Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Feeding and Management
2.2. Oregano Essential Oil Organic Cobalt Treatment
2.3. In Vitro Fermentation
2.4. Sampling
2.5. DNA Extraction and High-Throughput Sequencing
2.6. Bioinformatic Analysis
2.7. Chemical Analysis
2.8. Statistical Analysis
3. Results
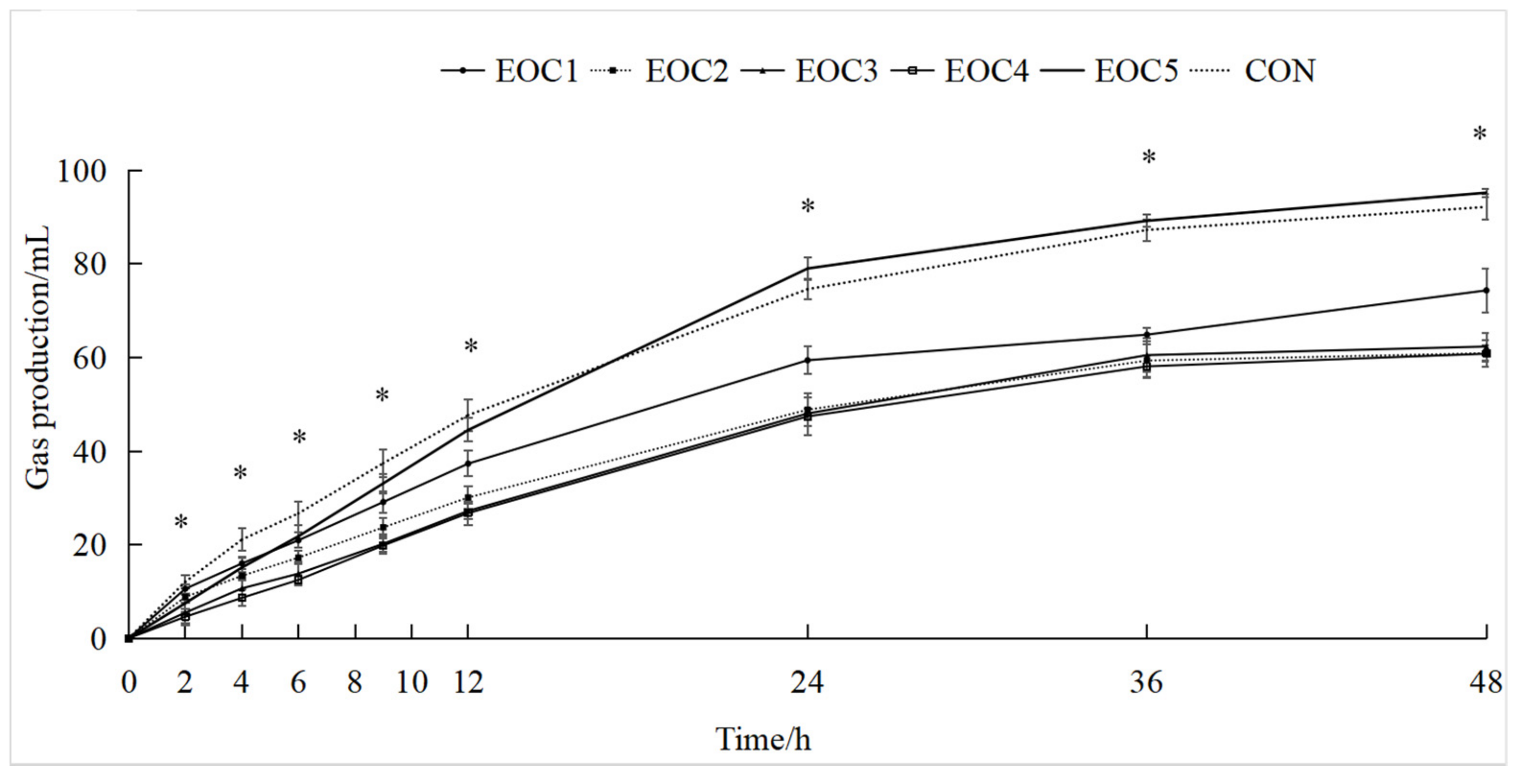
3.1. Effect of In Vitro Fermentation Total Gas Production by EOC
3.2. Changes in Feed Digestibility and Fermentation Parameters
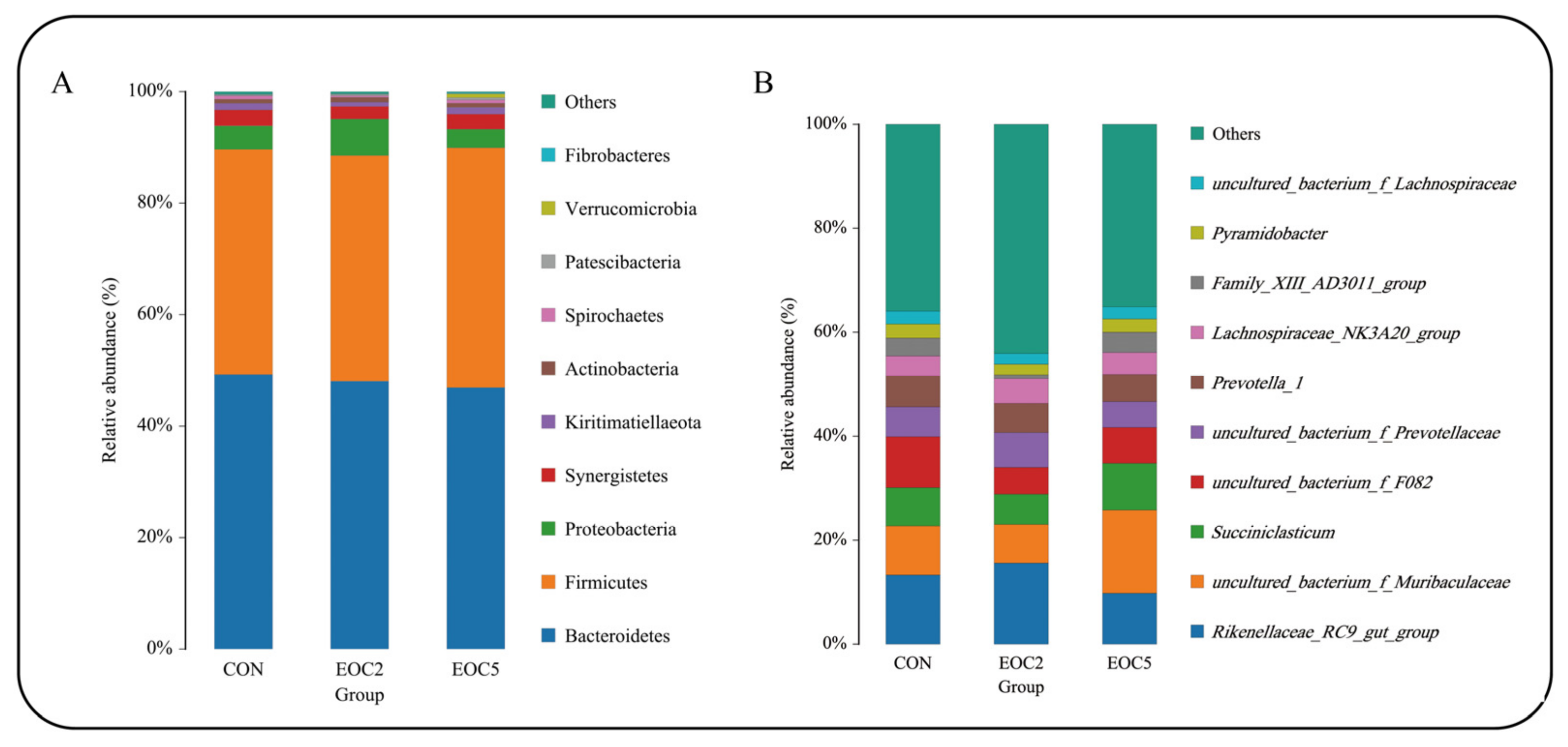
3.3. Alteration in the Composition of the Rumen Bacterial Community
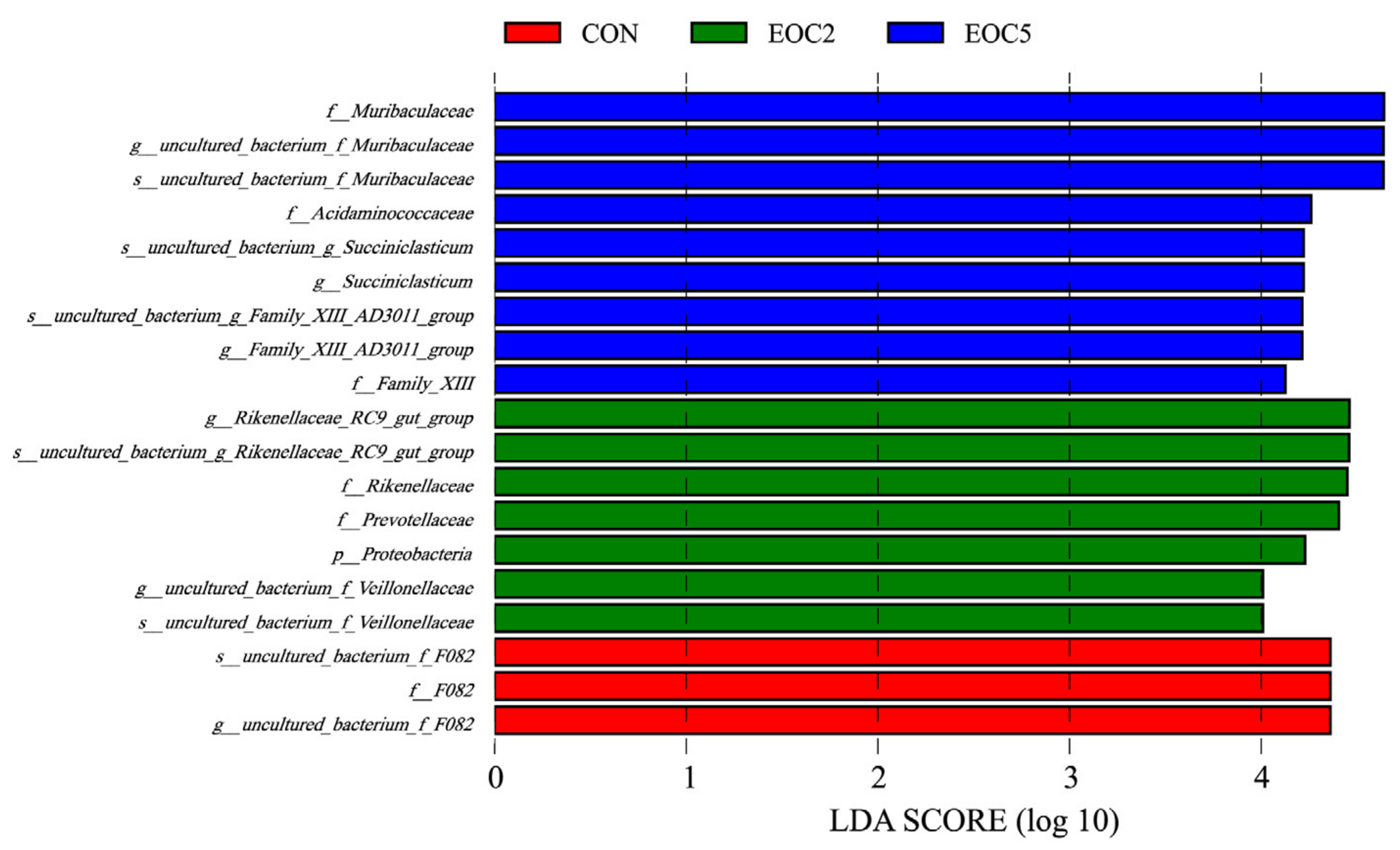
3.4. Alteration of Rumen Microbial Composition Caused from EOC
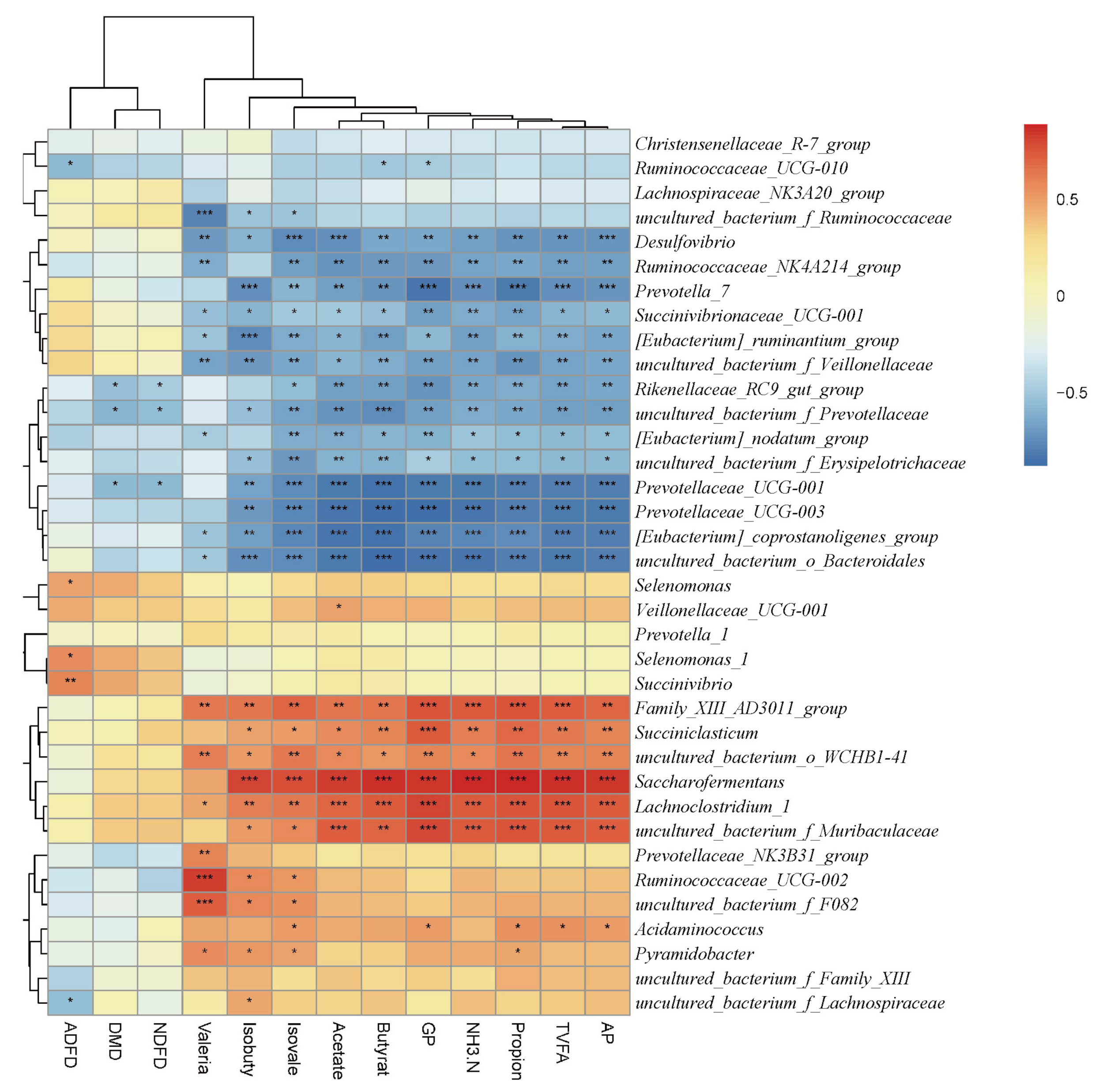
3.5. Interactions among In Vitro Digestibility, Fermentation Parameters and Rumen Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.; Cai, Y.; Zhu, H.; Tan, Z. Potential and constraints in the development of animal industries in China. J. Sci. Food Agric. 2012, 92, 1025–1030. [Google Scholar] [CrossRef]
- Agrawal, A.R.; Karim, S.A.; Kumar, R.; Sahoo, A.; John, P.J. Sheep and goat production: Basic differences, impact on climate and molecular tools for rumen microbiome study. Curr. Microbiol. 2014, 3, 2275–2284. [Google Scholar]
- Morgavi, D.P.; Kelly, W.J.; Janssen, P.H.; Attwood, G.T. Rumen microbial (meta)genomics and its application to ruminant production. Animal 2012, 7, 184–201. [Google Scholar] [CrossRef] [Green Version]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Simitzis, P.E. Enrichment of animal diets with essential oils—A great perspective on improving animal performance and quality characteristics of the derived products. Medicines 2017, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Valdivieso, U.M.; Gomez, L.C.; Plaza, D.J.; Gil, A. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Nagy, J.G.; Tengerdy, R.P. Antibacterial action of essential oils of artemisia as an ecological factor. Appl. Microbiol. 1967, 15, 819–821. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Lang, X.; Liu, L.; Casper, D.P.; Wang, C.; Zhang, L.; Wei, S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J. Dairy Sci. 2020, 103, 2303–2314. [Google Scholar] [CrossRef]
- Claudia, T.; Florian, C.S. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar]
- Lei, Z.; Zhang, K.; Li, C.; Wu, J.; Davis, D.; Casper, D.; Jiang, H.; Jiao, T.; Wang, X.; Wang, J. Dietary supplementation with essential-oils-cobalt for improving growth performance, meat quality and skin cell capacity of goats. Sci. Rep. 2018, 8, 11634. [Google Scholar] [CrossRef]
- Poudel, P. An Evaluation of Rumen Modifiers for Lactational Performance and Nutrient Digestibility by Cows. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 2016. [Google Scholar]
- Pretz, J.P. Improving Feed Efficiency through Forage Strategies for Increasing Dairy Profitability and Sustainability. Ph.D. Thesis, South Dakota State University, Brookings, SD, USA, 2016. [Google Scholar]
- Liu, T.; Chen, H.; Bai, Y.; Wu, J.; Cheng, S.; He, B.; Casper, D.P. Calf starter containing a blend of essential oils and prebiotics affects the growth performance of Holstein calves—ScienceDirect. J. Dairy Sci. 2020, 103, 2315–2323. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Deligeorgis, S.G.; Bizelis, J.A.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat. Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef]
- Hassan, F.U.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet-microbe interaction. Front. Vet. Sci. 2020, 7, 575801. [Google Scholar] [CrossRef]
- Jiao, T.; Wu, J.; Casper, D.P.; Davis, D.I.; Brown, M.A.; Zhao, S.; Liang, J.; Lei, Z.; Holloway, B. Feeding sheep cobalt and oregano essential oil alone or in combination on ruminal nutrient digestibility, fermentation, and fiber digestion combined with scanning electron microscopy. Front. Vet. Sci. 2021, 8, 639432. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 21th ed.; AOAC International: Rockville, MD, USA, 2019; Volume 2. [Google Scholar]
- Wang, Q.; Gong, J.; Huang, X.; Yu, H.; Xue, F. In vitro evaluation of the activity of microencapsulated carvacrol against Escherichia coli with K88 pili. J. Appl. Microbiol. 2009, 107, 1781–1788. [Google Scholar] [CrossRef]
- Zhou, Z.; Meng, Q.; Yu, Z. Effects of methanogenic inhibitors on methane production and abundances of methanogens and cellulolytic bacteria in in vitro ruminal cultures. Appl. Environ. Microbiol. 2011, 77, 2634–2639. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, F.M.; Williams, P.; Losa, R.; Wallace, R.J.; Beever, D.A.; Newbold, C.J. Effects of essential oils on ruminal microorganisms and their protein metabolism. Appl. Environ. Microbiol. 2003, 69, 5011–5014. [Google Scholar] [CrossRef] [Green Version]
- Macheboeuf, D.; Morgavi, D.; Papon, J. Dose–response effects of essential oils on in vitro fermentation activity of the rumen microbial population. Anim. Feed Sci. Technol. 2008, 145, 335–350. [Google Scholar] [CrossRef]
- Patra, A.; Yu, Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl. Environ. Microbiol. 2012, 78, 4271–4280. [Google Scholar] [CrossRef] [Green Version]
- Cobellis, G.; Trabalza-Marinucci, M.; Marcotullio, M.C.; Yu, Z. Evaluation of different essential oils in modulating methane and ammonia production, rumen fermentation, and rumen bacteria in vitro. Anim. Feed Sci. Technol. 2016, 215, 25–36. [Google Scholar] [CrossRef] [Green Version]
- González, J.; Escalera, F.; Alonso, A. Relationship between Vitamin B12 and Cobalt Metabolism in Domestic Ruminant: An Update. Animals 2020, 10, 1855. [Google Scholar] [CrossRef]
- Herdt, T.H.; Hoff, B. The Use of Blood Analysis to Evaluate Trace Mineral Status in Ruminant Livestock. Vet. Clin. N. Am.-Food A 2011, 27, 255–283. [Google Scholar] [CrossRef]
- Xue, H.F.; Meng, Q.X. Recent Nutritional Advances of Neutral Detergent Fiber in Dairy Cow. Chin. J. Anim. Nutr. 2007, 19, 454–458. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-DWYX2007S1007.htm (accessed on 21 December 2021).
- Cobellis, G.; Trabalza, M.; Yu, Z. Critical evaluation of essential oils as rumen modifiers in ruminant nutrition: A review. Sci. Total Environ. 2016, 3, 545–546. [Google Scholar] [CrossRef]
- Patra, A.K. Effects of Essential Oils on Rumen Fermentation, Microbial Ecology and Ruminant Production. Asian J. Anim. Vet. Adv. 2011, 6, 416–428. [Google Scholar] [CrossRef]
- Lopez, J.M.; Satter, L.D. Effect of copper and cobalt addition on digestion and growth in heifers fed diets containing alfalfa silage or corn crop residues. J. Dairy Sci. 1992, 75, 247–256. [Google Scholar] [CrossRef]
- Kumar, S.; Dagar, S.S.; Sirohi, S.K. Microbial profiles, in vitro gas production and dry matter digestibility based on various ratios of roughage to concentrate. Ann. Microbiol. 2013, 63, 541–545. [Google Scholar] [CrossRef]
- Khiaosa, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef]
- Poudel, P.; Froehlich, K.; Casper, D.P. Feeding Essential Oils to Neonatal Holstein Dairy Calves Results in Increased Ruminal Prevotellaceae Abundance and Propionate Concentrations. Microorganisms 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, J.; Wang, C. Effects of folic acid and cobalt sulphate supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Holstein calves. Br. J. Nutr. 2021, 21, 1–7. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [Green Version]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, M.J.; Andrade, S.; Coelho, M.A.N.; Loureiro, J.A.; Pereira, M.C. Molecular interactions between Vitamin B12 and membrane models: A biophysical study for new insights into the bioavailability of Vitamin. Colloids Surf. B Biointerfaces 2020, 194, 111187. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Kaakoush, N.O. Insights into the Role of Erysipelotrichaceae in the Human Host. Front. Cell. Infect. Microbiol. 2015, 5, 84. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.A.; Yang, C.; Zhang, J.; Kalwar, Q.; Liang, Z.; Li, C. Effects of Dietary Energy Levels on Rumen Fermentation, Microbial Diversity, and Feed Efficiency of Yaks (Bos grunniens). Front. Microbiol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Cai, B.; Pan, J.; Chen, H.; Chen, X.; Ye, Z.; Yuan, H.; Sun, H.; Wan, P. Oyster polysaccharides ameliorate intestinal mucositis and improve metabolism in 5-fluorouracil-treated S180 tumour-bearing mice. Carbohydr. Polym. 2021, 256, 117545. [Google Scholar] [CrossRef]
- Ormerod, K.L.; Wood, D.L.; Lachner, N.; Gellatly, S.L.; Daly, J.N.; Parsons, J.D. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 2016, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.; Marcondes, M.I.; Noronha, M.F.; Resende, R.T.; Machado, F.S.; Mantovani, H.C. Effect of Pre-weaning Diet on the Ruminal Archaeal, Bacterial, and Fungal Communities of Dairy Calves. Front. Microbiol. 2017, 8, 1553. [Google Scholar] [CrossRef]
- Shabat, S.K.; Sasson, G.; Doron, A.; Durman, T.; Yaacoby, S.; Berg, M.E.; White, B.A. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Tsedan, G.; Liu, Y.; Hou, F. Shrub coverage alters the rumen bacterial community of yaks (Bos grunniens) grazing in alpine meadows. J. Anim. Sci. Technol. 2020, 62, 504–520. [Google Scholar] [CrossRef]
- Benchaar, C.; Greathead, H. Essential oils and opportunities to mitigate enteric methane emissions from ruminants. Anim. Feed Sci. Technol. 2011, 166, 338–355. [Google Scholar] [CrossRef]
- Franco, J.; Duplessis, M.; Bui, A.; Reymond, C. Correlations between the Composition of the Bovine Microbiota and Vitamin B(12) Abundance. mSystems 2020, 5, e00107–e00120. [Google Scholar] [CrossRef] [Green Version]

| Ingredients | Content (%) | Nutrient | Content |
|---|---|---|---|
| Whole corn silage | 72.96 | Digestible energy, ME/(M J kg−1) | 11.58 |
| Corn | 14.41 | Digestible energy, DE/(M J kg−1) | 14.12 |
| Wheat bran | 4.00 | Dry matter, DM (%) | 36.23 |
| Cottonseed meal | 3.86 | Crude Protein, CP (%) | 14.73 |
| Rapeseed meal | 3.59 | Calcium, Ca (%) | 0.76 |
| Calcium Hydrogen Phosohate | 0.31 | Phosphorus P (%) | 0.65 |
| Limestone | 0.31 | Ether extract, EE (%) | 2.50 |
| Salt | 0.27 | Neutral detergent fiber, NDF (%) | 35.81 |
| 1% Premix 1 | 0.29 | Acid detergent fiber, ADF (%) | 20.01 |
| Total | 100 |
| Items | EOC1 | EOC2 | EOC3 | EOC4 | EOC5 | CON | SEM | p-Valve |
|---|---|---|---|---|---|---|---|---|
| GP24 h 1 (mL) | 59.4 c | 48.83 d | 48.03 d | 47.4 d | 79.00 a | 74.58 b | 2.79 | <0.001 |
| IVDMD 2 (%) | 67.99 | 68.02 | 67.98 | 68.02 | 69.44 | 67.3 | 1.14 | 0.062 |
| IVNDFD 3 (%) | 59.27 | 58.8 | 57.98 | 59.68 | 60.79 | 58.4 | 2.06 | 0.21 |
| IVADFD 4 (%) | 35.76 | 34.94 | 33.07 | 35.49 | 34.5 | 32.89 | 3.57 | 0.654 |
| Items | EOC1 | EOC2 | EOC3 | EOC4 | EOC5 | CON | SEM | p-Valve |
|---|---|---|---|---|---|---|---|---|
| NH3-N (mg dL−1) | 27.42 b | 24.76 c | 27.33 b | 27.32 b | 28.84 a | 27.04 b | 1.53 | <0.001 |
| TVFA (mmol l−1) 1 | 78.44 d | 68.89 e | 84.63 c | 77.01 d | 104.56 a | 95.55 b | 3.78 | <0.001 |
| MCP (mg mL−1) 2 | 3.48 b | 2.16 c | 3.59 b | 3.43 b | 3.91 a | 3.58 b | 0.16 | <0.001 |
| pH | 6.41 | 6.42 | 6.38 | 6.45 | 6.30 | 6.35 | 0.17 | 0.313 |
| Acetate (% 3) | 46.1 c | 46.39 c | 47.77 b | 48.02 b | 48.53 b | 50.97 a | 0.81 | <0.001 |
| Propionate (%) | 31.89 b | 34.01 a | 33.75 a | 32.46 b | 29.76 c | 28.75 d | 0.68 | <0.001 |
| Isobutyric acid (%) | 1.98 | 1.88 | 1.89 | 1.84 | 1.99 | 1.96 | 0.15 | 0.008 |
| Butyrate (%) | 11.66 b | 10.88 c | 10.88 c | 11.69 b | 12.65 a | 10.59 c | 0.52 | <0.001 |
| Isovaleric acid (%) | 3.84 a | 3.26 b | 3.03 b | 3.2 b | 4.04 a | 3.84 a | 0.28 | <0.001 |
| Valerianic acid (%) | 4.53 a | 3.69 b | 3.48 b | 3.48 b | 3.51 b | 3.53 b | 0.41 | <0.001 |
| A/P 4 | 1.45 cd | 1.37 e | 1.42 de | 1.48 c | 1.63 b | 1.77 a | 0.05 | <0.001 |
| Index Type | EOC2 | EOC5 | CON | SEM | p-Valve |
|---|---|---|---|---|---|
| Shannon | 6.83 | 6.93 | 6.77 | 0.134 | 0.163 |
| Simpson | 0.978 | 0.979 | 0.975 | 0.003 | 0.17 |
| ACE | 558.09 | 575.31 | 573.13 | 14.01 | 0.057 |
| Chao1 | 564.1 | 577.34 | 576.17 | 11.26 | 0.112 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, X.; Zhang, L.; Wu, J.; Zhao, S.; Jiao, T. Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community. Animals 2022, 12, 118. https://doi.org/10.3390/ani12010118
Wang Z, Li X, Zhang L, Wu J, Zhao S, Jiao T. Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community. Animals. 2022; 12(1):118. https://doi.org/10.3390/ani12010118
Chicago/Turabian StyleWang, Zhengwen, Xiongxiong Li, Lingyun Zhang, Jianping Wu, Shengguo Zhao, and Ting Jiao. 2022. "Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community" Animals 12, no. 1: 118. https://doi.org/10.3390/ani12010118






