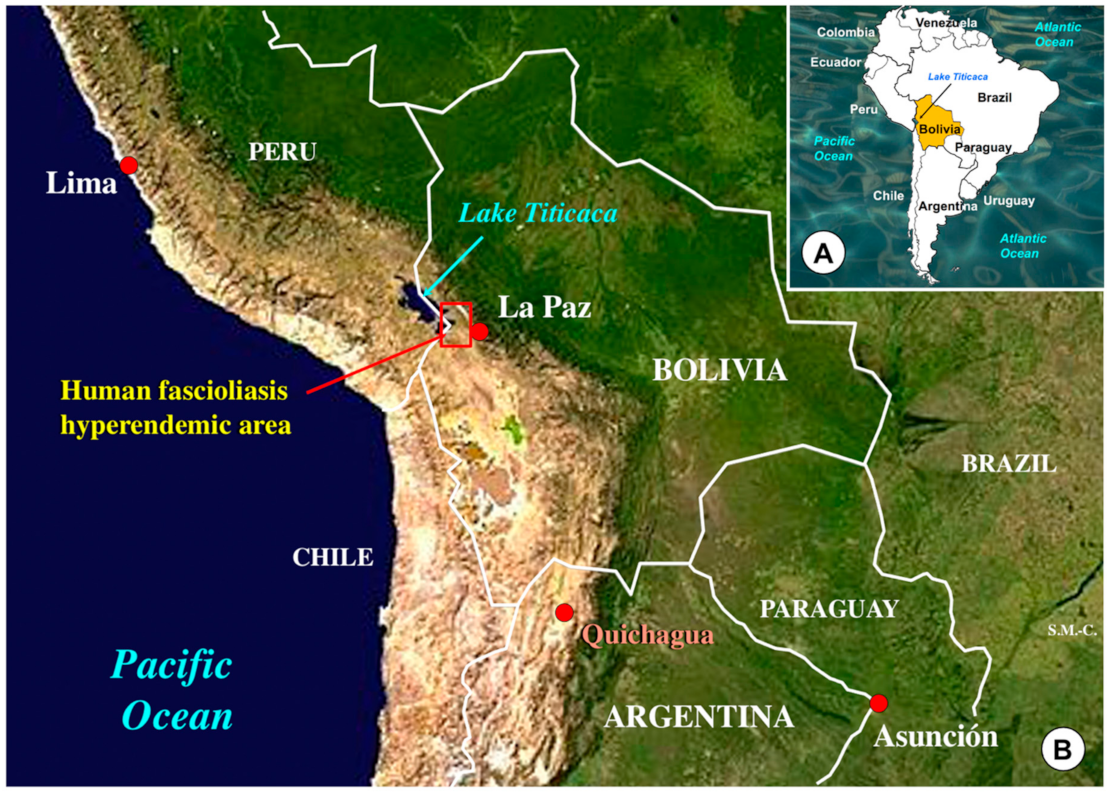
- in Bolivia, the human fascioliasis hyperendemic area where the highest prevalences (up to 72% by coprology and 100% by serology) and intensities (up to 8,000 eggs per gram of feces—epg—in children) have been reported in the Northern Bolivian Altiplano, at 3800–4100 m a.s.l., in between Lake Titicaca and the city of El Alto and the capital La Paz [12,42,43]; F. hepatica infection has been reported in alpacas raised on a farm of Belen, northward of the human endemic area [44,45];
- in Peru, human fascioliasis endemic areas have been described throughout many valleys along the whole north-south distributed Andean chain [13,46,47,48,49,50,51,52,53] and also in the Peruvian Altiplano [54]; the camelid species having been found infected by the liver fluke in Peru include the llama [55,56], alpaca [56,57,58,59], and vicuña [60,61].
2. Materials and Methods
2.1. Fasciolid Materials
2.2. Embryogenesis of Fluke Eggs
2.3. Experimental Infection of Snails
2.4. Laboratory Infections of Wistar Rats
2.5. Field Surveys of Llamas
2.6. Statistical Analyses
3. Results
3.1. Egg Embryonation
3.2. Snail Infectivity and Intramolluscan Development
3.3. Chronobiological Pattern of The Cercarial Emergence
3.4. Experimental Infectivity of Mammal Host
3.5. Prevalence, Intensity, Egg Measurements and Shedding Rates
4. Discussion
4.1. Egg Embryonation
4.1.1. Egg Embryonation in the Llama Isolate
4.1.2. Miracidial Infectivity, Intramolluscan Development and Cercarial Chronobiology
4.1.3. Cercarial Production, Lymnaeid Survival and Metacercarial Infectivity
4.2. Epidemiological Role of the Llama
- the zone located north of the endemic area, at a higher altitude on the way to the Eastern Andean Chain; it should be considered that there is no fascioliasis transmission in places located at an altitude higher than 4000 m a.s.l. due to the inability of the transmitting lymnaeids to survive at the temperature of such extreme altitudes [28];
- the altiplanic zone located south of the endemic area, on the way to Oruro, where temperatures are colder owing to the absence of the milder climatic influence of Lake Titicaca [97], which similarly explain the absence of lymnaeid vectors [28]; indeed, none among a total of 404 llamas from the Oruro department (82.4% from Sajama province, 8.7% from San Pedro de Totora province, 5.4% from Carangas province, and 3.5% from Litoral province) analyzed in the slaughterhouse of the locality of Turco, close to the city of Oruro, were found to be infected [98].
- Group I: It includes early resistance hosts characterized by possessing tissues that are not suitable for the fluke and resulting in a high degree of natural resistance. The infection is self-limiting without harming the host. The domestic pig is an example.
- Group II: This concerns the delayed resistance hosts characterized by a resistance that is acquired during the first weeks of a primary infection or during challenge infection. A delayed host reaction controls flukes during tissue migration, and chronic reactions including bile duct calcification lead to the eventual elimination of infection. Mortality is not common. Cattle and horses represent this group.
- Group III: Host species of this group have low resistance resulting in severe tissue reactions that do not immobilize or eliminate the parasites. In the chronic condition, there is no calcification of the bile ducts and flukes often survive the life of the host. Mortality in both the acute and chronic phases is common. Sheep and goats are hosts included in this group.
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schweizer, G.; Braun, U.; Deplazes, P.; Torgerson, P.R. Estimating the financial losses due the bovine fasciolosis in Switzerland. Vet. Rec. 2005, 157, 188–193. [Google Scholar] [CrossRef]
- Mazeri, S.; Rydevik, G.; Handel, I.; Bronsvoort, B.M.; de Sargison, C.N. Estimation of the impact of Fasciola hepatica infection on time taken for UK beef cattle to reach slaughter weight. Sci. Rep. 2017, 7, 7319. [Google Scholar] [CrossRef]
- Espinoza, J.R.; Terashima, A.; Herrera-Velit, P.; Marcos, L.A. Fasciolosis humana y animal en el Perú: Impacto en la economía de las zonas endémicas. Rev. Peru. Med. Exp. Salud Públ. 2010, 27, 604–612. [Google Scholar] [CrossRef]
- Copeman, D.B.; Copland, R.S. Importance and potential impact of liver fluke in cattle and buffalo. In Overcoming Liver Fluke as a Constraint to Ruminant Production in South-East Asia; Gray, G.D., Copland, R.S., Copeman, D.B., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2008; Volume 133, pp. 21–36. [Google Scholar]
- Mas-Coma, S.; Agramunt, V.H.; Valero, M.A. Neurological and ocular fascioliasis in humans. Adv. Parasitol. 2014, 84, 27–149. [Google Scholar]
- Valero, M.A.; Bargues, M.D.; Khoubbane, M.; Artigas, P.; Quesada, C.; Berinde, L.; Ubeira, F.M.; Mezo, M.; Hernandez, J.L.; Agramunt, V.H.; et al. Higher physiopathogenicity by Fasciola gigantica than by the genetically close F. hepatica: Experimental long-term follow-up of biochemical markers. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 55–66. [Google Scholar] [CrossRef]
- Gonzalez-Miguel, J.; Valero, M.A.; Reguera-Gomez, M.; Mas-Bargues, C.; Bargues, M.D.; Simon-Martin, F.; Mas-Coma, S. Numerous Fasciola plasminogen-binding proteins may underlie blood-brain barrier leakage and explain neurological disorder complexity and heterogeneity in the acute and chronic phases of human fascioliasis. Parasitology 2019, 146, 284–298. [Google Scholar] [CrossRef] [Green Version]
- Rondelaud, D.; Dreyfuss, G.; Vignoles, P. Clinical and biological abnormalities in patients after fasciolosis treatment. Med. Mal. Infect. 2006, 36, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.G.; Mott, K.E. Progress in assessment of morbidity due to Fasciola hepatica infection: A review of recent literature. Trop. Dis. Bull. 1990, 87, R1–R38. [Google Scholar]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Diagnosis of human fascioliasis by stool and blood techniques: Update for the present global scenario. Parasitology 2014, 141, 1918–1946. [Google Scholar] [CrossRef] [PubMed]
- Girones, N.; Valero, M.A.; Garcia-Bodelon, M.A.; Chico-Calero, M.I.; Punzon, C.; Fresno, M.; Mas-Coma, S. Immune suppression in advanced chronic fascioliasis: An experimental study in a rat model. J. Infect. Dis. 2007, 195, 1504–1512. [Google Scholar] [CrossRef]
- Esteban, J.G.; Flores, A.; Angles, R.; Strauss, W.; Aguirre, C.; Mas-Coma, S. A population-based coprological study of human fascioliasis in a hyperendemic area of the Bolivian Altiplano. Trop. Med. Int. Health 1997, 2, 695–699. [Google Scholar] [CrossRef]
- Gonzalez, L.C.; Esteban, J.G.; Bargues, M.D.; Valero, M.A.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Hyperendemic human fascioliasis in Andean valleys: An altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop. 2011, 120, 119–129. [Google Scholar] [CrossRef]
- Brady, M.T.; O’Neill, S.M.; Dalton, J.P.; Mills, K.H. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 1999, 67, 5372–5378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, M.A.; Navarro, M.; Garcia-Bodelon, M.A.; Marcilla, A.; Morales, M.; Garcia, J.E.; Hernandez, J.L.; Mas-Coma, S. High risk of bacterobilia in advanced experimental chronic fasciolosis. Acta Trop. 2006, 100, 17–23. [Google Scholar] [CrossRef]
- Ollerenshaw, C.B. The ecology of the liver fluke (Fasciola hepatica). Vet. Rec. 1959, 71, 957–965. [Google Scholar]
- Afshan, K.; Fortes-Lima, C.A.; Artigas, P.; Valero, M.A.; Qayyum, M.; Mas-Coma, S. Impact of climate change and man-made irrigation systems on the transmission risk, long-term trend and seasonality of human and animal fascioliasis in Pakistan. Geospat. Health 2014, 8, 317–334. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, M.A.; Bargues, M.D. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasitol. 2009, 69, 41–146. [Google Scholar]
- De, N.V.; Le, T.H.; Agramunt, V.H.; Mas-Coma, S. Early postnatal and preschool age infection by Fasciola spp.: Report of five cases from Vietnam and worldwide review. Am. J. Trop. Med. Hyg. 2020, 103, 1578–1589. [Google Scholar] [CrossRef]
- Mas-Coma, S. Human fascioliasis emergence risks in developed countries: From individual patients and small epidemics to climate and global change impacts. Enf. Emerg. Microbiol. Clín. 2020, 38, 253–256. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases; Department of Control of Neglected Tropical Diseases, World Health Organization: Geneva, Switzerland, 2013; p. 128. [Google Scholar]
- Gandhi, P.; Schmitt, E.K.; Chen, C.W.; Samantray, S.; Venishetty, V.K.; Hughes, D. Triclabendazole in the treatment of human fascioliasis: A review. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 797–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals. A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020; p. 47. Available online: https://www.who.int/neglected_diseases/Ending-the-neglect-to-attain-the-SDGs--NTD-Roadmap.pdf (accessed on 23 July 2020).
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Artigas, P.; Valero, M.A.; Bargues, M.D. Sheep and cattle reservoirs in the highest human fascioliasis hyperendemic area: Experimental transmission capacity, field epidemiology and control within a One Health initiative in Bolivia. Front. Vet. Sci. 2020, 7, 583204. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Buchon, P.; Funatsu, I.R.; Angles, R.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Donkey fascioliasis within a One Health control action: Transmission capacity, field epidemiology, and reservoir role in a human hyperendemic area. Front. Vet. Sci. 2020, 7, 591384. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Funatsu, I.R.; Angles, R.; Buchon, P.; Mas-Bargues, C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Domestic pig prioritized in one health action against fascioliasis in human endemic areas: Experimental assessment of transmission capacity and epidemiological evaluation of reservoir role. ONE Health 2021, 13, 100249. [Google Scholar] [CrossRef] [PubMed]
- Mas-Coma, S.; Bargues, M.D.; Valero, M.A. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures. Parasitology 2018, 145, 1665–1699. [Google Scholar] [CrossRef] [Green Version]
- Bargues, M.D.; Artigas, P.; Angles, R.; Osca, D.; Duran, P.; Buchon, P.; Gonzales-Pomar, R.K.; Pinto-Mendieta, J.; Mas-Coma, S. Genetic uniformity, geographical spread and anthropogenic habitat modifications of lymnaeid vectors found in a One Health initiative in the highest human fascioliasis hyperendemic of the Bolivian Altiplano. Parasit Vectors 2020, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Angles, R.; Coello, J.; Artigas, P.; Funatsu, I.R.; Cuervo, P.F.; Buchon, P.; Mas-Coma, S. One Health initiative in the Bolivian Altiplano human fascioliasis hyperendemic area: Lymnaeid biology, population dynamics, microecology and climatic factor influences. Braz. J. Vet. Parasitol. 2021, 30, e025620. [Google Scholar] [CrossRef]
- Fassi-Fehri, M.M. Las enfermedades de los camélidos. Rev. Sci. Tech. Off. Int. Epiz. 1987, 6, 355–373. [Google Scholar]
- Malandrini, J.B.; Carnevale, S.; Velazquez, J.; Soria, C.C. Diagnóstico de Fasciola hepatica con la técnica de ELISA en el Departamento de Tinogasta. Ciencia 2009, 4, 143–151. [Google Scholar]
- Mera y Sierra, R.; Agramunt, V.H.; Cuervo, P.; Mas-Coma, S. Human fascioliasis in Argentina: Retrospective overview, critical analysis and baseline for future research. Parasit. Vectors 2011, 4, 104. [Google Scholar] [CrossRef] [Green Version]
- Bargues, M.D.; Malandrini, J.B.; Artigas, P.; Soria, C.C.; Velasquez, J.N.; Carnevale, S.; Mateo, L.; Khoubbane, M.; Mas-Coma, S. Human fascioliasis endemic areas in Argentina: Multigene characterisation of the lymnaeid vectors and climatic-environmental assessment of the transmission pattern. Parasit. Vectors 2016, 9, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafrune, M.M.; Rebuffi, G.E.; Cabrera, R.H.; Aguirre, D.H. Fasciola hepatica en llamas (Lama glama) de la puna argentina. Vet. Arg. 1996, 13, 570–574. [Google Scholar]
- Cafrune, M.M.; Rebuffi, G.E.; Gaido, A.B.; Aguirre, D.H. Fasciola hepatica in Semi-Captive Vicuñas (Vicugna vicugna) in North West Argentina. Vet. Rec. 1996, 139, 97. [Google Scholar] [CrossRef] [PubMed]
- Cafrune, M.M.; Aguirre, D.H.; Freytes, I. Fasciolosis en vicuñas (Vicugna vicugna) en semi-cautiverio de Molinos, Salta, Argentina, con notas de otros helmintos en este hospedador. Vet. Arg. 2004, 21, 513–520. [Google Scholar]
- Olaechea, F.V.; Abad, M. An outbreak of fascioliasis in semicaptive guanacos (Lama guanicoe) in Patagonia (Argentina). First report. In XX International Conference of the World Association for the Advancement of Veterinary Parasitology; WAAVP: Christchurch, New Zealand, 2005. [Google Scholar]
- Issia, L.; Ovejero, R.; Carmanchahi, P.; Pietrokovsky, S.; Wisnivesky-Colli, C. Primer registro de Fasciola hepatica en guanacos silvestres de Mendoza, Argentina. V Congreso Latinoamericano de Especialistas en Pequeños Rumiantes y Camélidos Sudamericanos. Mendoza 2007, 1–2. Available online: www.produccion-animal.com.ar (accessed on 23 January 2021).
- Apt, W.; Aguilera, X.; Vega, F.; Zulantay, I.; Retamal, C.; Apt, P.; Sandoval, J. Fascioliasis en la población rural de las provincias de Curico, Talca y Linares. Rev. Méd. Chile 1992, 120, 621–626. [Google Scholar]
- Artigas, P.; Bargues, M.D.; Mera y Sierra, R.; Agramunt, V.H.; Mas-Coma, S. Characterisation of fascioliasis lymnaeid intermediate hosts from Chile by DNA sequencing, with emphasis on Lymnaea viator and Galba truncatula. Acta Trop. 2011, 120, 245–257. [Google Scholar] [CrossRef]
- Alcaino, H.; Gorman, T. Parásitos de los animales domésticos en Chile. Parasitol. Día 1999, 23, 33–41. [Google Scholar] [CrossRef]
- Hillyer, G.V.; Soler de Galanes, M.; Rodriguez-Perez, J.; Bjorland, J.; Silva de Lagrava, M.; Ramirez Guzman, S.; Bryan, R.T. Use of the Falcon Assay Screening Test—Enzyme-Linked Immunosorbent Assay (FAST-ELISA) and the Enzyme-Linked Immunoelectrotransfer Blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am. J. Trop. Med. Hyg. 1992, 46, 603–609. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Angles, R.; Esteban, J.G.; Bargues, M.D.; Buchon, P.; Franken, M.; Strauss, W. The Northern Bolivian Altiplano: A region highly endemic for human fascioliasis. Trop. Med. Int. Health 1999, 4, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Arandia, C.R.; Morales, L.G.; Medina, M.G. Fascioliasis of livestock and snail host for Fasciola in Altiplano region of Bolivia. Natl. Inst. Anim. Hlth. Quart. 1975, 15, 61–67. [Google Scholar]
- Mas-Coma, S.; Angles, R.; Strauss, W.; Esteban, J.G.; Oviedo, J.A.; Buchon, P. Human fasciolasis in Bolivia: A general analysis and a critical review of existing data. Res. Rev. Parasitol. 1995, 55, 73–93. [Google Scholar]
- Raymundo, L.A.M.; Maco Flores, V.; Terashima, A.; Samalvides, F.; Miranda, E.; Tantalean, M.; Espinoza, J.R.; Gotuzzo, E. Hiperendemicidad de fasciolosis humana en el Valle del Mantaro, Perú: Factores de riesgo de la infección por Fasciola hepatica. Rev. Gastroenterol. Perú 2004, 24, 158–164. [Google Scholar]
- Valencia, N.; Pariona, A.; Huaman, M.; Miranda, F.; Quintanilla, S.; Gonzales, A. Seroprevalencia de fasciolosis en escolares y en ganado vacuno en la provincia de Huancavelica, Perú. Rev. Peru. Med. Exp. Salud Publ. 2005, 22, 96–102. [Google Scholar]
- Espinoza, J.R.; Maco, V.; Marcos, L.; Saez, S.; Neyra, V.; Terashima, A.; Samalvides, F.; Gotuzzo, E.; Chavarry, E.; Huaman, C.; et al. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am. J. Trop. Med. Hyg. 2007, 76, 977–982. [Google Scholar] [CrossRef]
- Valero, M.A.; Perez-Crespo, I.; Khoubbane, M.; Artigas, P.; Panova, M.; Ortiz, P.; Maco, V.; Espinoza, J.R.; Mas-Coma, S. Fasciola hepatica phenotypic characterisation in Andean human endemic areas: Valley versus altiplanic patterns analysed in liver flukes from sheep from Cajamarca and Mantaro, Peru. Infect. Genet. Evol. 2012, 12, 403–410. [Google Scholar] [CrossRef]
- Valero, M.A.; Periago, M.V.; Perez-Crespo, I.; Angles, R.; Villegas, F.; Aguirre, C.; Strauss, W.; Espinoza, J.R.; Herrera, P.; Terashima, A.; et al. Field evaluation of a coproantigen detection test for fascioliasis diagnosis and surveillance in human hyperendemic areas of Andean countries. PLoS Negl. Trop. Dis. 2012, 6, e1812. [Google Scholar] [CrossRef]
- Lopez, M.; White, A.C., Jr.; Cabada, M.M. Burden of Fasciola hepatica infection among children from Paucartambo in Cusco, Peru. Am. J. Trop. Med. Hyg. 2012, 86, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Bargues, M.D.; Artigas, P.; Khoubbane, M.; Ortiz, P.; Naquira, C.; Mas-Coma, S. Molecular characterisation of Galba truncatula, Lymnaea neotropica and L. schirazensis from Cajamarca, Peru and their potential role in transmission of human and animal fascioliasis. Parasit. Vectors 2012, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Bardales-Valdivia, J.N.; Bargues, M.D.; Hoban-Vergara, C.; Bardales-Bardales, C.; Goicoechea-Portal, C.; Bazan-Zurita, H.; Del Valle-Mendoza, J.; Ortiz, P.; Mas-Coma, S. Spread of the fascioliasis endemic area assessed by seasonal follow-up of rRNA ITS-2 sequenced lymnaeid populations in Cajamarca, Peru. One Health 2021, 13, 100265. [Google Scholar] [CrossRef]
- Esteban, J.G.; Gonzalez, C.; Bargues, M.D.; Angles, R.; Sanchez, C.; Naquira, C.; Mas-Coma, S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop. Med. Int. Health 2002, 7, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Leguia, G. The epidemiology and economic impact of llama parasites. Parasitol. Today 1991, 7, 54–56. [Google Scholar] [CrossRef]
- Flores, B.; Pinedo, R.; Suárez, F.; Angelats, R.; Chávez, A. Prevalencia de fasciolosis en llamas y alpacas en dos comunidades rurales de Jauja, Peru. Rev. Investig. Vet. Perú 2014, 25, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, J.; Condorena, N. Fasciola hepatica en higado de alpaca. Rev. Fac. Med. Vet. Lima 1967, 21, 138–139. [Google Scholar]
- Leguia, G. Acute and subacute fasciolosis of alpacas and treatment with triclabendazole. Trop. Anim. Health Prod. 1997, 29, 31–32. [Google Scholar]
- Neyra, V.; Chavarry, E.; Espinoza, J.R. Cysteine proteinases Fas1 and Fas2 are diagnostic markers of Fasciola hepatica infection in alpacas (Lama pacos). Vet. Parasitol. 2002, 105, 21–32. [Google Scholar] [CrossRef]
- Del Pizarro, R.P.; Puray, N. Huevos de Fasciola hepatica en heces de vicuña ((Vicugna vicugna) en Tullpacancha Huancavelica-Perú. Enfoque Veterinario 2014, 1, 1–5. [Google Scholar]
- Samamé, L.M.; Chávez, A.; Pinedo, R. Fasciolosis en vicuñas (Vicugna vicugna) de la Sierra Central del Perú. Rev. Investig. Vet. Perú 2016, 27, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Kühne, G.I. Parásitos diagnosticados en el decenio 1976–198 en la Unidad Regional de Investigación en Sanidad Animal del Noroeste Argentino. I. Helmintos y protozoarios. Rev. Investig. Agropec. INTA 1986, 21, 73–79. [Google Scholar]
- Boray, J.C.; Enigk, K. Laboratory studies on the survival and infectivity of Fasciola hepatica and F. gigantica metacercariae. Zeitsch. Tropenmed. Parasitol. 1964, 15, 324–331. [Google Scholar]
- Bargues, M.D.; Mangold, A.J.; Muñoz-Antoli, C.; Pointier, J.P.; Mas-Coma, S. SSU rDNA characterization of lymnaeid snails transmitting human fascioliasis in South and Central America. J. Parasitol. 1997, 83, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Bargues, M.D.; Artigas, P.; Mera y Sierra, R.; Pointier, J.P.; Mas-Coma, S. Characterisation of Lymnaea cubensis, L. viatrix and L. neotropica n. sp., the main vectors of Fasciola hepatica in Latin America, by analysis of their ribosomal and mitochondrial DNA. Ann. Trop. Med. Parasitol. 2007, 101, 621–641. [Google Scholar] [CrossRef]
- Bargues, M.D.; Gayo, V.; Sanchis, J.; Artigas, P.; Khoubbane, M.; Birriel, S.; Mas-Coma, S. DNA multigene characterization of Fasciola hepatica and Lymnaea neotropica and its fascioliasis transmission capacity in Uruguay, with historical correlation, human report review and infection risk analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005352. [Google Scholar] [CrossRef]
- Bargues, M.D.; Artigas, P.; Khoubbane, M.; Flores, R.; Glöer, P.; Rojas-Garcia, R.; Ashrafi, K.; Falkner, G.; Mas-Coma, S. Lymnaea schirazensis, an overlooked snail distorting fascioliasis data: Genotype, phenotype, ecology, worldwide spread, susceptibility, applicability. PLoS ONE 2011, 6, e24567. [Google Scholar] [CrossRef]
- Valero, M.A.; Panova, M.; Comes, A.M.; Fons, R.; Mas-Coma, S. Patterns in size and shedding of Fasciola hepatica eggs by naturally and experimentally infected murid rodents. J. Parasitol. 2002, 88, 308–313. [Google Scholar] [CrossRef]
- Valero, M.A.; Mas-Coma, S. Comparative infectivity of Fasciola hepatica metacercariae from isolates of the main and secondary reservoir animal host species in the Bolivian Altiplano high human endemic region. Folia Parasitol. 2000, 47, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valero, M.A.; Girones, N.; Garcia-Bodelon, M.A.; Periago, M.V.; Chico-Calero, I.; Khoubbane, M.; Fresno, M.; Mas-Coma, S. Anaemia in advanced chronic fasciolosis. Acta Trop. 2008, 108, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dwinger, R.H.; Le Riche, P.D.; Kühne, G.I. Fascioliasis in beef cattle in North-west Argentina. Trop. Anim. Health Prod. 1982, 14, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Viñabal, A.E.; Aguirre, D.H. Modificación de una técnica coprológica para el diagnóstico de Fasciola hepatica. In Memorias de la 8ª Reunión Anual de la Asociación Argentina de Veterinarios de Laboratorio de Diagnóstico; AAVLD: Corrientes, Argentina, November 1992; p. 64. [Google Scholar]
- Dennis, W.R.; Stone, W.M.; Swanson, L.E. A new laboratory and field diagnostic test for fluke ova in feces. J. Am. Vet. Med. Ass. 1954, 124, 47–50. [Google Scholar]
- Cheriuyot, H.K.; Jordan, H.E. Potential for the spread of Fasciola hepatica in cattle in Oklahoma. J. Am. Vet. Med. Ass. 1990, 196, 1090–1094. [Google Scholar]
- Valero, M.A.; Panova, M.; Mas-Coma, S. Phenotypic analysis of adults and eggs of Fasciola hepatica by computer image analysis system. J. Helminthol. 2005, 79, 217–225. [Google Scholar] [CrossRef]
- Anderson, D.E.; Environmental Impact of Camelids. Big Meadow Creek Farm, Troy. 2009. Available online: http://bigmeadowcreekalpacas.com/About%20Alpacas/environmentalimpact.htm (accessed on 16 January 2021).
- Adam, K.; Adam, E.; Alpaca and Llama Beans. Serendipity Farm and Sanctuary, Lanark. 2014. Available online: http://www.serendipityalpacas.ca/pages/3755/alpaca--llama-beans (accessed on 16 January 2021).
- Fairweather, I.; Brennan, G.P.; Hanna, R.E.B.; Robinson, M.W.; Skuce, P.J. Drug resistance in liver flukes. Int. J. Parasitol. Drugs Drug Res. 2020, 12, 39–59. [Google Scholar] [CrossRef]
- Wilson, R.A.; Smith, G.; Thomas, M.R. Fascioliasis. In The Population Dynamics of Infectious Diseases: Theory and Applications, Anderson, R.M., Ed.; Chapman and Hall: New York, NY, USA, 1982; pp. 262–319. [Google Scholar]
- Diez-Baños, M.A.; Rojo-Vázquez, F.A. Influencia de la temperatura en el desarrollo de los huevos de Fasciola hepatica. An. Fac. Vet. León 1976, 22, 65–75. [Google Scholar]
- Kendall, S.B. Nutritional factors affecting the rate of development of Fasciola hepatica in Limnaea truncatula. J. Helminthol. 1993, 23, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Rondelaud, D. Variabilité interpopulationelle de l’infestation fasciolenne chez le mollusque Lymnaea truncatula Müller. Influence du contact préalable de la population avec le parasite. Bull. Soc. Zool. Franc. 1993, 118, 185–193. [Google Scholar]
- Vignoles, P.; Dreyfuss, G.; Rondelaud, D. Larval development of Fasciola hepatica in experimental infections: Variations with populations of Lymnaea truncatula. J. Helminthol. 2002, 76, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.E. Studies on the life-cycle of Fasciola hepatica (Linnaeus) and of its snail host Limnaea (Galba) truncatula (Müller) in the field and under controlled conditions in the laboratory. Ann. Trop. Med. Parasitol. 1950, 44, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Rondelaud, D.; Barthe, D. Les générations rédiennes de Fasciola hepatica L. Premières observations chez des Limnées tronquées en fin de cycle parasitaire. Bull. Soc. Franç. Parasitol. 1986, 4, 29–38. [Google Scholar]
- Rondelaud, D.; Dreyfuss, G. Fasciola hepatica: The influence of the definitive host on the characteristics of the infection in the snail Lymnaea truncatula. Parasite 1995, 2, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Mas-Coma, S.; Funatsu, I.R.; Bargues, M.D. Fasciola hepatica and lymnaeid snails occurring at very high altitude in South America. Parasitology 2001, 123, S115–S127. [Google Scholar] [CrossRef]
- Dreyfuss, G.; Rondelaud, D. Fasciola hepatica: A study of the shedding of cercariae from Lymnaea truncatula raised under constant conditions of temperature and photoperiod. Parasite 1994, 4, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Audousset, J.C.; Rondelaud, D.; Dreyfuss, G.; Vareille-Morel, C. Les émissions cercariennes de Fasciola hepatica L. chez le mollusque Lymnaea truncatula Müller. A propos de quelques observations chronobiologiques. Bull. Soc. Franç. Parasitol. 1989, 7, 217–224. [Google Scholar]
- Hodasi, J.K.M. The output of cercariae of Fasciola hepatica by Lymnaea truncatula and the distribution of metacercariae on grass. Parasitology 1972, 63, 431–456. [Google Scholar] [CrossRef] [Green Version]
- Bargues, M.D.; Oviedo, J.A.; Funatsu, I.R.; Rodriguez, A.; Mas-Coma, S. Survival of lymnaeid snails from the Bolivian Northern Altiplano after the parasitation by different Bolivian isolates of Fasciola hepatica (Linnaeus, 1758) (Trematoda: Fasciolidae). In Unitas Malacologica; Guerra, A., Rolán, E., Rocha, F., Eds.; Instituto de Investigaciones Marinas, CSIC: Vigo, Spain, 1995; pp. 443–445. [Google Scholar]
- Kimura, S.; Shimizu, A. Studies on the survival and infectivity of Fasciola gigantica metacercariae. Sci. Rep. Fac. Agr. Kobe Univ. 1979, 13, 347–349. [Google Scholar]
- Leguía, G. Enfermedades Parasitarias de Camélidos Sudamericanos; Del Mar: Lima, Peru, 1999; p. 190. [Google Scholar]
- Rickard, L.G. Development and application of a dot-ELISA test for the detection of serum antibodies to Fasciola hepatica antigens in llamas. Vet. Parasitol. 1995, 58, 9–15. [Google Scholar] [CrossRef]
- Cafrune, M.M.; Marin, R.E.; Auad, G.T.; Aguirre, D.H. Coprología parasitaria en llamas (Lama glama) de la Puna de Jujuy, Argentina. In IV Congr. Mund. Camélidos; Oliveira, D., Miragaya, M., Puig, S., Eds.; Resumenes y Trabajos: Santa María, Argentina, 2006; p. 44. [Google Scholar]
- Cafrune, M.M.; Romero, S.R.; Rigalt, F.A.; Marin, R.E.; Aguirre, D.H. Coprological prevalence of gastrointestinal helminths in South American camelids of Northwest Argentina. In Proceedings of the 23th International Conference of the World Association for the Advancement of Veterinary Parasitology (WAAVP), Buenos Aires, Argentina, 21–25 August 2011; p. 20. [Google Scholar]
- Lorini, J.; Liberman, M. El clima de la provincia Aroma del departamento de La Paz, Bolivia. Ecología Bolivia 1983, 4, 19–29. [Google Scholar]
- Mina, Q.J.C.; Santa Cruz, G.S.; Guzman, C.J. Prevalencia de Fasciola hepatica en Llamas Faenadas en el Matadero de Turco (Provincia Sajama, Departamento de Oruro); Facultad de Ciencias Veterinarias, Universidad Autónoma Gabriel René Moreno; Santa Cruz de la Sierra, Bolivia. 2010, pp. 1–33. Available online: https://docplayer.es/docview/77/75317131/#file=/storage/77/75317131/75317131.pdf (accessed on 23 January 2021).
- Rickard, L.R.; Foreyt, W.J. Experimental fascioliasis in llamas. J. Helminthol. Soc. Wash. 1992, 59, 140–144. [Google Scholar]
- Duff, J.P.; Maxwell, A.J.; Claxton, J.R. Chronic and fatal fascioliasis in llamas in the UK. Vet. Rec. 1999, 145, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.A.; Darce, N.A.; Panova, M.; Mas-Coma, S. Relationships between host species and morphometric patterns in Fasciola hepatica adults and eggs from the Northern Bolivian Altiplano hyperendemic region. Vet. Parasitol. 2001, 102, 85–100. [Google Scholar] [CrossRef]
- Valero, M.A.; Perez-Crespo, I.; Chillon-Marinas, C.; Khoubbane, M.; Quesada, C.; Reguera-Gomez, M.; Mas-Coma, S.; Fresno, M.; Girones, N. Fasciola hepatica reinfection potentiates a mixed Th1/Th2/Th17/Treg response and correlates with the clinical phenotypes of anemia. PLoS ONE 2017, 12, e0173456. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.A.; Girones, N.; Reguera-Gomez, M.; Perez-Crespo, I.; Lopez-Garcia, M.P.; Quesada, C.; Bargues, M.D.; Fresno, M.; Mas-Coma, S. Impact of fascioliasis reinfection on Fasciola hepatica egg shedding: Relationship with the immune-regulatory response. Acta Trop. 2020, 209, 105518. [Google Scholar] [CrossRef] [PubMed]
- Boray, J.C. Experimental fascioliasis in Australia. Adv. Parasitol. 1969, 7, 95–210. [Google Scholar]
- Gunsser, I.; Hänichen, T.; Maier, J. Liver fluke infestation in New World camelids. Parasitology, pathology, clinical findings and therapy. Tierarztl. Prax. Ausg. G. Grosstiere Nutztiere 1999, 27, 187–192. (In German) [Google Scholar]
- Cornick, J.L. Gastric squamous cell carcinoma and fascioliasis in a llama. Cornell Vet. 1988, 78, 235–241. [Google Scholar] [PubMed]
- Mera y Sierra, R.; Neira, G.; Bargues, M.D.; Cuervo, P.F.; Artigas, P.; Logarzo, L.; Cortiñas, G.; Ibaceta, D.E.J.; Lopez Garrido, A.; Bisutti, E.; et al. Equines as reservoirs of human fascioliasis: Transmission capacity, epidemiology and pathogenicity in Fasciola hepatica infected mules. J. Helminthol. 2020, 94, e189. [Google Scholar] [CrossRef] [PubMed]
- Hamir, A.N.; Smith, B.B. Severe biliary hyperplasia associated with liver fluke infestation in an adult alpaca. Vet. Pathol. 2002, 39, 592–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, T.; Alfonso, Y.; Lafaye, P.; García Lazaro, M.d.P.; Agueda Perez, L.; Herrera-Velit, P.; Espinoza, J.R. Anticuerpos de cadena única de alpaca para la detección de antígenos de Fasciola hepatica. Rev. Peru. Med. Exp. Salud Publica 2018, 35, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogrzewalska, M.; Nieri-Bastos, F.A.; Marcili, A.; Nava, S.; González-Acuña, D.; Muñoz-Leal, S.; Ruiz-Arrondo, I.; Venzal, J.M.; Mangold, A.; Labruna, M.B. A novel spotted fever group Rickettsia infecting Amblyomma parvitarsum (Acari: Ixodidae) in highlands of Argentina and Chile. Ticks Tick Borne Dis. 2016, 7, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. The Donkey in Human History. An. Archaelogical Perspective; Oxford University Press: Oxford, UK, 2018; p. 306. [Google Scholar]




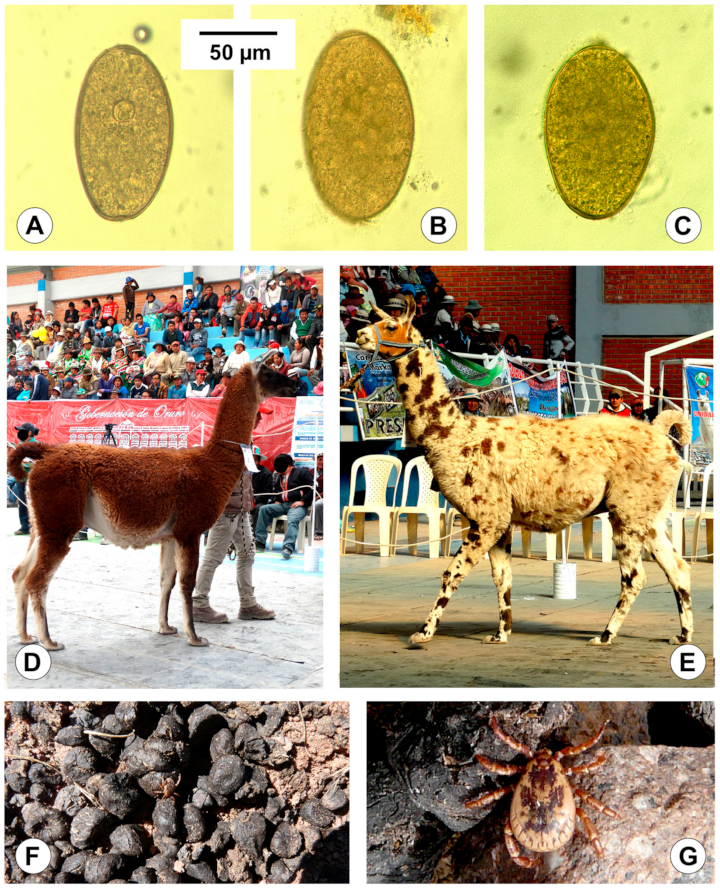

| Host Isolate | Llama | Sheep * | Cattle * |
|---|---|---|---|
| F. hepatica geographical origin | Quichagua, Argentina | Batallas, Bolivia | Batallas, Bolivia |
| Lymnaeid geographical origin | Ancocagua, Bolivia | Huacullani, Bolivia | Huacullani, Bolivia |
| Miracidial dose | mono-miracidial | mono-miracidial | mono-miracidial |
| Temperature (12h day/12h night) | 20 °C/20 °C | 20 °C/20 °C | 20 °C/20 °C |
| No. lymnaeids infected | 45 | 62 | 55 |
| No. survivor snails at beginning of shedding (%) | 30 (66.7%) | 54 (87.1%) | 48 (87.3%) |
| No. shedding snails (%) | 10 (33.3%) | 28 (51.8%) | 12 (25.0%) |
| Prepatent period in dpi (mean) | 37–50 (39.4) | 48–92 (55.6) | 49–76 (55.5) |
| Shedding end in dpi (mean) | 37–74 (50.6) | 52–136 (89.4) | 58–135 (101.6) |
| Shedding length in days (mean) | 1–38 (12.2) | 1–88 (34.7) | 1–85 (47.1) |
| No. total cercariae shed | 513 | 5542 | 3672 |
| No. cercariae/snail (mean) | 1–180 (51.3) | 8–562 (197.9) | 8–581 (306.0) |
| Snail survival after shedding end in days (mean) | 1–31 (7.3) | 1–132 (24.5) | 1–133 (42.3) |
| Longevity of shedding snails in dpi (mean) | 50–76 (57.9) | 53–192 (113.8) | 76–268 (143.9) |
| Longevity of non-shedding snails in dpi (mean) | 37–122 (70.8) | 49–196 (139.1) | 31–209 (105.4) |
| Host Isolate | Llama | Sheep *** | Cattle *** | ||
|---|---|---|---|---|---|
| F. hepatica geographical origin | Quichagua, Argentina | Batallas, Bolivia | Ancocagua, Bolivia | Kallutaca, Bolivia | Batallas, Bolivia |
| Age of metacercariae | 8–11 weeks | 1 week | 2 weeks | 6 weeks | 8 weeks |
| No. metacercariae inoculated per rat | 20 | 20 | 20 | 20 | 20 |
| No. inoculated rats | 25 | 14 | 23 | 4 | 4 |
| No. rats infected (%) | 13 (52.0%) | 11 (78.6%) | 18 (78.3%) | 4 (100%) | 2 (50.0%) |
| No. flukes recovered per rat (mean) | 1–10 (5.2) | 1–8 (3.6) | 1–10 (3.7) | 1–2 (1.7) | 1–2 (1.5) |
| Intensity * | 13.6% | 14.3% | 14.6% | 8.8% | 3.7% |
| Mean % flukes recovered/rat ** | 26.1% | 18.2% | 18.6% | 8.8% | 7.5% |
| Host | Llama | Sheep | Cattle | |||||
|---|---|---|---|---|---|---|---|---|
| (A) Egg Measurements | ||||||||
| Eggs | Fecal Samples (n = 36) | Bile Samples (n = 37) | Fecal Samples (n = 104) | Fecal Samples (n = 168) | ||||
| Measure-ments (µm) | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD |
| EL | 103.8–139.9 | 124.2 ± 9.1 | 105.7–146.7 | 120.9 ± 9.2 | 114.8–151.2 | 130.8 ± 7.1 | 105.3–155.9 | 132.0 ± 10.5 |
| EW | 57.6–74.5 | 66.6 ± 3.5 | 54.9–76.3 | 65.5 ± 5.4 | 65.5–81.4 | 72.6 ± 3.9 | 61.7–82.5 | 71.1 ± 4.4 |
| EPe | 284.3–350.6 | 321.8 ± 17.6 | 277.4–363.2 | 314.7 ± 19.5 | 294.2–368.2 | 327.6 ± 15.0 | 270.6–422.9 | 340.0 ± 33.4 |
| EA | 5022.4– 7269.5 | 6399.9 ± 625.1 | 4761.5–7570.7 | 6108.8 ± 728.2 | 5998.2–8608.4 | 7238.0 ± 532.8 | 5286.5–9676.8 | 7170.2 ± 802.5 |
| EL/EW | 1.52–2.13 | 1.8 ± 0.15 | 1.5–2.3 | 1.8 ± 0.2 | 1.5–2.1 | 1.8 ± 0.1 | 1.6–2.3 | 1.8 ± 0.2 |
| (B) Egg Shedding | ||||||||
| Intensity (epg) | 1–10 | 3–241 (a) | 1–96 (a) | |||||
| Stools/day (kg) | 0.7–3.9 (c) | 1–3 (b) | 15–35 (b) | |||||
| No.eggs/animal/day (d) | 700–39,000 | 3000–723,000 | 15,000–3360,000 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Coma, S.; Cafrune, M.M.; Funatsu, I.R.; Mangold, A.J.; Angles, R.; Buchon, P.; Fantozzi, M.C.; Artigas, P.; Valero, M.A.; Bargues, M.D. Fascioliasis in Llama, Lama glama, in Andean Endemic Areas: Experimental Transmission Capacity by the High Altitude Snail Vector Galba truncatula and Epidemiological Analysis of Its Reservoir Role. Animals 2021, 11, 2693. https://doi.org/10.3390/ani11092693
Mas-Coma S, Cafrune MM, Funatsu IR, Mangold AJ, Angles R, Buchon P, Fantozzi MC, Artigas P, Valero MA, Bargues MD. Fascioliasis in Llama, Lama glama, in Andean Endemic Areas: Experimental Transmission Capacity by the High Altitude Snail Vector Galba truncatula and Epidemiological Analysis of Its Reservoir Role. Animals. 2021; 11(9):2693. https://doi.org/10.3390/ani11092693
Chicago/Turabian StyleMas-Coma, Santiago, Maria Mercedes Cafrune, Ilra Renata Funatsu, Atilio Jose Mangold, Rene Angles, Paola Buchon, Maria Cecilia Fantozzi, Patricio Artigas, Maria Adela Valero, and Maria Dolores Bargues. 2021. "Fascioliasis in Llama, Lama glama, in Andean Endemic Areas: Experimental Transmission Capacity by the High Altitude Snail Vector Galba truncatula and Epidemiological Analysis of Its Reservoir Role" Animals 11, no. 9: 2693. https://doi.org/10.3390/ani11092693






