1. Introduction
Adequate nutrition is essential for human health and normal development (World Health Organization,
https://www.who.int/news-room/facts-in-pictures/detail/nutrition, accessed on 20 October 2021). Turkey meat (the second most widely consumed poultry meat in the world) is characterized by high-protein (up to 28%), low-fat (2% to 5%), and low-cholesterol content, which makes it an important component of a balanced diet [
1]. Turkey meat is also a good source of vitamins and minerals, especially B complex vitamins, which prevent anemia and maintain the normal functioning of the cardiovascular and nervous system [
2]. In recent years, turkey production and meat processing have turned into a fast-growing branch of poultry industry [
3]. Rising meat consumption and customers’ expectations are drawing attention to the issue of meat quality [
4]. Across developed countries, poultry producers and researchers try to increase the quality of meat by replacing intensive farming systems with organic farming, placing even greater emphasis on animal well-being [
5]. According to the Regulations of the European Community (EC) Commission (no. 889/2008), organic poultry production is defined as the production system that prevents poultry from being reared too quickly; therefore, it mainly utilizes slow-growing poultry strains. In organic poultry farming, animal housing conditions must comply with a high level of standards for animal welfare (e.g., permanent access to open air areas). In addition, animals should only feed on feedstuff produced in accordance with the rules of organic farming. Production systems can impinge on meat quality [
6]. Prolonged rearing periods positively affect the chemical composition of breast and leg muscles in poultry, which results in the more desirable aroma and taste of the meat (i.e., better sensory attributes; [
7]). Meat from poultry raised in pasture-based or pasture-enriched rearing systems is characterized by higher protein content and low-fat content (both features preferred by consumers) compared with that obtained after intensive rearing [
7,
8]. Meat from organic chickens has lower pH and water holding capacity, higher shear values, iron content, n-3 polyunsaturated fatty acids and oxidative status, as well as improved color, giving it better overall sensory quality [
8,
9]. Komprda et al. [
10] showed that the content of omega-3 fatty acids in turkey meat can vary widely depending on diet and farming system; the most favorable ratio of omega-6 to omega-3 fats was found in the skinned breast from organic, pasture-raised turkeys [
11].
Food production processes include a series of activities and technological operations that require constant control and supervision to ascertain their appropriate progression [
12]. One of the challenges facing the contemporary meat industry is to obtain reliable information on meat quality throughout the entire production cycle, which would ultimately provide a guaranteed quality of final products to consumers. To meet this challenge, a fast, accurate and non-invasive technique for determining the physical properties and chemical composition of skeletal muscles in live animals is urgently needed.
Ultrasonography is a non-invasive, inexpensive, widely available, painless (non-sedation-requiring), and real-time imaging and diagnostic technique [
13]. Sound waves and their reflections are the basis for displaying ultrasound images [
13]. The proportions of reflected and non-reflected waves determine the appearance of ultrasound images composed of numerous pixels ranging in their intensity from 0 (absolute black) to 255 (absolute white; [
14]). Ultrasound wave propagation in skeletal muscles depends not only on their composition but also on the structural organization (e.g., orientation of muscle fibers, distribution of connective tissue). However, significant changes in chemical composition may profoundly alter tissue organization and generate multiple acoustic impedance sites that increase backscatter of the echoes (ultrasound waves; [
15]). Combining the ultrasound technique with computer-assisted image analysis allows us to extract information from ultrasound images such as the first order textural attributes (mean pixel intensity or numerical values of image brightness elements, and pixel heterogeneity or standard deviation of numerical pixel values; [
16,
17]). A few experimental and clinical studies have reported a link between echogenicity and proximate chemical composition of various tissues (e.g., human dystrophic muscles [
18]; ram testes [
19]; and chicken pectoralis major muscle [
20]). Image-processing analysis of ultrasonograms could potentially be used to predict intramuscular fat content in live beef cattle; however, the accuracy of this method appears to decline with the increasing fat content [
21,
22,
23]. A personal computer-based image analysis software called USOFT was developed to track the changes in the intramuscular fat content of cattle in the range of 2 to 8% [
24], but outside of this range, the software was not able to provide any predictions. An attempt to use real-time ultrasound to predict intramuscular fat content was also made in swine [
25] and lambs [
26]. Results of those earlier studies showed that computerized analysis of muscle ultrasonograms was a promising tool to predict intramuscular fat percentage in live animals. In the poultry industry, it is possible to estimate carcass cut weights [
27] and overall meat content [
28,
29,
30] using in situ ultrasonographic measurements of birds’ skeletal muscles. However, the most advantageous application of ultrasonography would be ability to determine present and future (post-slaughter) physicochemical properties of skeletal muscles. In the most recent study in broiler chickens, Schwarz et al. [
20] reported a strong relationship between echotextural characteristics of pectoral muscles and several important meat quality traits such as cutting force, hardness, and chewiness as well as crude fat content. Similar studies do not exist for other poultry species.
Therefore, the primary aim of this study was to examine the echotexture of pectoral muscles (
M. pectoralis superficialis) for quantitative relationships with their physicochemical and sensory properties in organic turkeys receiving three different types of diet varying in the proportion of cereal (complete feed only or complete feed supplemented with wheat and oats). Wheat has long been considered a primary choice in poultry nutrition, although a proportion of course grains has usually been added to the rations containing wheat [
31]. Oats vary considerably in feeding value, due to differences in hull, but several studies and trials have shown that oats are excellent grain for young growing chicks, laying hens and turkeys [
32].
4. Discussion
Replacing a proportion of complete feed mix with wheat and oats resulted in a proportional reduction in body mass, carcass and pectoral muscle weight, and muscle fiber diameter, albeit the latter was statistically significant only between Groups C and Exp2 of 17-week-old turkeys. These changes were accompanied by a decline in muscle moisture in Group Exp1, but by a significant rise in the moisture content in Group Exp2 birds. The plane of nutrition had no apparent effect on intramuscular fat or protein content of pectoral muscles in the organic turkeys studied. Skeletal muscles of vertebrates are characterized by a specific profile of muscle fibers determining their biomechanical function [
38]. The number, size and composition of muscle fibers are genetically determined, but they can be changed in response to various extrinsic factors including nutrition [
39]. It appears that the addition of large proportions of wheat and oats to daily feed rations had a positive impact on muscle fiber size without altering the crude fat and protein composition of the entire pectoralis major muscle of turkeys. The mechanism of this tropic effects of cereal grains remains to be determined.
Only four of fifteen physical variables analyzed (
Table 5) were affected by the change in turkey nutrition, namely pH
0h, L*
0h, a*
0h and C*
24h. pH values immediately after slaughter were lower in both treatment groups compared with the control, whereas muscle coloration varied significantly among all three groups of organic turkeys studied, with L*0h/a*0 h and C*24 h being lowest/highest in Groups Exp1 and highest/lowest in Group C. pH has a high influence on water holding capacity (WHC) and the color of meat in different animal species. Low meat pH is often associated with low WHC and pale meat color, whereas high meat pH often causes a dark meat color [
40]. These observations are consistent with our present observations on meat coloration immediately after slaughter, but not with the moisture content of the pectoralis major muscles or their color 24 h post-mortem.
Changes in sensory characteristics of meat samples recorded in the present study were remarkably consistent. Apart from meat tenderness and flavor (intensity) that did not vary among the three groups of birds, and flavor (desirability), which only differed between Groups C and Exp2, all other attributes were greater for both experimental groups compared with a control subset of organic turkeys. Meat flavor can be affected by many factors including, but not limited to, feed used, aging after slaughter and/or cooking method [
41]. Clearly, an increased proportion of cereal in organic turkeys’ nutrition had a positive impact on meat characteristics assessed by the expert sensory panel.
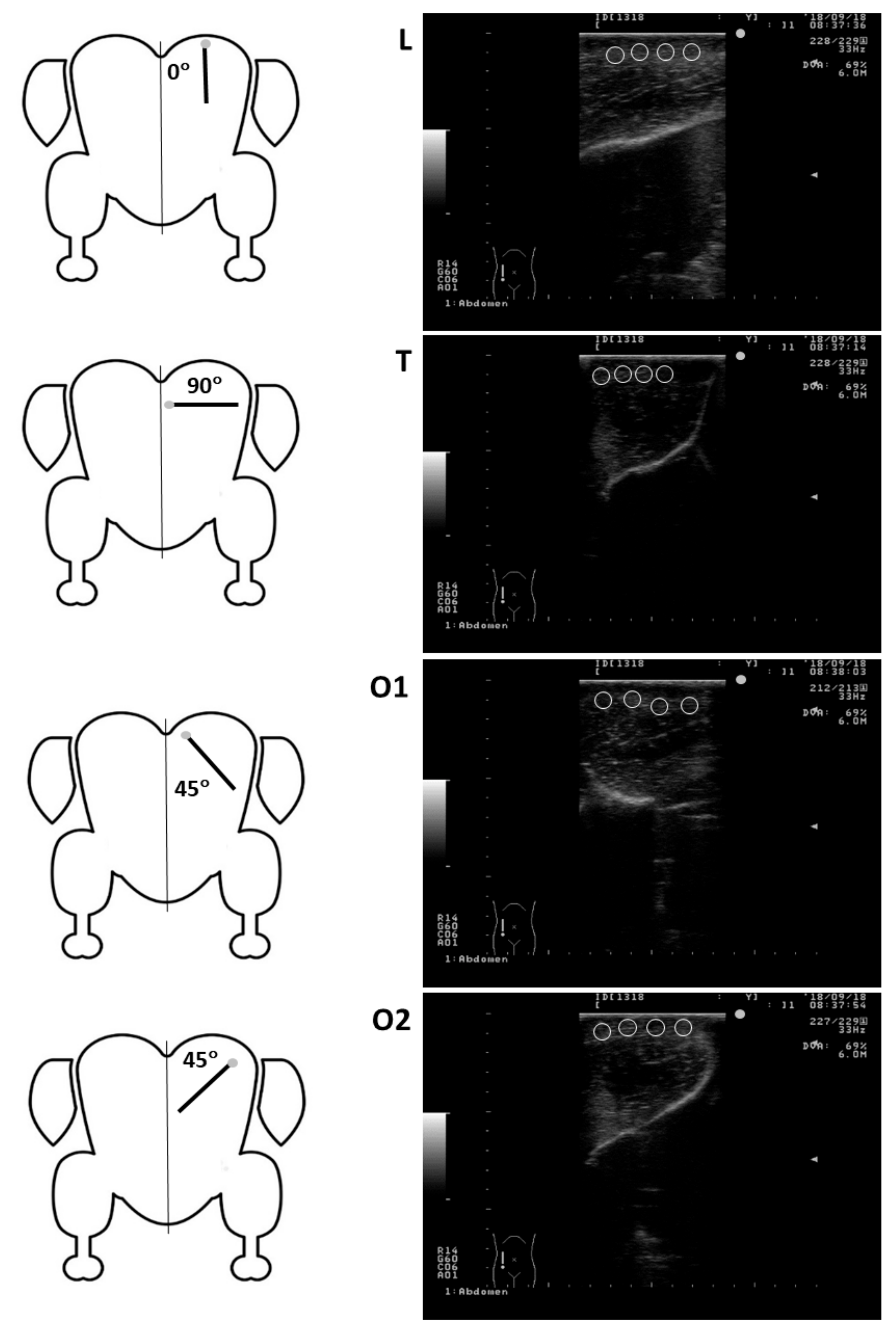
Mean pixel intensity and heterogeneity of the pectoralis major muscle in turkeys of the present study was generally greater for images obtained in L and O2 compared with T and O1 planes. The pectoralis muscle attaches to the humerus of the wing at the deltopectoral crest, its main portion (
sternobrachialis) originates from an enlarged sternal keel, with more anterior fibers arising from the furcular, and a much smaller portion (
thoracobrachialis) originates dorsally from ribs [
42,
43]. Therefore, the course of the fibres of the pectoralis major muscle was largely perpendicular to the probe held in L or O2 position and more parallel when the probe was held in T and O1 planes (
Figure 2). Due to reflections of ultrasound waves by the perimysial connective tissue of the fibers, which is moderately echogenic, the muscle has a “linear” and less echogenic appearance in the longitudinal plane (parallel to the muscle fibers) and “speckled” or more echogenic appearance in the transverse plane (perpendicular to muscle fibers; [
14]). Interestingly, the only MPI and MPH values that differed among the three groups of birds were obtained in O2 and O1 scanning planes. All echotextural characteristics of the pectoralis major muscles in the turkeys of the present study were numerically higher in Group C compared with both Exp groups, and the differences for O1-MPI and O2-MPI were statistically significant. The reason for this is difficult to explain. Although O2-MPI and O2-MPH values were consistently highest among the four scanning planes, O1-MPI and O1-MPH showed either intermediate or lowest values. In healthy humans, an increase in skeletal muscle echointensity usually results from loss in muscle mass (quantitative change), declining number and size of muscle fibers [
44] and/or age-related accumulation of fat and fibrous tissue (qualitative change; [
45]). The diameter of muscle fibers is higher is animals fed high-protein feed compared with those receiving low-protein diets, while the concurrent change in density of muscle fibers has shown the opposite trend [
46,
47]. Skeletal muscles with larger and less densely distributed muscle fibers generally exhibit higher pixel intensity of skeletal muscles [
48]. In the turkeys of the present study, lower pixel intensity of pectoralis major muscles in experimental groups of turkeys were associated with lower (numerically or significantly) body weight and muscle fiber size, but there were no differences in crude fat or protein content of the muscles among the three groups of birds studied. Therefore, muscle fiber diameter may be one of the most important factors determining echotextural properties of pectoralis major muscles in turkeys.
The analyses of quantitative correlations among echotextural and sensory/physicochemical characteristics of the pectoral muscle revealed different numbers of significant correlations within each group, ranging from one correlation in Group Exp1 and 8 to 10 correlations in Groups C and Exp2. This is puzzling and a reason for such an uneven distribution of significant correlations remains unknown. A large number of significant correlations (
n = 12) were recorded with the data pooled for all turkeys studied. Most correlations were with meat physical properties, luminosity, color, and sensory characteristics (e.g., taste and aroma). The only chemical constituent that was significantly correlated with echotextural variables, within the groups and for all animals studied, was moisture. The latter contrasts with earlier ultrasonographic studies of the pectoral muscles in broiler chickens [
20] and different mammalian organs/tissues [
19,
49] where significant correlations were consistently seen between first order echotextural variables and fat/protein content. Although the fact that turkey meat is less fatty than that in other livestock species may explain a lack of correlations with crude fat content, a reason for a lack of significant correlations with crude protein remains unknown.
Higher numerical pixel values of ultrasonograms reflect the occurrence of harder and more compact tissue fragments [
50,
51]. Therefore, a positive correlation between MPI and cutting force seen for T and O2 scanning planes (Groups C and Exp2, respectively) seems logical. Usually, ultrasound image echointensity is adversely related to anechoic water content, or moisture, but in the present study, such correlations were restricted to pixel heterogeneity of pectoral muscles (MPH). A significant negative correlation was also noted between MPI/MPH and thermal loss in the control group of turkeys. The pectoral muscle in this group was characterized by highest fat content and largest muscle fiber diameter, causing lowest juiciness; this may explain the highest MPI/MPH values and their association with the thermal loss.