A Saccharomyces cerevisiae Fermentation Product (Olimond BB) Alters the Early Response after Influenza Vaccination in Racehorses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Samples
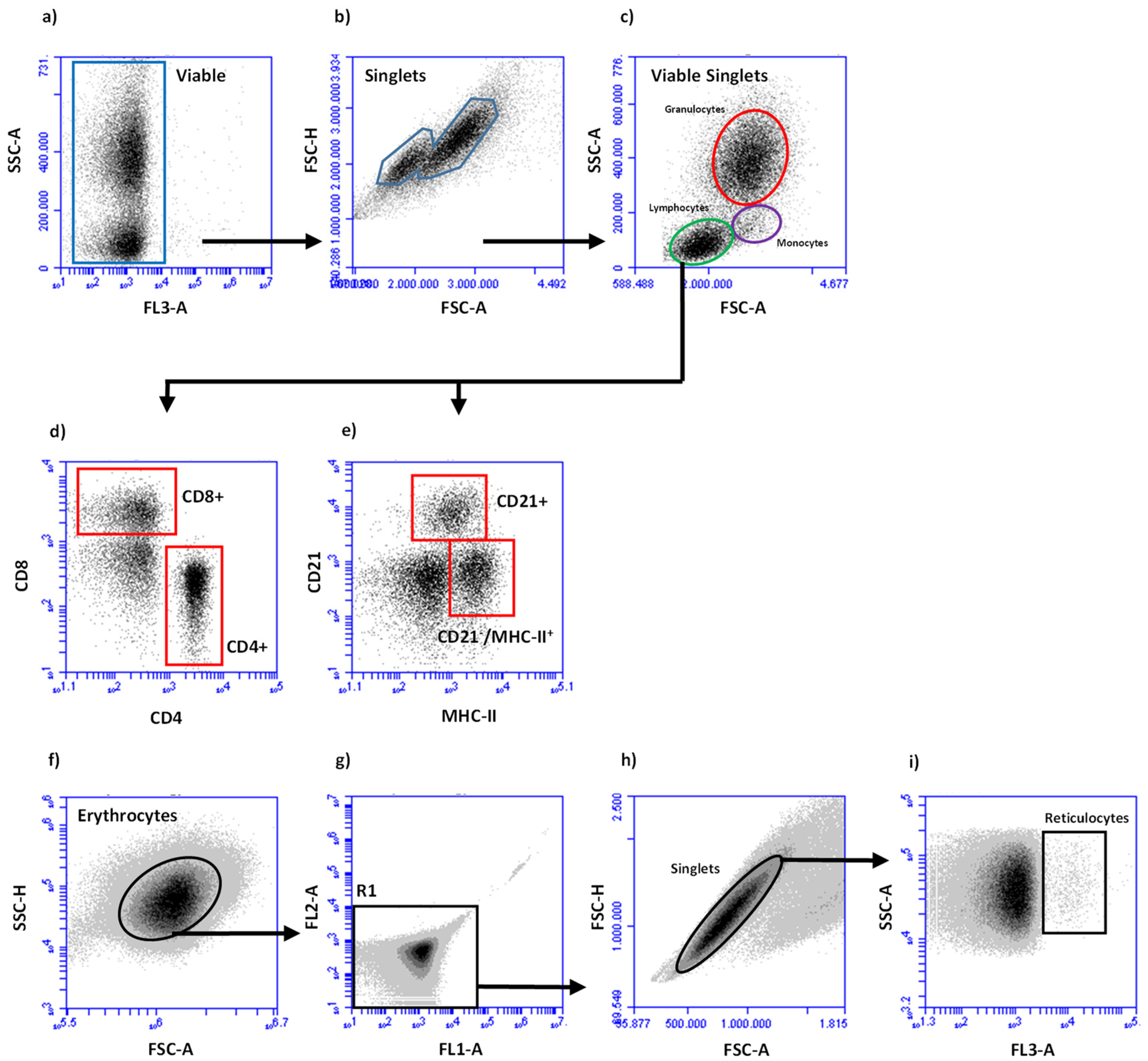
2.3. Leukocyte Preparation and Subpopulation Determination
2.4. Flow Cytometric Deterination of Lymphocyte Subpopulations
2.5. Flow Cytometric Deterination of Reticulocytes
2.6. Vaccination
2.7. Determination of Serum-Amyloid A and Influenza-Specific Antibodies
2.8. Statistical Analysis
3. Results
3.1. Clinical Response and Serum Amyloid A Concentrations
3.2. Blood Leukocyte Numbers and Reticulocyte Percentages
3.3. Vaccinaton-Induced Influenza-Specific Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chou, W.K.; Park, J.; Carey, J.B.; McIntyre, D.R.; Berghman, L.R. Immunomodulatory Effects of Saccharomyces cerevisiae Fermentation Product Supplementation on Immune Gene Expression and Lymphocyte Distribution in Immune Organs in Broilers. Front. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Lopreiato, V.; Mezzetti, M.; Cattaneo, L.; Ferronato, G.; Minuti, A.; Trevisi, E. Role of nutraceuticals during the transition period of dairy cows: A review. J. Anim. Sci. Biotechnol. 2020, 11, 96. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid. Based Complement. Alternat. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- Pujari, R.; Banerjee, G. Impact of prebiotics on immune response: From the bench to the clinic. Immunol. Cell. Biol. 2021, 99, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Ballou, M.A.; Davis, E.M.; Kasl, B.A. Nutraceuticals: An Alternative Strategy for the Use of Antimicrobials. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 507–534. [Google Scholar] [CrossRef]
- Pinheiro, I.; Robinson, L.; Verhelst, A.; Marzorati, M.; Winkens, B.; den Abbeele, P.V.; Possemiers, S. A yeast fermentate improves gastrointestinal discomfort and constipation by modulation of the gut microbiome: Results from a randomized double-blind placebo-controlled pilot trial. BMC Complement. Altern. Med. 2017, 17, 441. [Google Scholar] [CrossRef] [Green Version]
- Klemashevich, C.; Wu, C.; Howsmon, D.; Alaniz, R.C.; Lee, K.; Jayaraman, A. Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr. Opin. Biotechnol. 2014, 26, 85–90. [Google Scholar] [CrossRef]
- Grieshop, C.M.; Flickinger, E.A.; Bruce, K.J.; Patil, A.R.; Czarnecki-Maulden, G.L.; Fahey, G.C., Jr. Gastrointestinal and immunological responses of senior dogs to chicory and mannan-oligosaccharides. Arch. Anim. Nutr. 2004, 58, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Alexander, C.; Steelman, A.J.; Warzecha, C.M.; de Godoy, M.R.C.; Swanson, K.S. Effects of a Saccharomyces cerevisiae fermentation product on fecal characteristics, nutrient digestibility, fecal fermentative end-products, fecal microbial populations, immune function, and diet palatability in adult dogs1. J. Anim. Sci. 2019, 97, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Middelbos, I.S.; Godoy, M.R.; Fastinger, N.D.; Fahey, G.C., Jr. A dose-response evaluation of spray-dried yeast cell wall supplementation of diets fed to adult dogs: Effects on nutrient digestibility, immune indices, and fecal microbial populations. J. Anim. Sci. 2007, 85, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Burdick Sanchez, N.C.; Carroll, J.A.; Broadway, P.R.; Bass, B.E.; Frank, J.W. Modulation of the acute phase response following a lipopolysaccharide challenge in pigs supplemented with an all-natural Saccharomyces cerevisiae fermentation product. Livest. Sci. 2018, 208, 1–4. [Google Scholar] [CrossRef]
- Shen, Y.B.; Carroll, J.A.; Yoon, I.; Mateo, R.D.; Kim, S.W. Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 2011, 89, 2462–2471. [Google Scholar] [CrossRef]
- Ryman, V.E.; Nickerson, S.C.; Kautz, F.M.; Hurley, D.J.; Ely, L.O.; Wang, Y.Q.; Forsberg, N.E. Effect of dietary supplementation on the antimicrobial activity of blood leukocytes isolated from Holstein heifers. Res. Vet. Sci. 2013, 95, 969–974. [Google Scholar] [CrossRef]
- Wu, Z.H.; Yu, Y.; Alugongo, G.M.; Xiao, J.X.; Li, J.H.; Li, Y.X.; Wang, Y.J.; Li, S.L.; Cao, Z.J. Short communication: Effects of an immunomodulatory feed additive on phagocytic capacity of neutrophils and relative gene expression in circulating white blood cells of transition Holstein cows. J. Dairy Sci. 2017, 100, 7549–7555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.H.A.; Slate, J.R.; Hong, S.; Yoon, I.; McGill, J.L. Supplementing a Saccharomyces cerevisiae fermentation product modulates innate immune function and ameliorates bovine respiratory syncytial virus infection in neonatal calves. J. Anim. Sci. 2020, 98, skaa252. [Google Scholar] [CrossRef] [PubMed]
- Burdick Sanchez, N.C.; Carroll, J.A.; Broadway, P.R.; Edrington, T.S.; Yoon, I.; Belknap, C.R. Some aspects of the acute phase immune response to a lipopolysaccharide (LPS) challenge are mitigated by supplementation with a Saccharomyces cerevisiae fermentation product in weaned beef calves. Transl. Anim. Sci. 2020, 4, txaa156. [Google Scholar] [CrossRef]
- Moyad, M.A.; Robinson, L.E.; Kittelsrud, J.M.; Reeves, S.G.; Weaver, S.E.; Guzman, A.I.; Bubak, M.E. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: A randomized, double-blind, placebo-controlled trial. Adv. Ther. 2009, 26, 795–804. [Google Scholar] [CrossRef]
- Moyad, M.A.; Robinson, L.E.; Zawada, E.T.; Kittelsrud, J.; Chen, D.G.; Reeves, S.G.; Weaver, S. Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J. Altern. Complement. Med. 2010, 16, 213–218. [Google Scholar] [CrossRef]
- Willemse, E.; Bobel, J.M.; Russell, K.; Ferguson, D.; Clausen, S.; Warren, L.K. Impact of endurance exercise on fecal indicators of equine gut health. J. Eq. Vet. Sci. 2019, 76, 48. [Google Scholar] [CrossRef]
- Martinez, R.; Leatherwood, J.; Valigura, H.; Arnold, C.; Glass, K.; Much, M.; Owen, R.; Warzecha, C.; White, S. Responses to an intra-articular lipopolysaccharide challenge following dietary supplementation of Saccharomyces cerevisiae fermentation product in young horses. J. Eq. Vet. Sci. 2019, 76, 80. [Google Scholar] [CrossRef]
- Deters, E.L.; Stokes, R.S.; Genther-Schroeder, O.N.; Hansen, S.L. Effects of a Saccharomyces cerevisiae fermentation product in receiving diets of newly weaned beef steers. II. Digestibility and response to a vaccination challenge1. J. Anim. Sci. 2018, 96, 3906–3915. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Coronado, R.F.; Gómez-Rosales, S.; Angeles, M.d.L.; Casaubon-Huguenin, M.T.; Sørensen-Dalgaard, T. Influence of a yeast fermented product on the serum levels of the mannan-binding lectin and the antibodies against the Newcastle disease virus in Ross broilers. J. Appl. Poultry Res. 2017, 26, 38–49. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Wilkens, M.R.; Moller, B.; Ganter, M.; Breves, G.; Schuberth, H.J. Blood leukocyte composition and function in periparturient ewes kept on different dietary magnesium supply. BMC Vet. Res. 2020, 16, 484. [Google Scholar] [CrossRef] [PubMed]
- Viana, K.A.; Carvalho, M.d.G.; Dusse, L.M.S.A.; Fernandes, A.C.; Avelar, R.S.; Avelar, D.M.V.; Carvalho, B.; Ribeiro, C.M.F.; Antonelli, L.R.d.V.; Teixeira, A.; et al. Flow cytometry reticulocyte counting using acridine orange: Validation of a new protocol. J. Bras. Patol. Med. Lab. 2014, 50, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.M.; Youssef, I.M.I.; Abd El-Tawab, M.M.; Bakr, H.A.; Eissa, N.A.; Hassan, M.S.; Giadinis, N.D.; Milewski, S.; Baumgartner, W.; Sobiech, P. Influence of probiotic and yeast culture supplementation on selected biochemical and immunological parameters of growing lambs. Pol. J. Vet. Sci. 2020, 23, 5–12. [Google Scholar] [CrossRef]
- Al-Qaisi, M.; Horst, E.A.; Mayorga, E.J.; Goetz, B.M.; Abeyta, M.A.; Yoon, I.; Timms, L.L.; Appuhamy, J.A.; Baumgard, L.H. Effects of a Saccharomyces cerevisiae fermentation product on heat-stressed dairy cows. J. Dairy Sci. 2020, 103, 9634–9645. [Google Scholar] [CrossRef] [PubMed]
- Entenfellner, J.; Gahan, J.; Garvey, M.; Walsh, C.; Venner, M.; Cullinane, A. Response of Sport Horses to Different Formulations of Equine Influenza Vaccine. Vaccines 2020, 8, 372. [Google Scholar] [CrossRef]
- Andersen, S.A.; Petersen, H.H.; Ersbøll, A.K.; Falk-Rønne, J.; Jacobsen, S. Vaccination elicits a prominent acute phase response in horses. Vet. J. 2012, 191, 199–202. [Google Scholar] [CrossRef]
- Duran, M.C.; Dumrath, C.A.C.; Bartmann, C.P.; Medina Torres, C.E.; Moschos, A.; Goehring, L.S. Serum Amyloid A (SAA) Concentration after Vaccination in Horses and Mules. J. Eq. Vet. Sci. 2020, 92, 103165. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Hanson, N.Q.; Straka, R.J.; Hoke, T.R.; Ordovas, J.M.; Peacock, J.M.; Arends, V.L.; Arnett, D.K. Effect of influenza vaccine on markers of inflammation and lipid profile. J. Lab. Clin. Med. 2005, 145, 323–327. [Google Scholar] [CrossRef]
- Andersen-Nissen, E.; Fiore-Gartland, A.; Ballweber Fleming, L.; Carpp, L.N.; Naidoo, A.F.; Harper, M.S.; Voillet, V.; Grunenberg, N.; Laher, F.; Innes, C.; et al. Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS Pathog. 2021, 17, e1009363. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S. Systems biological assessment of human immunity to BNT162b2 mRNA vaccination. Res. Sq. 2021, rs.3.rs-438662. [Google Scholar] [CrossRef]
- Khuhapinant, A.; Bunyaratvej, A.; Tatsumi, N.; Pribwai, M.; Fucharoen, S. Number and maturation of reticulocytes in various genotypes of thalassaemia as assessed by flow cytometry. Acta Haematol. 1994, 91, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; McCullough, M.; O’Brien, P.J. Equine blood reticulocytes: Reference intervals, physiological and pathological changes. Comp. Clin. Pathol. 2019, 28, 53–62. [Google Scholar] [CrossRef]
- Delic, D.; Wunderlich, F.; Al-Quraishy, S.; Abdel-Baki, A.S.; Dkhil, M.A.; Araúzo-Bravo, M.J. Vaccination accelerates hepatic erythroblastosis induced by blood-stage malaria. Malar J. 2020, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Alamo, I.G.; Kannan, K.B.; Loftus, T.J.; Ramos, H.; Efron, P.A.; Mohr, A.M. Severe trauma and chronic stress activates extramedullary erythropoiesis. J. Trauma Acute Care Surg. 2017, 83, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Klei, T.R.L.; Meinderts, S.M.; van den Berg, T.K.; van Bruggen, R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front. Immunol. 2017, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Michael, C.F.; Waters, C.M.; LeMessurier, K.S.; Samarasinghe, A.E.; Song, C.Y.; Malik, K.U.; Lew, D.B. Airway Epithelial Repair by a Prebiotic Mannan Derived from Saccharomyces cerevisiae. J. Immunol Res. 2017, 2017, 8903982. [Google Scholar] [CrossRef] [Green Version]
- Rowson, A.D.; Wang, Y.Q.; Aalseth, E.; Forsberg, N.E.; Puntenney, S.B. Effects of an immunomodulatory feed additive on the development of mastitis in a mouse infection model using four bovine-origin isolates. Animal 2011, 5, 220–229. [Google Scholar] [CrossRef]
- Yuan, K.; Mendonca, L.G.; Hulbert, L.E.; Mamedova, L.K.; Muckey, M.B.; Shen, Y.; Elrod, C.C.; Bradford, B.J. Yeast product supplementation modulated humoral and mucosal immunity and uterine inflammatory signals in transition dairy cows. J. Dairy Sci. 2015, 98, 3236–3246. [Google Scholar] [CrossRef] [Green Version]
- Fuller, C.L.; Brittingham, K.C.; Porter, M.W.; Hepburn, M.J.; Petitt, P.L.; Pittman, P.R.; Bavari, S. Transcriptome analysis of human immune responses following live vaccine strain (LVS) Francisella tularensis vaccination. Mol. Immunol. 2007, 44, 3173–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobolev, O.; Binda, E.; O’Farrell, S.; Lorenc, A.; Pradines, J.; Huang, Y.; Duffner, J.; Schulz, R.; Cason, J.; Zambon, M.; et al. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat. Immunol. 2016, 17, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.S.; Schwartzberg, P.L.; Kotliarov, Y.; Biancotto, A.; Xie, Z.; Germain, R.N.; Wang, E.; Olnes, M.J.; Narayanan, M.; Golding, H.; et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell 2014, 157, 499–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, T.M.; Reedy, S.E. Equine Influenza Serological Methods. Methods Mol. Biol. 2020, 2123, 401–412. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Wrammert, J.; Lee, E.K.; Racioppi, L.; Marie-Kunze, S.; Haining, W.N.; Means, A.R.; Kasturi, S.P.; Khan, N.; Li, G.-M.; et al. Systems biology of vaccination for seasonal influenza in humans. Nat. Immunol. 2011, 12, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Bucasas, K.L.; Franco, L.M.; Shaw, C.A.; Bray, M.S.; Wells, J.M.; Niño, D.; Arden, N.; Quarles, J.M.; Couch, R.B.; Belmont, J.W. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. J. Infect. Dis. 2011, 203, 921–929. [Google Scholar] [CrossRef] [Green Version]


| Ingredient | PLA 1 | OLI |
|---|---|---|
| Tocopherol extract | 0.05 g | 0.05 g |
| Coconut oil | 0.15 g | 0.15 g |
| Vitamin C | 0.15 g | 0.15 g |
| Dextrose | 1.00 g | - |
| Corn cob meal | 0.75 g | - |
| Linseed cake | 0.75 g | - |
| Microcrystalline cellulose | 1.03 g | 1.43 g |
| Minerals | 1.13 g | 1.23 g |
| inactivated yeasts | - | 2.00 g |
| Pellet | 5.01 g | 5.01 |
| Group | Horse | SAA (µg/mL) | Fold Increase 1 | |
|---|---|---|---|---|
| D40 | D41 | |||
| OLI | #1 | 8.8 | 69.8 | 7.9 |
| #2 | 10.7 | 11.8 | 1.1 | |
| #3 | 10.5 | 13.5 | 1.3 | |
| #4 | 233.7 | 141.9 | 0.6 | |
| #5 | 9.2 | 16.1 | 1.8 | |
| #6 | 10.9 | 11.0 | 1.0 | |
| PLA | #7 | 9.7 | 31.0 | 3.2 |
| #8 | 9.5 | 11.8 | 1.2 | |
| #9 | 11.0 | 14.8 | 1.3 | |
| #10 | 10.4 | 11.4 | 1.1 | |
| #11 | 10.9 | 58.9 | 5.4 | |
| Cell Type | OLI | PLA | p |
|---|---|---|---|
| Leucocytes (G/L) | 8.35 ± 1.79 | 8.88 ± 0.80 | 0.557 |
| PMN (G/L) | 3.69 ± 0.74 | 3.67 ± 1.45 | 0.971 |
| Monocytes (G/L) | 0.38 ± 0.09 | 0.36 ± 0.09 | 0.752 |
| Lymphocytes (G/L) | 4.28 ± 1.30 | 4.85 ± 1.12 | 0.460 |
| CD8+ T cells (G/L) | 1.51 ± 0.33 | 1.76 ± 0.51 | 0.086 |
| CD4+ T cells (G/L) | 1.11 ± 0.28 | 1.27 ± 0.45 | 0.471 |
| CD21+ B cells (G/L) | 0.49 ± 0.20 | 0.59 ± 0.47 | 0.633 |
| CD21−/MHCII+ cells (G/L) | 0.87 ± 0.52 | 0.78 ± 0.43 | 0.782 |
| Reticulocytes (%) | 0.34 ± 0.13 | 0.42 ± 0.15 | 0.371 |
| Antigen 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Florida Clade 1 (H3N8) | Newmarket (H3N8) | Prague (H7N7) | |||||
| D40 | D56 | p | D40 | D56 | p | D40 | D56 | |
| OLI | 5.13 ± 0.62 a2 | 5.96 ± 1.05 a | 0.033 | 4.71 ± 1.33 a | 5.27 ± 0.79 a | 0.373 | n.d. 3 | n.d. |
| PLA | 5.41 ± 0.58 a | 6.24 ± 0.49 a | 0.108 | 4.30 ± 0.58 a | 5.68 ± 0.31 a | 0.003 | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucassen, A.; Finkler-Schade, C.; Schuberth, H.-J. A Saccharomyces cerevisiae Fermentation Product (Olimond BB) Alters the Early Response after Influenza Vaccination in Racehorses. Animals 2021, 11, 2726. https://doi.org/10.3390/ani11092726
Lucassen A, Finkler-Schade C, Schuberth H-J. A Saccharomyces cerevisiae Fermentation Product (Olimond BB) Alters the Early Response after Influenza Vaccination in Racehorses. Animals. 2021; 11(9):2726. https://doi.org/10.3390/ani11092726
Chicago/Turabian StyleLucassen, Alexandra, Christa Finkler-Schade, and Hans-Joachim Schuberth. 2021. "A Saccharomyces cerevisiae Fermentation Product (Olimond BB) Alters the Early Response after Influenza Vaccination in Racehorses" Animals 11, no. 9: 2726. https://doi.org/10.3390/ani11092726





