Non-Antibiotics Strategies to Control Salmonella Infection in Poultry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Sources of the Data and Search Strategy
3. The Genus Salmonella and Its Relevance in Poultry
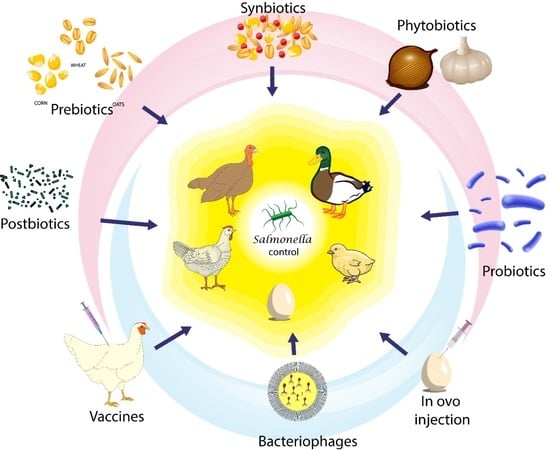
4. Feeding-Based Strategies to Control Salmonella Infection in Poultry
4.1. Prebiotics
4.2. Probiotics
4.3. Synbiotics
4.4. Postbiotics
4.5. Phytobiotics
5. Non-Feeding-Based Strategies
5.1. Bacteriophages
5.1.1. Bacteriophage Therapy
5.1.2. Phage Lytic Enzymes: Endolysins and Virion Associated Peptidoglycan Hydrolases (VAPGHs)
5.1.3. What Is Still Needed to Consolidate Bacteriophage/Endolysin Therapy for Salmonella in Poultry?
5.2. Vaccines
5.2.1. Live-Attenuated Vaccines
5.2.2. Killed or Inactivated Vaccine
5.2.3. Subunit Vaccines
5.3. In Ovo Strategies
6. The –Omics as a Tool for Salmonella Strategies Development
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Disease Control and Prevention. Salmonella. Available online: https://www.cdc.gov/Salmonella/index.html (accessed on 30 November 2021).
- Arya, G.; Holtslander, R.; Robertson, J.; Yoshida, C.; Harris, J.; Parmley, J.; Nichani, A.; Johnson, R.; Poppe, C. Epidemiology, pathogenesis, genoserotyping, antimicrobial resistance, and prevention and control of non-typhoidal Salmonella serovars. Curr. Clin. Microbiol. Rep. 2017, 4, 43–53. [Google Scholar] [CrossRef]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet. 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Gast, R.K.; Regmi, P.; Guraya, R.; Jones, D.R.; Anderson, K.E.; Karcher, D.M. Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poult. Sci. 2019, 98, 5023–5027. [Google Scholar] [CrossRef]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Kudirkiene, E.; Akinlabi, O.C.; Bello, M.B.; Olsen, J.E. Prevalence and risk factors of Salmonella in commercial poultry farms in nigeria. PLoS ONE. 2020, 15, e0238190. [Google Scholar] [CrossRef]
- Knap, I.; Kehlet, A.B.; Bennedsen, M.; Mathis, G.F.; Hofacre, C.L.; Lumpkins, B.S.; Jensen, M.M.; Raun, M.; Lay, A. Bacillus Subtilis (DSM17299) Significantly reduces Salmonella in broilers. Poult. Sci. 2011, 90, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Merino, L.; Trejo, F.M.; de Antoni, G.; Golowczyc, M.A. Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Int. Food Res. J. 2019, 123, 258–265. [Google Scholar] [CrossRef]
- Dhanani, A.S.; Block, G.; Dewar, K.; Forgetta, V.; Topp, E.; Beiko, R.G.; Diarra, M.S. genomic comparison of non-typhoidal Salmonella Enterica serovars Typhimurium, Enteritidis, Heidelberg, Hadar and Kentucky isolates from broiler chickens. PLoS ONE 2015, 10, e0128773. [Google Scholar] [CrossRef]
- Guillén, S.; Marcén, M.; Álvarez, I.; Mañas, P.; Cebrián, G. Stress resistance of emerging poultry-associated Salmonella serovars. Int. J. Food Microbiol. 2020, 335, 108884. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chousalkar, K.K. Transcriptome profiling analysis of caeca in chicks challenged with Salmonella Typhimurium reveals differential expression of genes involved in host mucosal immune response. Appl. Microbiol. Biotechnol. 2020, 104, 9327–9342. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef]
- Buncic, S.; Sofos, J. Interventions to control Salmonella contamination during poultry, cattle and pig slaughter. Int. Food Res. J. 2012, 45, 641–655. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Lohakare, J.D. Effect of dietary supplementation of peppermint on performance, egg quality, and serum metabolic profile of hy-line brown hens during the late laying period. Anim. Feed Sci. Technol. 2014, 197, 114–120. [Google Scholar] [CrossRef]
- Grant, A.; Hashem, F.; Parveen, S. Salmonella and Campylobacter: Antimicrobial resistance and bacteriophage control in poultry. Food Microbiol. 2016, 53, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, R.P. Methods for early nutrition and their potential. Worlds Poult. Sci. 2004, 60, 101–111. [Google Scholar] [CrossRef]
- Givisiez, P.E.N.; Moreira Filho, A.L.B.; Santos, M.R.B.; Oliveira, H.B.; Ferket, P.R.; Oliveira, C.J.B.; Malheiros, R.D. Chicken embryo development: Metabolic and morphological basis for in ovo feeding technology. Poult. Sci. 2020, 99, 6774–6782. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Rosales, A.G.; da Costa, M.; Cookson, K.; Schaeffer, J.; Jones, M.K. Immunity and protection provided by live modified vaccines against paratyphoid Salmonella in poultry—an applied perspective. Avian Dis. 2021, 65, 295–302. [Google Scholar] [CrossRef]
- Revolledo, L.; Ferreira, A.J.P. Current perspectives in avian salmonellosis: Vaccines and immune mechanisms of protection. J. Appl. Poult. Res. 2012, 21, 418–431. [Google Scholar] [CrossRef]
- Renu, S.; Markazi, A.D.; Dhakal, S.; Shaan Lakshmanappa, Y.; Shanmugasundaram, R.; Selvaraj, R.K.; Renukaradhya, G.J. Oral deliverable mucoadhesive chitosan-Salmonella subunit nanovaccine for layer chickens. Int. J. Nanomed. 2020, 15, 761–777. [Google Scholar] [CrossRef] [Green Version]
- Vaid, R.K.; Thakur, Z.; Anand, T.; Kumar, S.; Tripathi, B.N. Comparative genome analysis of Salmonella enterica serovar Gallinarum biovars Pullorum and Gallinarum decodes strain specific genes. PLoS ONE 2021, 16, e0255612. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.K. Salmonella Enterica. In Foodborne Microbial Pathogens; Springer New York: New York, NY, USA, 2008; pp. 201–216. [Google Scholar]
- Pui, C.F.; Wong, W.C.; Chai, L.C.; Robin, T.; Ponniah, J.; Hidayah, M.S.; Anyi, U.; Mohamad Ghazali, F.; Cheah, Y.K.; Son, R. Review article Salmonella: A foodborne pathogen. Int. Food Res. J. 2011, 18, 465–473. [Google Scholar]
- Abatcha, M.G.; Goni, M.D.; Abbas, M.A.; Jalo, I.M.; Mohammed, G. A review of Listeria and Salmonella: An update on description, characteristics, incidence, and antibiotic susceptibility. Adv. Anim. Vet. Sci. 2020, 8, 1232–1249. [Google Scholar] [CrossRef]
- Gast, R.K. Serotype-specific and serotype-independent strategies for preharvest control of food-borne Salmonella in poultry. Avian Dis. 2007, 51, 817–828. [Google Scholar] [CrossRef]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [Green Version]
- Popa, G.L.; Popa, M.I. Salmonella spp. Infection—A continuous threat worldwide. Germs 2021, 11, 88–96. [Google Scholar] [CrossRef]
- Sumano López, H.; Gutiérrez Olvera, L. Farmacología Clínica en Aves comerciales; Sumano López, H., Gutiérrez Olvera, L., Eds.; Mc Graw Hill: Mexico City, Mexico, 2010. [Google Scholar]
- Desin, T.S.; Köster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Expert Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Crouch, C.F.; Pugh, C.; Patel, A.; Brink, H.; Wharmby, C.; Watts, A.; van Hulten, M.C.W.; de Vries, S.P.W. reduction in intestinal colonization and invasion of internal organs after challenge by homologous and heterologous serovars of Salmonella Enterica following vaccination of chickens with a novel trivalent inactivated Salmonella vaccine. Avian Pathol. 2020, 49, 666–677. [Google Scholar] [CrossRef]
- Cadirci, O.; Gucukoglu, A.; Gulel, G.T.; Gunaydin, E.; Uyanik, T.; Kanat, S. Determination and antibiotic resistance profiles of Salmonella serotypes isolated from poultry meat. Fresenius Environ. Bull. 2021, 30, 4251–4261. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricke, S.C.; Lee, S.I.; Kim, S.A.; Park, S.H.; Shi, Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020, 99, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Potential of fructooligosaccharide prebiotics in alternative and nonconventional poultry production systems. Poult. Sci. 2015, 94, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef]
- Adhikari, P.; Cosby, D.E.; Cox, N.A.; Franca, M.S.; Williams, S.M.; Gogal, R.M.; Ritz, C.W.; Kim, W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella Enteritidis. Poult. Sci. 2018, 97, 2525–2533. [Google Scholar] [CrossRef]
- Wu, Y.T.; Yang, W.Y.; Samuel Wu, Y.H.; Chen, J.W.; Chen, Y.C. Modulations of growth performance, gut microbiota, and inflammatory cytokines by trehalose on Salmonella Typhimurium-challenged broilers. Poult. Sci. 2020, 99, 4034–4043. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.E.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals 2020, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Londero, A.; Menconi, A.; Reginatto, A.R.; Bacocina, I.; Wolfenden, A.; Shivaramaiah, S.; Hargis, B.M.; Tellez, G. Effect of an aspergillus meal prebiotic on Salmonella infection in turkeys and broiler chickens. Int. J. Poult. Sci. 2011, 10, 946–951. [Google Scholar] [CrossRef] [Green Version]
- Santana, E.S.; Andrade, M.A.; da Silveira Neto, O.J.; de Sa Jayme, V.; de Camargo, J.N.C.; de Souza Barnabe, A.C. Intestinal integrity and performance of turkeys subjected to inoculation of Salmonella Enteritidis and a diet supplemented with lactulose. Pesqui. Agropecu. Bras. 2020, 55. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Praharaj, I.; John, S.M.; Bandyopadhyay, R.; Kang, G. Probiotics, antibiotics and the immune responses to vaccines. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140144. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Arif, M.; Sajjad, N.; Al-Ghadi, M.Q.; Alagawany, M.; Abd El-Hack, M.E.; Alhimaidi, A.R.; Elnesr, S.S.; Almutairi, B.O.; Amran, R.A.; et al. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 2020, 99, 6946–6953. [Google Scholar] [CrossRef]
- Khan, S.; Chousalkar, K.K. Salmonella Typhimurium infection disrupts but continuous feeding of bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Beirão, B.C.B.; Ingberman, M.; Fávaro, C.; Mesa, D.; Bittencourt, L.C.; Fascina, V.B.; Caron, L.F. Effect of an Enterococcus faecium probiotic on specific IgA Following Live Salmonella Enteritidis vaccination of layer chickens. Avian Pathol. 2018, 47, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Price, P.T.; Gaydos, T.A.; Berghaus, R.D.; Baxter, V.; Hofacre, C.L.; Sims, M.D. Salmonella Enteritidis reduction in layer ceca with a bacillus probiotic. Vet. World. 2020, 13, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.A.; Shawrang, P.; Shakorzadeh, S. Immune response of Salmonella challenged broiler chickens fed diets containing gallipro®, a Bacillus subtilis probiotic. Probiotics Antimicrob. Proteins 2015, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, W.A.A.; S.A. El-Shafii, S.; Hatem, M.E.; E. Dawood, R. A Trial to prevent Salmonella Enteritidis infection in broiler chickens using autogenous bacterin compared with probiotic preparation. J. Agric. Sci. 2012, 4, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ishfaq, M.; Wang, J. Effects of Lactobacillus salivarius supplementation on the growth performance, liver function, meat quality, immune response and Salmonella Pullorum infection resistance of broilers challenged with Aflatoxin B1. Poult Sci. 2021, 101651. [Google Scholar] [CrossRef]
- Koenen, M.E.; Kramer, J.; Van Der Hulst, R.; Heres, L.; Jeurissen, S.H.M.; Boersma, W.J.A. Immunomodulation by probiotic Lactobacilli in layer—And meat-type chickens. British Poult. Sci. 2004, 45, 355–366. [Google Scholar] [CrossRef]
- Groves, P.J.; Williamson, S.L.; Ahaduzzaman, M.; Diamond, M.; Ngo, M.; Han, A.; Sharpe, S.M. Can a combination of vaccination, probiotic and organic acid treatment in layer hens protect against early life exposure to Salmonella Typhimurium and challenge at sexual maturity? Vaccine 2021, 39, 815–824. [Google Scholar] [CrossRef]
- Oh, J.K.; Pajarillo, E.A.B.; Chae, J.P.; Kim, I.H.; Kang, D.K. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of hy-line brown layers. Asian-Australas. J. Anim. Sci. 2017, 30, 1332–1339. [Google Scholar] [CrossRef] [Green Version]
- Villagran-de la Mora, Z.; Nuño, K.; Olga, V.; Avalos, H.; Castro-rosas, J.; Carlos, G.; Angulo, C.; Ascencio, F. Effect of a synbiotic mix on intestinal structural changes, and Salmonella Typhimurium and Clostridium perfringens colonization in broiler chickens. Animals 2019, 9, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimminau, E.A.; Karnezos, T.P.; Berghaus, R.D.; Jones, M.K.; Baxter, J.A.; Hofacre, C.L. Combination of probiotic and prebiotic impacts Salmonella Enteritidis infection in layer hens. J. Appl. Poult. Res. 2021, 30, 100200. [Google Scholar] [CrossRef]
- Suganuma, K.; Hamasaki, T.; Hamaoka, T. Effect of dietary direct-fed microbial and yeast cell walls on cecal digesta microbiota of layer chicks inoculated with nalidixic acid resistant Salmonella Enteritidis. Poult. Sci. 2021, 100, 101385. [Google Scholar] [CrossRef] [PubMed]
- Ajiguna, J.C.; Prakasita, V.C.; Nahak, T.E.M.; Tabbu, C.R.; Santosa, C.M.; Wahyuni, A.E.T.H. The role of synbiotics (commercial product) as a substitute for antibiotic growth promotor (AGP) in the performance and blood values of cobb-strain broilers challenged with Salmonella Enteritidis. Adv. Biol. Res. 2021, 15, 59–66. [Google Scholar]
- Gingerich, E.; Frana, T.; Logue, C.M.; Smith, D.P.; Pavlidis, H.O.; Chaney, W.E. Effect of feeding a postbiotic derived from Saccharomyces Cerevisiae fermentation as a preharvest food safety hurdle for reducing Salmonella Enteritidis in the ceca of layer pullets. J. Food Prot. 2021, 84, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Yu, C.; Hsieh, Y.H.; Chen, S.W.; Chen, B.J.; Chen, C.Y. Effects of albusin B (a bacteriocin) of Ruminococcus Albus 7 expressed by yeast on growth performance and intestinal absorption of broiler chickens-its potential role as an alternative to feed antibiotics. J. Sci. Food Agric. 2011, 91, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Svetoch, E.A.; Eruslanov, B.V.; Levchuk, V.P.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Stepanshin, J.; Dyatlov, I.; Seal, B.S.; Stern, N.J. Isolation of Lactobacillus Salivarius 1077 (NRRL B-50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Appl. Environ. Microbiol. 2011, 77, 2749–2754. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.A. Lactic acid bacteria:bacteriocin producer: A mini review. IOSR J. Pharm. 2013, 3, 44–50. [Google Scholar] [CrossRef]
- Kuralkar, P.; Kuralkar, S.V. Role of herbal products in animal production—An updated review. J. Ethnopharmacol. 2021, 278, 114246. [Google Scholar] [CrossRef]
- Yıldız, A.Ö.; Şentürk, E.T.; Olgun, O. Use of alfalfa meal in layer diets—A review. Poult. Sci. J. 2020, 76, 134–143. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Navarro-Cruz, A.R.; Sosa-Morales, M.E.; López-Malo, A.; Palou, E. Chapter 27—Bergamot (Citrus bergamia) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 247–252. [Google Scholar]
- Sadarman; Febrina, D.; Yendraliza; Shirothul Haq, M.; Amalia Nurfitriani, R.; Nurmilati Barkah, N.; Miftakhus Sholikin, M.; Yunilas; Qomariyah, N.; Jayanegara, A.; et al. Effect of dietary black cumin seed (Nigella sativa) on performance, immune status, and serum metabolites of small ruminants: A meta-analysis. Small Rumin. Res. 2021, 204, 106521. [Google Scholar] [CrossRef]
- Vicente, J.L.; Lopez, C.; Avila, E.; Morales, E.; Hargis, B.M.; Tellez, G. Effect of dietary natural capsaicin on experimental Salmonella Enteritidis infection and yolk pigmentation in laying hens. Int. J. Poult. Sci. 2007, 6, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, M.R.; Mahdavi, A.H.; Rahmani, H.R.; Jahanian, E. Effects of different levels of clove bud (Syzygium aromaticum) on yolk biochemical parameters and fatty acids profile, yolk oxidative stability, and ovarian follicle numbers of laying hens receiving different n-6 to n-3 ratios. Anim. Feed Sci. Technol. 2015, 206, 67–75. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Q.Y.; Liu, Y.R.; Ma, Q.G.; Zhang, J.Y.; Guo, Y.P.; Xue, Z.; Zhao, L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021, 100, 101163. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, W.M.; Zeweil, H.S.; Ahmed, M.H.; Zahran, S.M.; Shaalan, M.M.; Abdelsalam, N.R.; Abdel-Moneim, A.-M.E.; Taha, A.E.; El-Tarabily, K.A.; Abd El-Hack, M.E. Impacts of onion and cinnamon supplementation as natural additives on the performance, egg quality and immunity in laying japanese quail. Poult. Sci. 2021, 100, 101482. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Miyata, G. The nutraceutical benefit, part iv: Garlic. Nutrition 2000, 16, 787–788. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Barkat, R.A.; Gabr, A.A.; Foda, M.A.; Noreldin, A.E.; Khafaga, A.F.; El-Sabrout, K.; et al. Potential role of important nutraceuticals in poultry performance and health—A comprehensive review. Vet. Sci. Res. J. 2021, 137, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Salem, W.M.; Shibat El-hamed, D.M.W.; Sayed, W.F.; Elamary, R.B. Alterations in virulence and antibiotic resistant genes of multidrug-resistant Salmonella serovars isolated from poultry: The bactericidal efficacy of Allium sativum. Microb. Pathog. 2017, 108, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Orndorff, B.W.; Novak, C.L.; Pierson, F.W.; Caldwell, D.J.; Mcelroy, A.P. Comparison of prophylactic or therapeutic dietary administration of capsaicin for reduction of Salmonella in broiler chickens. Avian Dis. 2005, 49, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kollanoor-Johny, A.; Mattson, T.; Baskaran, S.A.; Amalaradjou, M.A.; Babapoor, S.; March, B.; Valipe, S.; Darre, M.; Hoagland, T.; Schreiber, D.; et al. Reduction of Salmonella Enterica serovar Enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012, 78, 2981–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alali, W.Q.; Hofacre, C.L.; Mathis, G.F.; Faltys, G. Effect of essential oil compound on shedding and colonization of Salmonella Enterica serovar Heidelberg in broilers. Poult. Sci. 2013, 92, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.H.; Gebert, R.R.; Barreta, M.; Baldissera, M.D.; dos Santos, I.D.; Wagner, R.; Campigotto, G.; Jaguezeski, A.M.; Gris, A.; de Lima, J.L.F.; et al. Effects of phytogenic feed additive based on thymol, carvacrol and cinnamic aldehyde on body weight, blood parameters and environmental bacteria in broilers chickens. Microb. Pathog. 2018, 125, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Laptev, G.Y.; Filippova, V.A.; Kochish, I.I.; Yildirim, E.A.; Ilina, L.A.; Dubrovin, A.V.; Brazhnik, E.A.; Novikova, N.I.; Novikova, O.B.; Dmitrieva, M.E.; et al. Examination of the expression of immunity genes and bacterial profiles in the caecum of growing chickens infected with Salmonella Enteritidis and fed a phytobiotic. Animals 2019, 9, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Rubio, L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Alkhulaifi, M.M.; Aljumaah, R.S.; Abudabos, A.M.; Abdullatif, A.A.; Suliman, G.M.; Al-Ghadi, M.Q.; Stanley, D. Influence of sanguinarine-based phytobiotic supplementation on post necrotic enteritis challenge recovery. Heliyon 2020, 6, e05361. [Google Scholar] [CrossRef] [PubMed]
- Aljumaah, M.R.; Suliman, G.M.; Abdullatif, A.A.; Abudabos, A.M. Effects of phytobiotic feed additives on growth traits, blood biochemistry, and meat characteristics of broiler chickens exposed to Salmonella Typhimurium. Poult. Sci. 2020, 99, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Kutter, E. Bacteriophage therapy in humans. In Bacteriophages; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Melo, L.D.R.; Oliveira, H.; Pires, D.P.; Dabrowska, K.; Azeredo, J. Phage therapy efficacy: A review of the last 10 years of preclinical studies. Crit. Rev. Microbiol. 2020, 46, 78–99. [Google Scholar] [CrossRef]
- Weinbauer, M.G. Ecology of Prokaryotic Viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rubio, L.; Gutiérrez, D.; Donovan, D.M.; Martínez, B.; Rodríguez, A.; García, P. Phage lytic proteins: Biotechnological applications beyond clinical antimicrobials. Crit. Rev. Biotechnol. 2016, 36, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Fiorentin, L.; Vieira, N.D.; Barioni, W. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 2005, 34, 258–263. [Google Scholar] [CrossRef]
- Lim, T.H.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Youn, H.N.; Kim, M.S.; Lee, H.J.; Yang, S.Y.; Cho, Y.W.; Lee, J.B.; et al. Efficacy of bacteriophage therapy on horizontal transmission of Salmonella Gallinarum on commercial layer chickens. Avian Dis. 2011, 55, 435–438. [Google Scholar] [CrossRef]
- Andreatti Filho, R.L.; Higgins, J.P.; Higgins, S.E.; Gaona, G.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar Enteritidis in vitro and in vivo. Poult. Sci. 2007, 86, 1904–1909. [Google Scholar] [CrossRef]
- Atterbury, R.J.; van Bergen, M.A.P.; Ortiz, F.; Lovell, M.A.; Harris, J.A.; de Boer, A.; Wagenaar, J.A.; Allen, V.M.; Barrow, P.A. Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Appl. Environ. Microbiol. 2007, 73, 4543–4549. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Amir Karimi Torshizi, M.; Rahimi, S.; Dennehy, J.J. Prophylactic bacteriophage administration more effective than post-infection administration in reducing Salmonella enterica serovar Enteritidis shedding in quail. Front. Microbiol. 2016, 7, 1253. [Google Scholar] [CrossRef] [Green Version]
- Toro, H.; Price, S.B.; McKee, S.; Hoerr, F.J.; Krehling, J.; Perdue, M.; Bauermeister, L. Use of bacteriophages in combination with competitive exclusion to reduce Salmonella from infected chickens. Avian Dis. 2005, 49, 118–124. [Google Scholar] [CrossRef]
- Borie, C.; Albala, I.; Sànchez, P.; Sánchez, M.L.; Ramírez, S.; Navarro, C.; Morales, M.A.; Retamales, J.; Robeson, J. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008, 52, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Borie, C.; Sánchez, M.L.; Navarro, C.; Ramírez, S.; Morales, M.A.; Retamales, J.; Robeson, J. El tratamiento por aerosol con bacteriófagos y exclusión competitiva reduce la infección con Salmonella Enteritidis en pollos. Avian Dis. 2009, 53, 250–254. [Google Scholar] [CrossRef]
- Lim, T.H.; Kim, M.S.; Lee, D.H.; Lee, Y.N.; Park, J.K.; Youn, H.N.; Lee, H.J.; Yang, S.Y.; Cho, Y.W.; Lee, J.B.; et al. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Vet. Sci. Res. J. 2012, 93, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Bardina, C.; Spricigo, D.A.; Cortés, P.; Llagostera, M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012, 78, 6600–6607. [Google Scholar] [CrossRef] [Green Version]
- Clavijo, V.; Torres-Acosta, M.A.; Vives-Flórez, M.J.; Rito-Palomares, M. Aqueous two-phase systems for the recovery and purification of phage therapy products: Recovery of Salmonella bacteriophage ΦSan23 as a case study. Sep. Purif. Technol. 2019, 211, 322–329. [Google Scholar] [CrossRef]
- Kimminau, E.A.; Russo, K.N.; Karnezos, T.P.; Oh, H.G.; Lee, J.J.; Tate, C.C.; Baxter, J.A.; Berghaus, R.D.; Hofacre, C.L. Bacteriophage in-feed application: A novel approach to preventing Salmonella Enteritidis colonization in chicks fed experimentally contaminated feed. J. Appl. Poult. Res. 2020, 29, 930–936. [Google Scholar] [CrossRef]
- Li, M.; Lin, H.; Jing, Y.; Wang, J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020, 99, 3643–3654. [Google Scholar] [CrossRef]
- Vaz, C.S.L.; Voss-Rech, D.; Alves, L.; Coldebella, A.; Brentano, L.; Trevisol, I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet. Microbiol. 2020, 240, 108527. [Google Scholar] [CrossRef]
- Sorour, H.K.; Gaber, A.F.; Hosny, R.A. Evaluation of the efficiency of using Salmonella Kentucky and Escherichia coli O119 bacteriophages in the treatment and prevention of salmonellosis and colibacillosis in broiler chickens. Lett. Appl. Microbiol. 2020, 71, 345–350. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Rivas, C.I.; Valle-Hurtado, N.A.; González-Lugo, G.M.; Baizabal-Aguirre, V.M.; Bravo-Patiño, A.; Cajero-Juárez, M.; Valdez-Alarcón, J.J. Bacteriophage Therapy: An alternative for the treatment of Staphylococcus aureus infections in animals and animal models. In Frontiers in Staphylococcus aureus; InTech: London, UK, 2017. [Google Scholar]
- Oliveira, H.; Thiagarajan, V.; Walmagh, M.; Sillankorva, S.; Lavigne, R.; Neves-Petersen, M.T.; Kluskens, L.D.; Azeredo, J. A thermostable Salmonella phage endolysin, Lys68, with broad bactericidal properties against gram-negative pathogens in presence of weak acids. PLoS ONE 2014, 9, e108376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonova, N.P.; Vasina, D.v.; Lendel, A.M.; Usachev, E.v.; Makarov, V.v.; Gintsburg, A.L.; Tkachuk, A.P.; Gushchin, V.A. Broad bactericidal activity of the myoviridae bacteriophage lysins LysAm24, LysECD7, and LysSi3 against gram-negative ESKAPE pathogens. Viruses 2019, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Yang, E.; Chang, P.S.; Ryu, S. Preparation and characterization of endolysin-containing liposomes and evaluation of their antimicrobial activities against gram-negative bacteria. Enzyme Microb. Technol. 2019, 128, 40–48. [Google Scholar] [CrossRef]
- Sarjoughian, M.R.; Rahmani, F.; Abolmaali, S.; Darvish, S.; Astaneh, A. Bacillus phage endolysin, lys46, bactericidal properties against gram-negative bacteria. Iran J. Microbiol. 2020, 12, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, X.; Zhang, T.; Wang, X.; Zou, J.; Zhang, C.; Tang, H.; Zou, Y.; Cheng, B.; Wang, R. Bioinformatic analyses of a potential Salmonella-virus-FelixO1 Biocontrol Phage BPS15S6 and the characterisation and anti-Enterobacteriaceae-Pathogen activity of its endolysin LyS15S6. Antonie Leeuwenhoek J. Microbiol. 2019, 112, 1577–1592. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Y.; Huang, C.; Wang, J.; Wang, X. An endolysin LysSE24 by bacteriophage LPSE1 confers cpecific bactericidal activity against multidrug-resistant Salmonella strains. Microorganisms 2020, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.W.; Jin, J.S.; Kim, J. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2020, 22, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, X.; Wang, L.; Li, G.; Cong, C.; Li, R.; Cui, H.; Murtaza, B.; Xu, Y. The Endolysin of the Acinetobacter Baumannii Phage VB_AbaP_D2 Shows Broad Antibacterial Activity. Microb. Biotechnol. 2021, 14, 403–418. [Google Scholar] [CrossRef]
- Vorob’ev, A.M.; Anurova, M.N.; Aleshkin, A.V.; Gushchin, V.A.; Vasina, D.V.; Antonova, N.P.; Kiseleva, I.A.; Rubalskii, E.O.; Zul’karneev, E.R.; Laishevtsev, A.I.; et al. Determination of bactericidal activity spectrum of recombinant endolysins of ECD7, Am24, Ap22, Si3, and St11 Bacteriophages. Bull. Exp. Biol. Med. 2021, 170, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Qadir, S.; Qureshi, S.; Rehman, S.U. Cloning and expression analysis of fused holin-endolysin from RL bacteriophage; exhibits broad activity against multi drug resistant pathogens. Enzyme Microb. Technol. 2021, 149, 109846. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, D.; Wang, L.; Qu, M.; Li, F.; Tan, Z.; Yao, L. Characterization of a broad-spectrum endolysin LysSP1 encoded by a Salmonella bacteriophage. Appl. Microbiol. Biotechnol. 2021, 105, 5461–5470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, H.H.; Duc, H.M.; Masuda, Y.; Honjoh, K.I.; Miyamoto, T. Endolysin LysSTG2: Characterization and application to control Salmonella Typhimurium biofilm alone and in combination with slightly acidic hypochlorous water. Food Microbiol. 2021, 98, 103791. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, A. Phage therapy regulation: From night to dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [Green Version]
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The bacterial ghost platform system: Production and applications. Bioeng. Bugs. 2010, 1, 326–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of chicken with flagellin adjuvanted Salmonella Enteritidis bacterial ghosts confers complete protection against chicken salmonellosis. Poult. Sci. 2021, 100, 101205. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.M. Controlling Salmonella in Poultry Production and Processing; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Machado Junior, P.C.; Chung, C.; Hagerman, A. Modeling Salmonella spread in broiler production: Identifying determinants and control strategies. Front. Vet. Sci. 2020, 7, 564. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahiman, U.A.; Nordin, N.F.H.; Abdul-Mutalib, N.-A.; Sanny, M. Holistic approaches to reducing Salmonella contamination in poultry industry. Pertanika J. Trop. Agric. Sci. 2021, 44, 2231–8534. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.Y.; Renu, S.; Shanmugasundaram, R.; Akerele, G.O.; Gourapura, R.J.; Selvaraj, R.K. Salmonella chitosan nanoparticle vaccine administration is protective against Salmonella Enteritidis in broiler birds. PLoS ONE 2021, 16, e0259334. [Google Scholar] [CrossRef]
- Berghaus, R.; Thayer, S.G.; Maurer, J.J.; Hofacre, C.L. Effect of vaccinating breeder chickens with a killed Salmonella vaccine on Salmonella prevalences and loads in breeder and broiler chicken flocks. J. Food Prot. 2011, 74, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ren, J.; Xian, H.; Yin, C.; Yuan, Y.; Li, Y.; Ji, R.; Chu, C.; Qiao, Z.; Jiao, X. ROmpF and OMVs as efficient subunit vaccines against Salmonella Enterica serovar Enteritidis infections in poultry farms. Vaccine 2020, 38, 7094–7099. [Google Scholar] [CrossRef]
- Acevedo-Villanueva, K.Y.; Akerele, G.O.; al Hakeem, W.G.; Renu, S.; Shanmugasundaram, R.; Selvaraj, R.K. A Novel approach against Salmonella: A review of polymeric nanoparticle vaccines for broilers and layers. Vaccines 2021, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Akter, T.; Nooruzzaman, N.; Mumu, T.T.; Ahammed, M.; Jalal, A.; Uddin, R.P.; Khan, M.A.H.N.A.; Hossain, M.M. Development of an effective vaccination protocol to produce Salmonella-free layer flock. GMPC TOP. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Zhang-Barber, L.; Turner, A.K.; Barrow, P.A. Vaccination for control of Salmonella in poultry. Vaccine 1999, 17, 2538–2545. [Google Scholar] [CrossRef]
- Crouch, C.F.; Nell, T.; Reijnders, M.; Donkers, T.; Pugh, C.; Patel, A.; Davis, P.; van Hulten, M.C.W.; de Vries, S.P.W. Safety and efficacy of a novel inactivated trivalent Salmonella Enterica vaccine in chickens. Vaccine 2020, 38, 6741–6750. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.W. The use of live vaccines in experimental Salmonella Gallinarum infection in chickens with observations on their interference effect. Am. J. Hyg. 1956, 54, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Methner, U.; Barrow, P.A.; Martin, G.; Meyer, H. Comparative study of the protective effect against Salmonella colonisation in newly hatched spf chickens using live, attenuated Salmonella vaccine strains, Wild-Type Salmonella Strains or a Competitive Exclusion Product. Int. J. Food Microbiol. 1997, 35, 223–230. [Google Scholar] [CrossRef]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Ter Veen, C.; Feberwee, A.; Augustijn, M.; de Wit, S. High specificity of the Salmonella Pullorum/Gallinarum rapid plate agglutination test despite vaccinations against Salmonella Enteritidis and Salmonella Typhimurium. Avian Pathol. 2021, 1–7. [Google Scholar] [CrossRef]
- Rabie, N.S.; Amin Girh, Z.M.S. Bacterial vaccines in poultry. Doc. Bull. Natl. Res. Cent. 2020, 44, 15. [Google Scholar] [CrossRef] [PubMed]
- Tennant, S.M.; Levine, M.M. Live attenuated vaccines for invasive Salmonella infections. Vaccine 2015, 33, C36–C41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marangon, S.; Busani, L. The use of vaccination in poultry production. Revue scientifique et technique. Int. Off. Epizoot. Rev. Sci. Tech. 2007, 26, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Buckley, A.M.; Wang, J.; Hudson, D.L.; Grant, A.J.; Jones, M.A.; Maskell, D.J.; Stevens, M.P. Evaluation of live-attenuated Salmonella vaccines expressing Campylobacter antigens for control of C. jejuni in Poultry. Vaccine 2010, 28, 1094–1105. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of chickens with Salmonella Gallinarium ghosts expressing Salmonella Enteritidis NFliC-FimAC and CD40LC fusion antigen enhances cell-mediated immune responses and protects against wild-type challenges with both species. Dev. Comp. Immunol. 2022, 126, 104265. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.J.; Sharpe, S.M.; Cox, J.M. response of layer and broiler strain chickens to parenteral administration of a live Salmonella Typhimurium vaccine. Poult. Sci. 2015, 94, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Haesebrouck, F.; Ducatelle, R.; van Immerseel, F. Oral vaccination with a live Salmonella Enteritidis/Typhimurium bivalent vaccine in layers induces cross-protection against caecal and internal organ colonization by a Salmonella Infantis strain. Vet. Microbiol. 2018, 218, 7–12. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Sharif, S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008, 9, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eren, U.; Kum, S.; Nazligul, A.; Gules, O.; Aka, E.; Zorlu, S.; Yildiz, M. The several elements of intestinal innate immune system at the beginning of the life of broiler chicks. Microsc. Res. Tech. 2016, 79, 604–614. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Methner, U.; Rychlik, I.; Nagy, B.; Velge, P.; Martin, G.; Foster, N.; Ducatelle, R.; Barrow, P.A. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: Exploitation of innate immunity and microbial activity. Epidemiol. Infect. 2005, 133, 959–978. [Google Scholar] [CrossRef]
- Kallerup, R.S.; Foged, C. Classification of vaccines. In Subunit Vaccine Delivery; Foged, C., Rades, T., Perrie, Y., Hooks, S., Eds.; Springer: New York, NY, USA, 2015; pp. 15–29. [Google Scholar]
- Deguchi, K.; Yokoyama, E.; Honda, T.; Mizuno, K. Efficacy of a novel trivalent inactivated vaccine against the shedding of Salmonella in a chicken challenge model. Avian Dis. 2009, 53, 281–286. [Google Scholar] [CrossRef]
- Foged, C.; Rades, T.; Perrie, Y.; Hook, S. (Eds.) Subunit Vaccine Delivery; Springer: New York, NY, USA, 2015. [Google Scholar]
- de Paiva, J.; Penha Filho, R.; Argüello, Y.; da Silva, M.; Gardin, Y.; Resende, F.; Berchieri Junior, A.; Sesti, L. Efficacy of several Salmonella vaccination programs against experimental challenge with Salmonella Gallinarum in commercial brown layer and broiler breeder hens. Rev. Bras. Cienc. Avic. 2009, 11. [Google Scholar] [CrossRef]
- Mares, M. (Ed.) Current Topics in Salmonella and Salmonellosis; InTech: London, UK, 2017. [Google Scholar]
- Liu, Q.; Tan, K.; Yuan, J.; Song, K.; Li, R.; Huang, X.; Liu, Q. Flagellin-deficient outer membrane vesicles as adjuvant induce cross-protection of Salmonella Typhimurium outer membrane proteins against infection by heterologous Salmonella serotypes. Int. J. Med. Microbiol. Suppl. 2018, 308, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Liljeqvist, S.; Ståhl, S. Production of recombinant subunit vaccines: Protein immunogens, live delivery systems and nucleic acid vaccines. J. Biotechnol. 1999, 73, 1–33. [Google Scholar] [CrossRef]
- Wang, S.; Kong, Q.; Curtiss, R. New technologies in developing recombinant attenuated Salmonella vaccine vectors. Microb. Pathog. 2013, 58, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roto, S.M.; Kwon, Y.M.; Ricke, S.C. Applications of in ovo technique for the optimal development of the gastrointestinal tract and the potential influence on the establishment of its microbiome in poultry. Front. Vet. Sci. 2016, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzała, M.; Janicki, B.; Czarnecki, R. Consequences of different growth rates in broiler breeder and layer hens on embryogenesis, metabolism and metabolic rate: A review. Poult. Sci. 2015, 94, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Tako, E.; Gal-Garber, O.; Sklan, D. Morphological, molecular, and functional changes in the chicken small intestine of the late-term embryo. Poult. Sci. 2003, 82, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.; Levanon, M. Research note: Effect of posthatch holding time on performance and on residual yolk and liver composition. Poult. Sci. 1993, 72, 1994–1997. [Google Scholar] [CrossRef]
- Noy, Y.P.; Geyra, A.; Sklan, D.J. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001, 80, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Halevy, O.; Geyra, A.; Barak, M.; Uni, Z.; Sklan, D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000, 130, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozdziak, P.E.; Dibner, J.J.; McCoy, D.W. The effect of early posthatch starvation on calpain mRNA levels. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 133, 221–226. [Google Scholar] [CrossRef]
- Distinguished, W.N.R. Embryo Epigenomic Response to Breeder Management and Nutrition; World’s Poultry Congress: Paris, France, 2012. [Google Scholar]
- Rousseau, X.; Valable, A.-S.; Létourneau-Montminy, M.-P.; Même, N.; Godet, E.; Magnin, M.; Nys, Y.; Duclos, M.J.; Narcy, A. Adaptive response of broilers to dietary phosphorus and calcium restrictions. Poult. Sci. 2016, 95, 2849–2860. [Google Scholar] [CrossRef]
- Ashwell, C.M.; Angel, R. Nutritional Genomics: A practical approach by early life conditioning with dietary phosphorus. Rev. Bras. Zootec. 2010, 39, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Akinyemi, F.T.; Ding, J.; Zhou, H.; Xu, K.; He, C.; Han, C.; Zheng, Y.; Luo, H.; Yang, K.; Gu, C.; et al. Dynamic distribution of gut microbiota during embryonic development in chicken. Poult. Sci. 2020, 99, 5079–5090. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, X.; Cheng, R.; Ansari, A.R.; Elokil, A.A.; Hu, Y.; Chen, Y.; Nafady, A.A.; Liu, H. Sex differences in growth performance are related to cecal microbiota in chicken. Microb. Pathog. 2021, 150, 104710. [Google Scholar] [CrossRef]
- Van der Eijk, J.A.J.; de Vries, H.; Kjaer, J.B.; Naguib, M.; Kemp, B.; Smidt, H.; Rodenburg, T.B.; Lammers, A. Differences in gut microbiota composition of laying hen lines divergently selected on feather pecking. Poult. Sci. 2019, 98, 7009–7021. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M.; Burmester, B.R. Resistance of Marek’s disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. 1982, 26, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Bavananthasivam, J.; Astill, J.; Matsuyama-Kato, A.; Taha-Abdelaziz, K.; Shojadoost, B.; Sharif, S. Gut Microbiota is associated with protection against marek’s disease virus infection in chickens. Virology. 2021, 553, 122–130. [Google Scholar] [CrossRef]
- Sun, X.; Liao, X.; Lu, L.; Zhang, L.; Ma, Q.; Xi, L.; Luo, X. Effect of in ovo zinc injection on the embryonic development, tissue zinc contents, antioxidation, and related gene expressions of broiler breeder eggs. J. Integr. Agric. 2018, 17, 648–656. [Google Scholar] [CrossRef] [Green Version]
- Zhai, W.; Rowe, D.E.; Peebles, E.D. Effects of commercial in ovo injection of carbohydrates on broiler embryogenesis. Poult. Sci. 2011, 90, 1295–1301. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Elwan, H.A.M.; Xu, Q.Q.; Xie, C.; Dong, X.Y.; Zou, X.T. Effects of in ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult. Sci. 2019, 98, 2290–2298. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, K.; Mishra, R.; Jha, R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020, 99, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, L.L.; Miao, L.P.; Zhang, N.N.; Zou, X.T. Effects of in ovo feeding of cationic amino acids on hatchability, hatch weights, and organ developments in domestic pigeon squabs (Columba livia). Poult. Sci. 2018, 97, 110–117. [Google Scholar] [CrossRef]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat Stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Castañeda, C.D.; Miotto, J.; McDaniel, C.; Kiess, A.S.; Zhang, L. Effects of in ovo probiotic administration on the incidence of avian pathogenic Escherichia coli in broilers and an evaluation on its virulence and antimicrobial resistance properties. Poult. Sci. 2021, 100, 100903. [Google Scholar] [CrossRef] [PubMed]
- Vaezirad, M.M.; Koene, M.G.; Wagenaar, J.A.; van Putten, J.P.M. Chicken immune response following in ovo delivery of bacterial flagellin. Vaccine 2018, 36, 2139–2146. [Google Scholar] [CrossRef]
- Yamawaki, R.A.; Milbradt, E.L.; Coppola, M.P.; Rodrigues, J.C.Z.; Andreatti Filho, R.L.; Padovani, C.R.; Okamoto, A.S. Effect of immersion and inoculation in ovo of Lactobacillus spp. in embryonated chicken eggs in the prevention of Salmonella Enteritidis after hatch. Poult. Sci. 2013, 92, 1560–1563. [Google Scholar] [CrossRef]
- Berrocoso, J.D.; Kida, R.; Singh, A.K.; Kim, Y.S.; Jha, R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017, 96, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Abousaad, S.; Lassiter, K.; Piekarski, A.; Chary, P.; Striplin, K.; Christensen, K.; Bielke, L.R.; Hargis, B.M.; Dridi, S.; Bottje, W.G. Effects of in ovo feeding of dextrin-iodinated casein in broilers: I. hatch weights and early growth performance. Poult. Sci. 2017, 96, 1473–1477. [Google Scholar] [CrossRef]
- Hashemzadeh, Z.; Torshizi, M.A.K.; Rahimi, S.; Razban, V.; Salehi, T.Z. Prevention of Salmonella colonization in neonatal broiler chicks by using different routes of probiotic administration in hatchery evaluated by culture and PCR techniques. Agric. Sci. Technol. 2010, 12, 425–432. [Google Scholar]
- McGruder, E.D.; Ramirez, G.A.; Kogut, M.H.; Moore, R.W.; Corrier, D.E.; Deloach, J.R.; Hargis, B.M. In ovo administration of Salmonella Enteritidis-immune lymphokines confers protection to neonatal chicks against Salmonella Enteritidis organ infectivity. Poult. Sci. 1995, 74, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.M.; Dalloul, R.A. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015, 6, 45–52. [Google Scholar] [CrossRef]
- Meijerhof, R.; Hulet, R.M. In ovo injection of competitive hatching eggs exclusion culture in broiler. J. Appl. Poult. Res. 1997, 6, 260–266. [Google Scholar] [CrossRef]
- Penha Filho, R.A.C.; Díaz, S.J.A.; Fernando, F.S.; Chang, Y.F.; Andreatti Filho, R.L.; Berchieri Junior, A. Immunomodulatory activity and control of Salmonella Enteritidis colonization in the intestinal tract of chickens by Lactobacillus based probiotic. Vet. Immunol. Immunopathol. 2015, 167, 64–69. [Google Scholar] [CrossRef]
- Teague, K.D.; Graham, L.E.; Dunn, J.R.; Cheng, H.H.; Anthony, N.; Latorre, J.D.; Menconi, A.; Wolfenden, R.E.; Wolfenden, A.D.; Mahaffey, B.D.; et al. In ovo evaluation of FloraMax®-B11 on Marek’s disease HVT vaccine protective efficacy, hatchability, microbiota composition, morphometric analysis, and Salmonella Enteritidis infection in broiler chickens. Poult. Sci. 2017, 96, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.S.; Line, E. In ovo gentamicin and mucosal starter culture to control Salmonella in broiler production. J. Appl. Poult. Res. 2001, 10, 376–379. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, C.D.; Dittoe, D.K.; Wamsley, K.G.S.; McDaniel, C.D.; Blanch, A.; Sandvang, D.; Kiess, A.S. In ovo inoculation of an Enterococcus faecium–based product to enhance broiler hatchability, live performance, and intestinal morphology. Poult. Sci. 2020, 99, 6163–6172. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.E.; van der Hoeven-Hangoor, E.; van de Linde, I.B.; Montijn, R.C.; van der Vossen, J.M.B.M. In ovo inoculation of chicken embryos with probiotic bacteria and its effect on posthatch Salmonella susceptibility. Poult. Sci. 2014, 93, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Pender, C.M.; Kim, S.; Potter, T.D.; Ritzi, M.M.; Young, M.; Dalloul, R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017, 96, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, K.M.; He, H.; Swaggerty, C.L.; McReynolds, J.L.; Genovese, K.J.; Duke, S.E.; Nerren, J.R.; Kogut, M.H. In ovo treatment with CpG oligodeoxynucleotides decreases colonization of Salmonella Enteriditis in broiler chickens. Vet. Immunol. Immunopathol. 2009, 127, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Sanger, F.; Coulson, A.R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J. Mol. Biol. 1975, 94, 441–448. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [Green Version]
- Schadt, E.E.; Turner, S.; Kasarskis, A. A window into third-generation sequencing. Hum. Mol. Genet. 2010, 19, R227–R240. [Google Scholar] [CrossRef]
- Pareek, C.S.; Smoczynski, R.; Tretyn, A. Sequencing technologies and genome sequencing. J. Appl. Genet. 2011, 52, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzker, M.L. Sequencing in real time. Nat. Biotechnol. 2009, 27, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.-J.; Zody, M.C.; Eriksson, J.; Meadows, J.R.S.; Sherwood, E.; Webster, M.T.; Jiang, L.; Ingman, M.; Sharpe, T.; Ka, S.; et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 2010, 464, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; He, Y.; Mann, D.A.; Deng, X. Global spread of Salmonella Enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chousalkar, K.K. Short-term feeding of probiotics and synbiotics modulates caecal microbiota during Salmonella Typhimurium infection but does not reduce shedding and invasion in chickens. Appl. Microbiol. Biotechnol. 2019, 104, 319–334. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Y.; Dong, Y.; Ito, K.; Zhang, B. Highly nutritious diet resists Salmonella Typhimurium infections by improving intestinal microbiota and morphology in broiler chickens. Poult. Sci. 2020, 99, 7055. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Zhu, B.; Wang, J.; Wang, Q.; Zheng, M.; Wen, J.; Li, Q.; Zhao, G. Transcriptome analysis of the cecal tonsil of jingxing yellow chickens revealed the mechanism of differential resistance to Salmonella. Genes 2019, 10, 979. [Google Scholar]
- Cadena, M.; Froenicke, L.; Britton, M.; Settles, M.L.; Durbin-Johnson, B.; Kumimoto, E.; Gallardo, R.A.; Ferreiro, A.; Chylkova, T.; Zhou, H.; et al. Transcriptome analysis of Salmonella Heidelberg after exposure to cetylpyridinium chloride, acidified calcium hypochlorite, and peroxyacetic Acid. J. Food Prot. 2019, 82, 109–119. [Google Scholar] [CrossRef]
| Target Species | Prebiotic | Experimental Procedure | Main Results | Ref. |
|---|---|---|---|---|
| 60–65 w old White Leghorn hens | FOS | Birds housed at 27 ± 2 °C under a photoperiod of 16 h light: 8 h dark and supplemented with 0.1% of the prebiotic into a diet based on corn/soybean meal. Challenged against a nalidixic acid-resistant S. Enteritidis strain (2.4 × 108 CFU) | FOS reduced fecal S. Enteritidis numbers and increased TLR-4, IFN γ, and IgA expression | [38] |
| Commercial meat-type broiler | Trehalose dihydrate | Broiler was supplemented with 5% w/w of the prebiotic and allocated at 22–30 °C (Humidity: 60 to 70%, dark-light 4/20 h photoperiod) and inoculated with S. Typhimurium (3.5 × 108 CFU) | Trehalose increased the abundance of lactobacilli and suppressed the growth and inflammation caused by S. Typhimurium in the cecum | [39] |
| One-day-old Cobb broilers | 2.6 Beta LevaFructan | Broilers were orally supplemented with the LevaFructan (100 gm on 1000 mL/0.5 mL per liter of drinking water) and maintained at 24 °C with a diet based on a balanced commercial ration. Challenge was performed by inoculation of S. Enteritidis (109 CFU/mL) and a live lyophilized attenuated vaccine (S. Enteritidis Sm24/Rif 12/Ssq) | Prebiotics had a synergistic effect whit the vaccine on the decreasing of fecal isolation of S. Enteritidis | [40] |
| 40 d old Cobb broilers | Aspergillus meal | Broilers were allocated on floor pens, fed with a commercial diet, and supplemented with 0.2% w/w of the Aspergillus meal. Challenge occurred by inoculation with S. Typhimurium (1.25 × 105 CFU) | Aspergillus meal reduced S. Typhimurium horizontal transmission | [41] |
| Turkeys | Aspergillus meal | Turkeys were housed on floor pens, fed with a commercial diet supplemented with 0.2% w/w of Aspergillus meal, and challenged against S. Enteritidis (1.5 × 105 CFU) | Aspergillus meal reduced S. Enteritidis colonization | [41] |
| Turkeys | Lactulose | Turkeys were fed with a corn and soybean meal diet, supplemented with lactulose (0.003 mL kg−1 body weight), and inoculated with S. Enteritidis (7.0 × 105 CFU). | Salmonella challenged Turkeys, but prebiotic supplemented increased weight gain | [42] |
| Target Species | Probiotic | Experimental Procedure | Main Results | Ref. |
|---|---|---|---|---|
| One-day-old Cobb Broilers | Lactobacillus acidophilus, Lactobacillus plantarum, Pediococcus pentosaceus, Saccharomyces cerevisiae, Bacillus subtilis, and Bacillus licheniformis | Broilers were supplemented with a commercial probiotic-based preparation (1.0 × 109 CFU/each strain), challenged against S. Enteritidis (0.5 mL, 109 CFU/mL), and inoculated with a live attenuated vaccine (S. enteritidis, strain Sm24/Rif 12/Ssq). Allocation was at 24 °C and feeding based on a commercial balanced ration | Probiotics exhibited a synergistic effect whit the vaccine and resulted in the decreasing of fecal isolation of S. Enteritidis | [44] |
| Broilers | Lactobacillus acidophilus, Enterococcus faecium, Lactobacillus plantarum, and Lactobacillus casei | Probiotic supplemented broilers (1 mg/4 L of drinking water of the commercial preparation) were challenged against S. Enteritidis (0.5 mL, 109 CFU/mL). Diet consisted of a standard commercial starter concentrate | Probiotics prevented Salmonella infections in broilers | [50] |
| Hy-Line Brown layer hens | Poultry Star® (Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, and Lactobacillus reuteri) | Layers housed on floor pens and fed with stem-pelleted pullet starter and grower rations. Challenge consisted in the inoculation with S. Typhimurium PT 135 (106 CFU per bird) | Probiotics enhanced the protection induced by vaccination with a live aro-A deletion mutant vaccine | [53] |
| Layer hens | Bacillus subtilis DSM 32324, Bacillus subtilis DSM 32325, and Bacillus amyloliquefaciens | Hens were allocated on floor pens and supplemented with the probiotic combination (1 g/kg of feed) to be challenged against S. Typhimurium (106 CFU/mL) | Probiotic supplementation decreased Salmonella counts in feces | [46] |
| Layer hens (Hy-Line Brown) | Bacillus amyloliquefaciens, B. licheniformis, and B. pumilus | Hens were supplemented with the commercial preparation (454 g/ton of feed) and challenged against S. Enteritidis (3 × 107 CFU/mL). Allocation consisted of floor pens and feed based on a basal diet, mash feed, and water offered ad libitum | Probiotics reduced the Salmonella recovery from layer ceca | [48] |
| Hy-Line Brown layer hens | Bacillus subtilis CSL2 | Hens were housed on floor pens, fed with an antibiotic and additive-free basal diet, and inoculated with S. Gallinarum KVCC-BA0700722 (1 × 108 CFU/mL) | Protective effects include improvement of bacterial diversity, enhanced metabolic activity and gut functionality, and reversal of the effects of S. Gallinarum infection | [54] |
| 1 d-old Arbor Acres broilers | Lactobacillus salivarius Erya | Broilers were fed basal diet supplemented with L. salivarius Erya 107, 108 and 109 CFU/kg of feed, vaccinated with attenuated infectious bursal disease virus vaccine and challenged against Salmonella Pullorum and exposed to aflatoxin B1 (AFB1). | L. salivarius degrade AFB1, enhanced antibody and IFN-γ production and lymphocite proliferaion, besides enhanced the resistance against S. Pullorum infection. | [51] |
| Broilers | Bacillus subtilis | Broilers were supplemented with the commercial probiotic preparation (0.2% w/w of feed) and challenged against S. Enteritidis (0.2 mL of 1.0 × 105 CFU/)mL. Housing consisted of floor pens and fed in a basal diet | Probiotic improved the immune response of Salmonella-infected broilers | [49] |
| Target Species | Synbiotic | Experimental Procedure | Main Results | Ref. |
|---|---|---|---|---|
| Hy-Line Brown laying hens | Commercial probiotic mix (Enterococcus faecium, Pediococcus acidilactici, Bifidobacterium animalis, Lactobacilus reuteri) + fructooligosaccharides | Symbiotic supplementation (20 g/1000 birds/day) of hens allocated on floor pens, orally-infected with S. Typhimurium PT 135 (106 CFU per bird), and vaccinated against Salmonella | The synbiotic enhanced the immune response in vaccinated hens, inhibiting Salmonella shedding pattern | [53] |
| Hy-Line pullets | Commercial symbiotic: Bacillus + mannooligosaccharide | Pullets were supplemented with the symbiotic (0.075% w/w of diet formulated with 113 g/ton of amprolium) and challenged against a Nalidixic acid-resistant S. Enteritidis strain (3 × 106 CFU) | The symbiotic-supplemented birds exhibited reduced colonization of ceca and ovary with S. Enteritidis | [56] |
| Hy-Line W-36 laying hens | Bacillus amyloliquefaciens, Bacillus licheniformis, and Bacillus pumilus (250 ppm) and yeast cell wall (mannan and β-glucan, 250 ppm) | Synbiotic were mixed with the commercial feed and supplemented to hens challenged against a nalidixic acid-resistant S. Enteritidis strain (7 × 107 CFU) | The synbiotic reduced the counts of S. Enteritidis from ceca | [48] |
| Dekalb White female chicks | Bacillus subtilis C-3102 (250,000 CFU/g) and 0.05% of yeast cell walls | Birds were allocated into floor cages and fed with a nonmedicated ration based on corn and soybean. A nalidixic acid-resistant strain of S. Enteritidis was inoculated to chicks (2.1 × 109 CFU) | A significantly lower abundance of Salmonella was found in the cecal microbiota of supplemented birds | [57] |
| Cobb broilers | Commercial synbiotic (Saccharomyces sp. and Lactobacillus sp.) | Birds feeding includes a commercial broiler feed supplemented with the commercial synbiotic. Broilers were allocated into battery cages and inoculated with S. Enteritidis (1 × 109 CFU) | Salmonella-challenged Broilers challenged, but synbiotic-supplemented increased the weight gain and maintained immunity response compared to its unsupplemented counterpart | [58] |
| COBB Avian48 broilers | Lactobacillus rhamnosus HN001 and Pediococcus acidilactici MA18/5M (7 log CFU, and fructans from Agave tequilana (4.5%) | Broilers were allocated into floor pens and fed with an antibiotic-free diet supplemented with the synbiotic. The challenge consisted of inoculation with S. Typhimurium PT 135 (105 log CFU per bird) | S. Typhimurium was inhibited in synbiotic-supplemented broilers and resulted in a decrease in the intensity and frequency of histopathological injuries | [55] |
| Target Species | Postbiotic Strategy | Experimental Procedure | Main Results | Ref. |
|---|---|---|---|---|
| Hy-Line W-36 laying pullets | Fermentation-based postbiotic from Saccharomyces cerevisiae | Birds were housed in rooms, fed with a standard commercial starter and grower, postibiotic-supplemented (1.5 kg/MT in the starter diet, 1.0 kg/MT in the grower diet), and challenged against S. Enteritidis (1.0 × 106 CFU/mL) | Postbiotics reduced S. Enteritidis concentrations in the ceca | [59] |
| Broilers | Semi-purified Albusin B from Ruminococcus albus 7 (2.5 g/kg) | Broilers were housed into pens under controlled conditions and fed with a basal diet based on corn/soybean meal. Challenge was performed by Salmonella spp. Inoculation (6.15 log CFU/g) | Salmonella colonization was reduced in postbiotic-supplemented broilers as well as nutrient absorption was improved | [60] |
| 43-day-old broilers | Bacteriocin L-1077 from Lactobacillus salivarius 1077 (12.5 mg/L of drinking water) | Birds were inoculated with an S. enterica serovar Enteritidis 0.2 mL suspension of 1011 CFU/mL for 3 days while feed and water were offered ad libitum | Bacteriocin supplementation reduced Salmonella counts | [61] |
| Broilers | Salmonella Enteritidis bacterin from an autogenous Salmonella Enteritidis (0.2 mL of/bird) | Broilers were fed with a standard commercial ration and challenged against S. Enteritidis (0.5 mL containing 109 CFU/mL) | Bacterin inactivated S. Enteritidis and avoided pathogenic infection in broilers | [50] |
| Target Species | Phytobiotic | Procedure | Main Results | Ref. |
|---|---|---|---|---|
| Cobb broiler chicks | Garlic extract | Five consecutive days of garlic extract orally administered (200 mm/)mL 24 h later of Salmonella infection | In vitro inhibition of S. Typhimurium. S. Papuana, S. Inganda, S. Kentucky, S. Enteritidis, S. Heidelberg, S. Molade, S. Tamale, S. Labadi (Minimum inhibitory concentration of 40–100 mg/)mL. Decrease in mortality and increase in body weight in supplemented chickens and challenged against S. Typhimurium | [73] |
| Cobb X Cobb broilers | Capsaicin | Inclusion of purified capsaicin (10 ppm), capsaicin oleoresin in finisher diet of S. Enteritidis challenged birds (5 or 20 ppm), or prophylactic use for prevention of S. Typhimurium infection (5 or 20 ppm) | Reduction in S. Enteritidis colonization in liver/spleen and ceca when used purified capsaicin. Inclusion of 5 ppm reduced S. Enteritidis colonization in ceca and decreased cecal lamina propia thickness. Prophylactic use of capsaicin induced resistance to S. Typhimurium | [74] |
| 20 d old Ross X Ross broilers | Plant-derived trans-Cinnamaldehyde (TC) and Eugenol (EG) | Birds were supplemented with TC (0.5 or 0.75%) or EG (0.75 or 1%) and inoculated with S. Enteritidis on day 8 | Both TC and EG reduced S. Enteritidis colonization of the cecum after 10 d of infection. TC did not affect feed intake and body weight; meanwhile, body weight was lower in EG supplemented birds | [75] |
| One-day-old male Cobb × Cobb broilers | Essential oil blend (carvacrol, thymol, eucalyptol, lemon) | Essential oil blend was administrated in drinking water to chicks (0–7 and 35–42 day), and a half of birds were challenged against S. Heidelberg | An inclusion of 0.05% of the essential oil blend reduced S. Heidelberg colonization in crops of challenged birds, but no effect was observed when 0.025 or 0.015% concentrations were used. The essential oil also lowered feed conversion ratio and improved weight gain | [76] |
| 1 d old male broiler Cobb 500 chicks | Phytogenic feed additive based in essential oils (Carvacrol, thymol, and cinnamic aldehyde) | Chickens were supplemented with 0.5 or 1% of the additive and monitored for the total bacterial count in bed samples on day 42 | Total bacterial count in bed samples was reduced by 1% of inclusion of the feed additive, and total erythrocyte counts and hemoglobin content increased, while lymphocyte counts decreased | [77] |
| Ross 308 chickens | Commercial phytobiotic based on a mix of essential oils Intebio (garlic, lemon, thyme, and eucalyptus) | Administration of the phytobiotic mixture since 1 d old and challenge against S. Enteritidis at day 19 | One day post infection, genes AvBD10, IL6, IL8L2, CASP6, and IRF7 were upregulated, and their expression was lower at day 23 in the infected birds. Intebio did not involve a pronounced change in microbiota but an earlier suppression of inflammatory reaction | [78] |
| Cobb broiler chickens | Propyl propane Thiosulfonate derived from garlic (PTS-O) | Feed inclusion of PTS-O (45 or 90 mg/kg of diet) | Both concentrations of the compound resulted in lower number of copies (log10) of ileal Salmonella sp., crop Enterobacteria, and Escherichia coli. Feed–gain ratios were improved as well as ileal villus height, width and surface area, mucosal thickness, and muscular layer thickness | [79] |
| Dekalb hens | Capsaicin | Two levels supplementation of the capsaicin (18 and 36 ppm). Hens were challenged against S. Enteritidis on day 25 | Salmonella liver and spleen invasion was reduced when hens were supplemented with 36 ppm of capsaicin. Capsaicin also increased the deposition of red pigment in egg yolk | [67] |
| Ross 308 broiler chicks | Sanguinarine, oregano. | Birds were supplemented with the phytobiotics and their combination con probiotic strains and challenged on day 2 against S. Typhimorium | Phytobiotics improved growth performance and gut health through the mitigation of the negative effect of the disease | [80] |
| Ross 308 broiler chicks | Commercial mixture of 7 plant extracts (oregano, eucalyptus, thyme, garlic, lemon, rosemary, and sweet orange) | Three presentations of phytobiotic mixture (Mix-Oil Mint, Mix-Oil Liquid, Sangrovit Extra) were administrated to birds infected with S. Typhimorium | Supplemented and Salmonella challenged birds exhibited growth performance and improvements in meat characteristics comparable with their counterpart treated with the antibiotic avilamycin | [81] |
| Target Species | Description b | Phage Application c | Results | Ref. |
|---|---|---|---|---|
| One-day-old chicken | Oral challenge S. Enteritidis PT4 108 CFU/bird | Single oral application of phage cocktail (CNPSA1, CNPSA3, and CNPSA4) 1011 PFU | Reduction in 3.5 orders of magnitude of CFU of S. Enteritidis PT4 per gram of cecal content | [87] |
| 6-week-old chickens | Oral challenge S. Gallinarum 5 × 108 CFU/mL | Bacteriophage CJø01 as food additive at 106 PFU/kg | Reduction from 30% to 5% of mortality | [88] |
| One-day-old chickens | Challenge with S. Enteritidis by oral gavage (0.25 mL) 9 × 103 CFU/chick | Cocktail of 4 bacteriophages (CB4φ) from commercial broiler houses; cocktail of 45 bacteriophages (WT45φ) from wastewater treatment plants; 108 PFU/chick | Short time (24–48 h) prevention of colonization. No long-term effect | [89] |
| 36-day-old chickens | Challenge with S. Enteritidis, 1 mL of 108 CFU/mL | Cocktail of three bacteriophages (151, 25, and 10) against S. Enteritidis, S. Hadar, S. Typhimurium, by oral gavage, 109 and 1011 PFU/ml | Reduction of 2–4 log units of S. Enteritidis and S. Typhimurium after 1011 PFU/ml | [90] |
| 33-day-old quails | Oral challenge, 100 mL S. Enteritidis, 1.2 × 109 CFU/ml | Single Salmonella lysing phage (PSE), 109 PFU/mL 100 µL by oral beverage for 2 days | 100% clearance of S. Enteritidis from tonsils after 6 h of treatment | [91] |
| One-day-old chickens | Oral challenge, 0.5 mL of S. Typhimurium, 2.4 × 105 or 7.9 × 105 CFU/mL | Bacteriophage cocktail (S2a, S9, S11), 106 PFU/bird at days 4–6 and 8–10 of age. Supplementation with commercial probiotic | 10-fold reduction of S. Typhimurium in ileum, ceca, liver, and spleen. Synergism with the probiotic | [92] |
| Ten-day-old chickens | Oral challenge, S. Enteritidis 9.6 × 105 CFU/mL | Cocktail of three bacteriophages from sewage system, 103 PFU by coarse spray or drinking water, 24 h prior to bacterial challenge | Reduction from 5.67 (control) to 4.04 (aerosol) and 4.25 (drinking water) log10 CFU/mLof S. Enteritidis | [93] |
| One-day-old chickens | Oral challenge, 2.5 × 105 CFU/mLof S. Enteritidis | Cocktail of three phages () 108 PFU//mLdose by aerosol at 6 days of age. Probiotic supplementation | Reduction of 100% of mortality | [94] |
| One-day-old chickens | Oral challenge, S. Enteritidis, 5 × 108 CFU/mL | Bacteriophage CJ07, 105, 107, 109 PFU/g, 21 days after challenge | Higher titers reduced replication of the pathogen in the digestive tract | [95] |
| Three-day-old, specific pathogen-free chickens | Oral challenge, S. Typhimurium 105 CFU/animal (10 times the lethal dose) | Cocktail of three bacteriophages (UAB_Phi20, UAB_Phi78, UAB_Phi87) lytic against S. Enteritidis and S. Typhimurium. 1010 PFU/animal. Treatment from day-1 to 15 post infection | Reduction in 2–4.4 log10 of S. Typhimurium. Repeated administration of the cocktail maintained bacteriophage titers by 104–105 PFU/g cecal content | [96] |
| 14-day-old broiler chickens | No challenge, prevalence evaluation in a large-scale study (more than 69,000 chicks) | SalmoFREE®, commercial cocktail of six bacteriophages in drinking water, 1 × 108 PFU/mL | Reduction to 0% of prevalence in cloacal swabs, PCR detection | [97] |
| 70-day-old broiler chickens | Feed challenge, S. Enteritidis 107 CFU/g food | Bacteriophage KCTC 12012BP, 108 PFU/g food | Reduction of the prevalence of S. Enteritidis in cloacal swabs, liver/spleen samples, and ceca | [98] |
| Two-week-old chickens | Feed challenge, S. Typhimurium, 1 × 108 CFU/g food | Bacteriophages STP4-a, 109 PFU/g food; pre-treated 7 days before bacterial challenge, treated 14 days after bacterial challenge | Pretreatment eliminated S. Typhimurium; treatment reduced bacterial counts in 2 log10 units | [99] |
| One-day-old chickens | Oral gavage challenge, S. Enteritidis, 6 × 106 CFU | Bacteriophage cocktail (BRM 13312, BRM 13313, BRM 13314) 6.8 × 1010 PFU/broiler. Early treatment (days 6–10 post-infection), late treatment (days 31–35 post-infection) | Later treatment was more effective, reducing by 1.08 log10 CFU/g cecal content. | [100] |
| One-day-old broiler chickens | Oral challenge, 0.1 mLat 108 CFU/,mL S. Kentucky | Oral administration, 0.1 mL at 108 PFU/mL | Reduction in 1.76 to 2.6 log10 units. No significant difference if bacteriophages were administrated before or after challenge | [101] |
| Target Pathogens | Endolysin Application | Main Results | Ref. |
|---|---|---|---|
| S. Typhimurium LT2, A. baumannii 2, P. aeruginosa PAO1, P. fluorescens 7A, Shigella sonnei ATCC 25931, E. coli O157:H7 CECT 4782, Cronobacter sakazakii CECT 858, Pantoea agglomerans SA5634, Enterobacter amnigenus CECT 4878, Proteus mirabilis SA5445, Salmonella bongori SGSC 3100, Klebsiella oxytoca ATCC 13182, Yersinia enterocolitica SA5429 | Recombinant endolysin Lys68 (phage from S. Enteritidis), 2 µM with EDTA and organic acids as permeabilizers | Broad spectrum of activity in the presence of malic acid as permeabilizer | [104] |
| Clinical or reference strains of Pseudomonas aeruginosa, Acinetobacter baumannii. Klebsiella pneumoniae, Escherichia coli, Salmonella typhi, Staphylococcus aureus, Staphylococcus haemolyticus | Recombinant endolysins LysAm24 (phage from A. baumannii), LysECD7 (phage from E. coli), LysSi3 (phage from S. Typhi), 5–15 µg/mL | Broad spectrum of specificity against all strains except those from the genus Staphylococcus, which showed total or partial resistance | [105] |
| Salmonella Typhimurium LT2, Escherichia coli DH5α | Recombinant endolysin BSP16Lys (phage from S. Typhiumurium) loaded into liposomes to trespass outer membrane barrier | BSP16Lys loaded into liposomes were active against S. Typhimurium and E. coli | [106] |
| Streptococcus pyogenes ATCC19615, Bacillus subtilis ATCC 12711, Bacillus sp., P. aeruginosa ATCC 27853, E. coli ATCC 25922, S. Typhimurium DDBCC1001, Proteus sp., K. pneumoniae ATCC 13883 | Purified endolysin Lys4630, from a lysogenic bacteriophage SPP1 against Bacillus subtilis | Lytic activity against Gram-negative pathogens tested | [107] |
| S. enterica ATCC13076, E. coli ATCC35150, Shigella flexneri CMCC51572 | Recombinant Endolysin LyS15S6 (phage Salmonella-virus-FelixO1) administrated with ε-poly-L-lysine as outer membrane permeabilizer | Reduction in 4.19, 3.18, and 3.00 log10 units, respectively | [108] |
| Multidrug-resistant strains of S. Enteritidis, S. Typhimurium, S. Agora, S. Indiana, S. Anatum, S. Dublin | Recombinant endolysin LysSE24 administrated with outer membrane permeabilizers | Broad spectrum of activity mainly against multidrug resistance Salmonella | [109] |
| A. baumanii, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella Enteritidis, S. Typhimurium, Staphylococcus aureus | Recombinant endolysis LysSS (phage from S. Enteritidis) | Broad spectrum of antibacterial activity. Active without a permeabilizer additive. Different minimal inhibitory concentrations for A. baumannii genotypes | [110] |
| Several strains of A. baumannii, Salmonella spp., Pseudomonas aeruginosa, Enterococcus faecium, Enterococcus faecalis, Staphylococcus aureus. | Recombinant endolysin Abtn-4 (phage from Acinetobacter baumannii), 5 µM. No permeabilizer added | Reduction of Gram-negatives in more than 3 log10 units, active against Gram-positives. Reduction of biofilm formation for both Gram-positives and Gram-negatives | [111] |
| Clinical isolates from S. Enteritidis, S. Infantis, S. Typhimurium, S. Dublin, S London, A. baumanii, Enterobacter spp., P. aeruginosa, K. pneumoniae, Campylobacter jejuni | Individually tested recombinant endolysins (100 µg/)mL LysAm24 (phage from A. baumannii), LysAp22 (phage from A. baumannii), LysSi3 (phage from S. Infantis), LysSt11 (phage from S. Typhimurium), LysECD7 (phage from E. coli) | All tested isolates showed broad spectrum of activity | [112] |
| Pseudomonas aeruginosa, S. Typhimurium, S. Enteritidis, S. Gallinarum Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive S. aureus (MSSA) | Combined use of recombinant endolysin RL-Lys and holin RLH-Lys (phage from P. aeruginosa) | The holin allows the entrance of the endolysin into the periplasmic space showing a broad-spectrum activity | [113] |
| S. Typhimurium, S. Enteritidis, S. Paratyphi A, S. Paratyphi B, Shigella dysenteriae, S. boydii, E. coli, Lysteria monocytogenes, S. aureus | Recombinant LysSp1 (phage from S. Typhimurium) 1–10 µg/mLin the presence of EDTA | Reduction in 1–6 log10 units, broad spectrum of specificity | [114] |
| S. Typhimurium NBRC 12529, S. Typhimurium FHC, S. Anatum, S. Braenderup, S. Derby, S. Enteritidis FHC, S. Hadar, S. Litchfield, S. Stanley, Escherichia coli NBRC 3301 (K-12), E. coli BW25113, Enterohemorrhagic E. coli O91:H-*1, Enterohemorrhagic E. coli O157: H7, Pseudomonas aeruginosa NBRC 13275, Staphylococcus aureus NCTC8325, Listeria monocytogenes No.180 | Recombinant endolysin LysSTG2 (phage from S. Typhimurium), 2–800 µg/mLin the presence of chloroform/Tris-HCl (spectrum activity assay) or slightly acidic hypochlorous water (SAHW; 40 mg/L chlorine, pH5.5) for biofilm assay in S. Typhimurium | Broad spectrum of activity on Gram-negative bacteria. Synergy with SAHW in biofilm assays | [115] |
| Target Species | Delivered Compound | Experimental Procedure | Main Results | Ref. |
|---|---|---|---|---|
| SPF Ross 308 broilers | Vaccine | Vaccination Salmonella flagellin to 18 day old embryonated eggs | Elevated pro-inflammatory chIL-6 and chIL-8 cytokine transcript levels 24 h post-vaccination. High titers of FliC-specific antibodies 21 day post-hatch | [173] |
| Cobb 500 embryonated eggs | Probiotics | Inoculation with a 3 × 1011 CFU/mL suspension of Lactobacillus acidophilus, L. fermentum, and L. salivarius in the air cell of 18 d embryonated eggs. S. Enteritidis inoculation 2 day after hatching | No decrease (p > 0.05) in S. Enteritidis colonization of chick ceca. | [174] |
| Coob 500 broiler fertil eggs | Prebiotics | In ovo injection of Raffinose (1.5, 3.0, and 4.5 mg in 0.2 mL of aqueous diluents) into the air sac of 12 day embryonated eggs | Increase of the villus height, the villus height–crypt depth ratio (p < 0.05), and the expression levels of CD3 and chB6 | [175] |
| Ross 308 hatching eggs | Prebiotics and synbiotics | Administration, in the air chamber at 12 day of incubation, of inulin, Bi2tos, inulin, and Lactococcus lactis subs. lactis or Bi2tos and Lactococcus lactis subs. lactis | Modulation of central and peripheral lymphatic organ development (cortex/medulla ratio in the thymus, development of cortex in bursal follicles, and germinal center’s formation in the spleens), especially through the use of symbiotics | [176] |
| Broilers’ embryonated eggs | Prebiotic | Commercial egg injector system (InovojectTM) to apply a dextrin solution (18% maltodextrin, 10% potato extract dextrin) containing iodinated casein (80, 240, 720, or 2160 µg/)mL | Improvement in hatchability and early growth attributable to iodinated casein in combination with Dextrin. No differences in Salmonella colonization after chicks were challenged | [177] |
| Ross 308 Salmonella free hatching eggs | Probiotic | In ovo injection, into the air cell, at 18 day incubation with 0.1 mL of a commercial probiotic suspension (7 × 107 CFU/mL in PBS). After hatching, chicks were challenged against S. Enteritidis (Se) (8 log CFU) | Reduction in the number of Se colonized chicks since day 1 post hatching. Reduction of Se colonization in the alimentary tract of chicks | [178] |
| Fertile broiler eggs | Probiotic | Probiotic administration, at 18 day of incubation, of Marek’s vaccine + one of three Bacillus subtilis strains (ATCC 6051, ATCC 8473, ATCC 9466) | Results regarding hatching were strain-dependent; however, probiotic strains reduced the bacterial counts (total aerobes and coliforms) in the ileum and ceca | [172] |
| White Leghorn hens | Immune lymphokines | On day 18 of embryogenesis, eggs were injected into the amnion with Immune (ILK) and nonimmune (NILK) Lymphokines. Post-hatch, chicks were orally challenged against S. Enteritidis (5 × 104 CFU) | In vitro bactericidal activity was higher, and organ invasion with S. Enteritidis decreased in ILK-treated chicks. Hatchability was not affected, although ILK-treated chicks were 1 g lighter than NILK-treated ones (p < 0.05) | [179] |
| Broiler embryonated eggs | Probiotic | Injection, at 18 day of incubation, of an undefined and anaerobically grown competitive exclusion culture into the air cells or beneath the inner air cell membrane | Evident resistance to S. Typhimorium of chicks challenged at day 7 post hatch | [180] |
| Broiler embryonated eggs | Probiotic | A competitive exclusion culture consisting of several species of unrevealed bacteria injected either the air cell or body of the 18 day of incubation embryos | Injection in the body proper resulted in losing almost all the hatchability. Hatchability was reduced and mortality during the first week increased in air cell injected embryos. No effects on Salmonella infection were observed when chicks were challenged 1 day after hatching | [181] |
| Ross embryonated eggs | Probiotic | 18 d incubating eggs were inoculated using cecal microbiota (total or diluted) and Lactobacillus salivarius into the inner air sac | Maximum hatchability observed was 65%. 2-d chicks were challenged against S. Enteritidis. Liver and cecum colonization was not reduced in the in ovo inoculated chicks | [182] |
| 18-d White Leghorn 15I5 × 71 embryonated eggs | Probiotic | Eggs were inoculated after 18 d of incubation with a commercial probiotic (FloraMax®-B11) through injection into the amnion. After hatching, chickens were orally infected with S. Enteritidis | Probiotic administration did not affect hatchability but increased body weight during first 7 d, increased the villi surface area in the ileum and reduced the presence of lactose-positive Gram-negative bacteria, as well as reduced the incidence of S. Enteritidis | [183] |
| Broiler embryonated eggs | Antibiotic | Gentamicin was administered at 18 d of incubation into the amnion. At hatching, chicks were gavaged with a commercial Competitive Exclusion Culture (MSC®. 0.2ml, 1 × 108 UFC/)mL and challenged against S. Typhimurium | A cumulative effect was observed by the in ovo application of Gentamicin and the supplementation with the Competitive Exclusion Culture at hatching in ceca colonization with S. Typhimurium | [184] |
| Ross × Ross 708 fertile eggs | Probiotic | 18 d of incubation eggs were inoculated with an Enterococcus faecium-based commercial probiotic (Galli-Pro Hatch) at three concentrations | Hatchability was not affected, and live performance in the first 21 days were improved as well as yolk absorption and intestinal and spleen morphology | [185] |
| Ross 308 broiler embryonated eggs | Probiotic | Injection was performed at 17.5 d of incubation for the inoculation of two probiotic strains (Enterococcus faecium and Bacillus subtilis). Chicks were orally challenged against S. Enteritidis 4 days post hatching | Probiotic administration, at a dose up to 109 CFU/egg) not only reduces but also eliminates the presence of Salmonella in broilers | [186] |
| Cobb 500 fertile eggs | Probiotic | On day 18 embryonic day, eggs were injected into the air cell with a commercial probiotic (Primalac W/S. Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Bifidobacterium bifidum) using three different concentrations | Hatchability, feed intake, and feed conversion ratio were not affected by the probiotic administration. The expression of immune-related genes in the ileum and cecal tonsils were modulated | [187] |
| Broiler fertilized eggs from a commercial breeder | Immune response stimulation | 18 d old embryos were injected in the amnion with CpG oliodeoxynucleotides (CpG-ODN) and orally infected with S. Enteritidis at day 10 post-hatch | Colonization of S. Enteritidis in ceca was reduced greater than 10-fold in comparison to placebo birds. CpG-ODN stimulates innate immune responsiveness of birds heterophils | [188] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. https://doi.org/10.3390/ani12010102
Ruvalcaba-Gómez JM, Villagrán Z, Valdez-Alarcón JJ, Martínez-Núñez M, Gomez-Godínez LJ, Ruesga-Gutiérrez E, Anaya-Esparza LM, Arteaga-Garibay RI, Villarruel-López A. Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals. 2022; 12(1):102. https://doi.org/10.3390/ani12010102
Chicago/Turabian StyleRuvalcaba-Gómez, José Martín, Zuamí Villagrán, Juan José Valdez-Alarcón, Marcelino Martínez-Núñez, Lorena Jacqueline Gomez-Godínez, Edmundo Ruesga-Gutiérrez, Luis Miguel Anaya-Esparza, Ramón Ignacio Arteaga-Garibay, and Angélica Villarruel-López. 2022. "Non-Antibiotics Strategies to Control Salmonella Infection in Poultry" Animals 12, no. 1: 102. https://doi.org/10.3390/ani12010102
APA StyleRuvalcaba-Gómez, J. M., Villagrán, Z., Valdez-Alarcón, J. J., Martínez-Núñez, M., Gomez-Godínez, L. J., Ruesga-Gutiérrez, E., Anaya-Esparza, L. M., Arteaga-Garibay, R. I., & Villarruel-López, A. (2022). Non-Antibiotics Strategies to Control Salmonella Infection in Poultry. Animals, 12(1), 102. https://doi.org/10.3390/ani12010102