Two Different Copy Number Variations of the CLCN2 Gene in Chinese Cattle and Their Association with Growth Traits
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Growth Traits Measurements
2.2. Preparation of Sample and Genomic DNA
2.3. Candidate Gene Identification and Primer Design
2.4. Detection of the CLCN2 Gene Copy Number
2.5. Statistical Analysis
3. Results
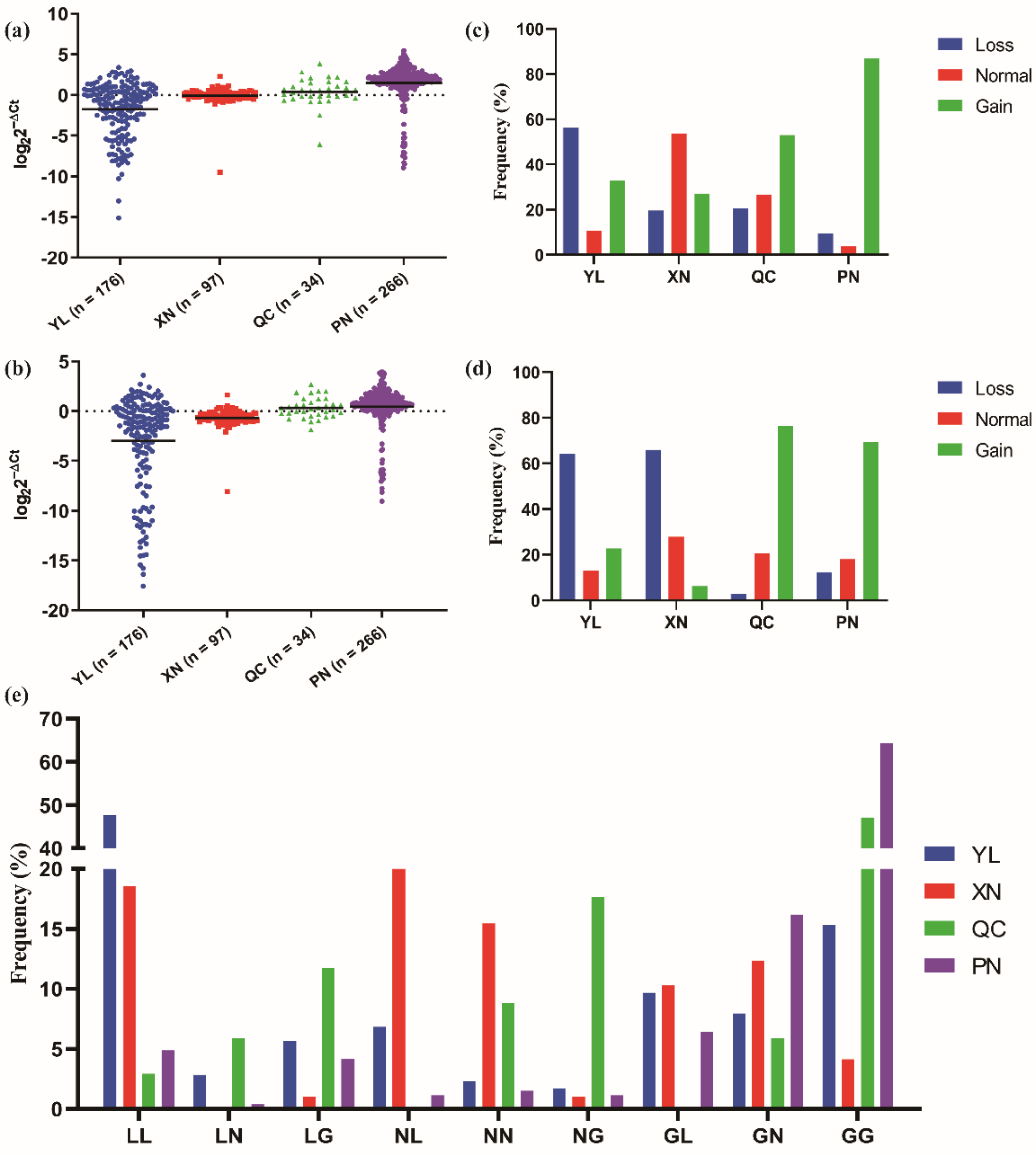
3.1. Distribution of CNVs of CLCN2 Gene in the Experimental Sample Group
3.2. Association Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.Z.; Yan, C.G.; Zan, L.S. Current situation and future prospects for beef production in China—A review. Asian-Australas J. Anim. Sci. 2018, 31, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Margawati, E. A Global Strategy of Using Molecular Genetic Information to Improve Genetics in Livestock. Reprod. Domest. Anim. 2012, 47, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Williams, J. The use of marker-assisted selection in animal breeding and biotechnology. Rev. Sci. Tech. (Int. Off. Epizoot.) 2005, 24, 379–391. [Google Scholar] [CrossRef]
- Mills, R.E.; Walter, K.; Stewart, C.; Handsaker, R.E.; Korbel, J.O. Mapping Copy Number Variation by Population-Scale Genome Sequencing. Nature 2011, 470, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.M.; Giachetto, P.F.; Da Silva, L.O.; Cintra, L.C.; Paiva, S.R.; Yamagishi, M.E.B.; Caetano, A.R. Genome-wide copy number variation (CNV) detection in Nelore cattle reveals highly frequent variants in genome regions harboring QTLs affecting production traits. BMC Genom. 2016, 17, 454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Xu, L.; Zhu, B.; Niu, H.; Zhang, W.; Miao, J.; Shi, X.; Zhang, M.; Chen, Y.; Zhang, L. Genome-wide analysis reveals differential selection involved with copy number variation in diverse Chinese Cattle. Sci. Rep. 2017, 7, 14299. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, A.; Gründer, S.; Pusch, M.; Jentsch, T.J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 1992, 356, 57. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Ritter, M.; Gamper, N.; Huber, S.; Fillon, S.; Tanneur, V.; Lepple-Wienhues, A.; Szabo, I.; Bulbins, E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell. Physiol. Biochem. 2000, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Maeno, E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 377–383. [Google Scholar] [CrossRef]
- Guan, Y.-Y.; Wang, G.-L.; Zhou, J.-G. The ClC-3 Cl− channel in cell volume regulation, proliferation and apoptosis in vascular smooth muscle cells. Trends Pharmacol. Sci. 2006, 27, 290–296. [Google Scholar] [CrossRef]
- Voets, T.; Szücs, G.; Droogmans, G.; Nilius, B. Blockers of volume-activated Cl currents inhibit endothelial cell proliferation. Pflügers Arch. Eur. J. Physiol. 1995, 431, 132–134. [Google Scholar] [CrossRef]
- Xiao, G.N.; Guan, Y.Y.; He, H. Effects of Cl− channel blockers on endothelin-1-induced proliferation of rat vascular smooth muscle cells. Life Sci. 2002, 70, 2233–2241. [Google Scholar] [CrossRef]
- Rouzaire-Dubois, B.; Milandri, J.B.; Bostel, S.; Dubois, J.M. Control of cell proliferation by cell volume alterations in rat C6 glioma cells. Pflügers Arch. Eur. J. Physiol. 2000, 440, 881–888. [Google Scholar] [CrossRef]
- O’Loughlin, E.V. Interleukin 2 modulates ion secretion and cell proliferation in cultured human small intestinal enterocytes. Gut 2001, 49, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Hattori, N.; Liu, B.; Nakayama, Y.; Kitagawa, K.; Inagaki, C. Suppression of cell proliferation with induction of p21 by Cl− channel blockers in human leukemic cells. Eur. J. Pharmacol. 2004, 488, 27–34. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Wang, J.; Li, Y.; Li, F.; Zhang, Y.; Zheng, X.; Shen, Y.; Wang, Y.; Zhou, L. CLC-2 is a positive modulator of oligodendrocyte precursor cell differentiation and myelination. Mol. Med. Rep. 2018, 17, 4515–4523. [Google Scholar] [CrossRef]
- Gang, C.; Kim, M.J.; Jia, G.; Agrawal, D.K. Involvement of Chloride Channels in IGF-I -induced Proliferation of Porcine Arterial Smooth Muscle Cells. Cardiovasc. Res. 2007, 73, 198–207. [Google Scholar]
- Sun, L.; Dong, Y.; Jing, Z.; Yin, Y.; Zheng, Y. The CLC-2 Chloride Channel Modulates ECM Synthesis, Differentiation, and Migration of Human Conjunctival Fibroblasts via the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2016, 17, 910. [Google Scholar] [CrossRef] [Green Version]
- Rapetti-Mauss, R.; Berenguier, C.; Allegrini, B.; Soriani, O. Interplay Between Ion Channels and the Wnt/β-Catenin Signaling Pathway in Cancers. Front. Pharmacol. 2020, 11, 525020. [Google Scholar] [CrossRef]
- Cid, L.P.; Montrose-Rafizadeh, C.; Smith, D.I.; Guggino, W.B.; Cutting, G.R. Cloning of a putative human voltage-gated chloride channel (CIC-2) cDNA widely expressed in human tissues. Hum. Mol. Genet. 1995, 4, 407–413. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef] [PubMed]
- Kchl, S.; Niedersttter, H.; Parson, W. DNA Extraction and Quantitation of Forensic Samples Using the Phenol–Chloroform Method and Real-Time PCR. Methods Mol. Biol. 2005, 297, 13–30. [Google Scholar]
- Yang, M.; Lv, J.; Zhang, L.; Li, M.; Hong, C. Association study and expression analysis of CYP4A11 gene copy number variation in Chinese cattle. Sci. Rep. 2017, 7, 46599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Xu, L.; Zhou, Y.; Liu, M.; Wang, L.; Kijas, J.W.; Zhang, H.; Li, L.; Liu, G.E. Diversity of copy number variation in a worldwide population of sheep. Genomics 2017, 110, 143–148. [Google Scholar] [CrossRef]
- Redon, R.; Ishikawa, S.; Fitch, K.R.; Feuk, L.; Perry, G.H.; Andrews, T.D.; Fiegler, H.; Shapero, M.H.; Carson, A.R.; Chen, W.; et al. Global variation in copy number in the human genome. Nature 2006, 444, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Feuk, L.; Carson, A.R.; Scherer, S.W. Structural variation in the human genome. Nat. Rev. Genet. 2006, 7, 85–97. [Google Scholar] [CrossRef]
- Griffin, D.K.; Robertson, L.B.; Tempest, H.G.; Vignal, A.; Fillon, V.; Crooijmans, R.P.M.A.; Groenen, M.A.M.; Deryusheva, S.; Gaginskaya, E.; Carré, W.; et al. Whole genome comparative studies between chicken and turkey and their implications for avian genome evolution. BMC Genom. 2008, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Ramayo-Caldas, Y.; Castelló, A.; Pena, R.N.; Alves, E.; Mercadé, A.; Souza, C.A.; Fernández, A.I.; Perez-Enciso, M.; Folch, J.M. Copy number variation in the porcine genome inferred from a 60 k SNP BeadChip. BMC Genom. 2010, 11, 593. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Liu, S.; Meng, Q. Genome-Wide Assessment Characteristics of Genes Overlapping Copy Number Variation Regions in Duroc Purebred Population. Front. Genet. 2021, 12. [Google Scholar] [CrossRef]
- Liu, G.E.; Hou, Y.; Zhu, B.; Cardone, M.F.; Jiang, L.; Cellamare, A.; Mitra, A.; Alexander, L.J.; Coutinho, L.L.; Dell’Aquila, M.E.; et al. Analysis of copy number variations among diverse cattle breeds. Genome Res. 2010, 20, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Mei, C.; Junjvlieke, Z.; Raza, S.H.A.; Wang, H.; Cheng, G.; Zhao, C.; Zhu, W.; Zan, L. Copy number variation detection in Chinese indigenous cattle by whole genome sequencing. Genomics 2020, 112, 831–836. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Xu, L.; Ren, H.; Lu, J.; Zhang, X.; Zhang, S.; Zhou, X.; Wei, C.; Zhao, F.; et al. Analysis of copy number variations in the sheep genome using 50K SNP BeadChip array. BMC Genom. 2013, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Lupski, J.R.; Stankiewicz, P. Genomic disorders: Molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005, 1, e49. [Google Scholar] [CrossRef] [Green Version]
- Strillacci, M.G.; Gorla, E.; Cozzi, M.C.; Vevey, M.; Bagnato, A. A Copy Number Variant (CNV) scan in the autochthonous Italian Valdostana Red Pied cattle and comparison with specialized dairy populations. PLoS ONE 2018, 13, e0204669. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Bosse, M.; Mullaart, E.; Groenen, M.A.M.; Veerkamp, R.F.; Bouwman, A.C. Functional and population genetic features of copy number variations in two dairy cattle populations. BMC Genom. 2020, 21, 89. [Google Scholar] [CrossRef] [Green Version]
- Jentsch, T.J.; Poët, M.; Fuhrmann, J.C.; Zdebik, A.A. PHYSIOLOGICAL FUNCTIONS OF CLC Cl−CHANNELS GLEANED FROM HUMAN GENETIC DISEASE AND MOUSE MODELS. Annu. Rev. Physiol. 2005, 67, 779–807. [Google Scholar] [CrossRef] [Green Version]
- Bösl, M.R.; Stein, V.; Hübner, C.; Zdebik, A.A.; Jordt, S.-E.; Mukhopadhyay, A.K.; Davidoff, M.S.; Holstein, A.-F.; Jentsch, T.J. Male germ cells and photoreceptors, both dependent on close cell–cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J. 2001, 20, 1289–1299. [Google Scholar] [CrossRef] [Green Version]
- Lang, F.; Föller, M.; Lang, K.; Lang, P.; Ritter, M.; Vereninov, A.; Szabo, I.; Huber, S.M.; Gulbins, E. Chapter Eleven—Cell Volume Regulatory Ion Channels in Cell Proliferation and Cell Death. In Methods in Enzymology; Häussinger, D., Sies, H., Eds.; Academic Press: Cambridge, MA, USA, 2007; Volume 428, pp. 209–225. [Google Scholar]
- Depienne, C.; Bugiani, M.; Dupuits, C.; Galanaud, D.; Touitou, V.; Postma, N.; Van Berkel, C.; Polder, E.; Tollard, E.; Darios, F.; et al. Brain white matter oedema due to ClC-2 chloride channel deficiency: An observational analytical study. Lancet Neurol. 2013, 12, 659–668. [Google Scholar] [CrossRef]
- Hanagasi, H.A.; Bilgiç, B.; Abbink, T.E.M.; Hanagasi, F.; Tüfekçioğlu, Z.; Gürvit, H.; Başak, N.; Van der Knaap, M.S.; Emre, M. Secondary paroxysmal kinesigenic dyskinesia associated with CLCN2 gene mutation. Parkinsonism Relat. Disord. 2015, 21, 544–546. [Google Scholar] [CrossRef]
- Saint-Martin, C.; Gauvain, G.; Teodorescu, G.; Gourfinkel-An, I.; Fedirko, E.; Weber, Y.G.; Maljevic, S.; Ernst, J.-P.; Garcia-Olivares, J.; Fahlke, C.; et al. Two novel CLCN2 mutations accelerating chloride channel deactivation are associated with idiopathic generalized epilepsy. Hum. Mutat. 2009, 30, 397–405. [Google Scholar] [CrossRef]
- Sartelet, A.; Stauber, T.; Coppieters, W.; Ludwig, C.F.; Fasquelle, C.; Druet, T.; Zhang, Z.; Ahariz, N.; Cambisano, N.; Jentsch, T.J.; et al. A missense mutation accelerating the gating of the lysosomal Cl−/H+-exchanger ClC-7/Ostm1 causes osteopetrosis with gingival hamartomas in cattle. Dis. Models Mech. 2014, 7, 119–128. [Google Scholar] [CrossRef] [Green Version]
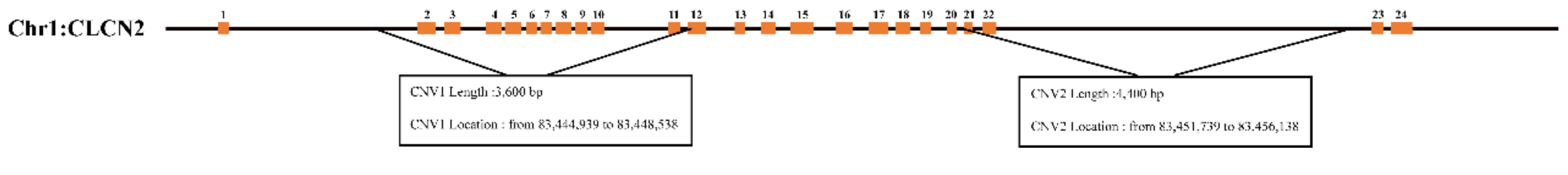

| Genes | Sequences (5′-3′) | Amplification Length |
|---|---|---|
| CLCN2-CNV1 | Forward primer: TTCAGCGCCTTCATCTTCCG | 104 bp |
| Reverse primer: GCCCCACCTCATCTGAAACAT | ||
| CLCN2-CNV2 | Forward primer: TGGGGAGTCTGGGGTCTAAC | 120 bp |
| Reverse primer: TCCTCACCAGGATAGGGCTG | ||
| BTF3 | Forward primer: AACCAGGAGAAACTCGCCAA | 120 bp |
| Reverse primer: TTCGGTGAAATGCCCTCTCG |
| Breed | Growth Traits | CNV Types (Mean ± SE) | p-Value | ||
|---|---|---|---|---|---|
| Loss (n = 99) | Normal (n = 19) | Gain (n = 58) | |||
| Yunling cattle | cannon circumference (cm) | 18.88 ± 0.140 A | 18.47 ± 0.269 AB | 17.74 ± 0.360 B | 0.002 ** |
| Breed | Growth Traits | CNV types (Mean ± SE) | p-Value | ||
|---|---|---|---|---|---|
| Loss (n = 64) | Normal (n = 27) | Gain (n = 6) | |||
| Xianan cattle | body slanting length (cm) | 158.09 ± 0.707 a | 161.59 ± 1.591 b | 155.50 ± 2.717 ac | 0.031 * |
| chest girth (cm) | 191.61 ± 1.019 a | 196.67 ± 1.768 b | 189.83 ± 3.156 ab | 0.025 * | |
| body weight (kg) | 543.19 ± 6.694 a | 574.48 ± 13.627 b | 523.00 ± 11.897 ab | 0.034 * | |
| Breed | Growth Traits | CNV types (Mean ± SE) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LL (84) | LN (5) | LG (10) | NL (12) | NN (4) | NG (3) | GL (17) | GN (14) | GG (27) | |||
| Yunling cattle | cannon circumference (cm) | 18.84 ± 0.147 Aab | 19.80 ± 0.917 Aab | 18.80 ± 0.442 Aab | 18.33 ± 0.284 ABa | 18.75 ± 0.946 ABa | 18.67 ± 0.667 ABab | 18.06 ± 0.369 ABa | 16.50 ± 1.300 Bb | 18.19 ± 0.283 ABa | 0.006 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Shen, X.; Yang, Y.; Yang, H.; Qi, A.; Yang, S.; Qu, K.; Lan, X.; Huang, B.; Chen, H. Two Different Copy Number Variations of the CLCN2 Gene in Chinese Cattle and Their Association with Growth Traits. Animals 2022, 12, 41. https://doi.org/10.3390/ani12010041
Tang J, Shen X, Yang Y, Yang H, Qi A, Yang S, Qu K, Lan X, Huang B, Chen H. Two Different Copy Number Variations of the CLCN2 Gene in Chinese Cattle and Their Association with Growth Traits. Animals. 2022; 12(1):41. https://doi.org/10.3390/ani12010041
Chicago/Turabian StyleTang, Jia, Xuemei Shen, Yu Yang, Haiyan Yang, Ao Qi, Shuling Yang, Kaixing Qu, Xianyong Lan, Bizhi Huang, and Hong Chen. 2022. "Two Different Copy Number Variations of the CLCN2 Gene in Chinese Cattle and Their Association with Growth Traits" Animals 12, no. 1: 41. https://doi.org/10.3390/ani12010041





