Growth Rate and Bone Hydroxyproline Concentration in Turkeys Fed with a Silage-Composed Diet Modified with Different Diet Cation–Anion Differences (DCADs)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds Husbandry
2.2. Diets
2.3. Performance Measurements
2.4. Analytical Procedures
2.5. Diet Ion Contents
2.6. Hydrolysis of Femur Samples
2.7. Blood Analysis
2.8. Statistical Analysis
3. Results
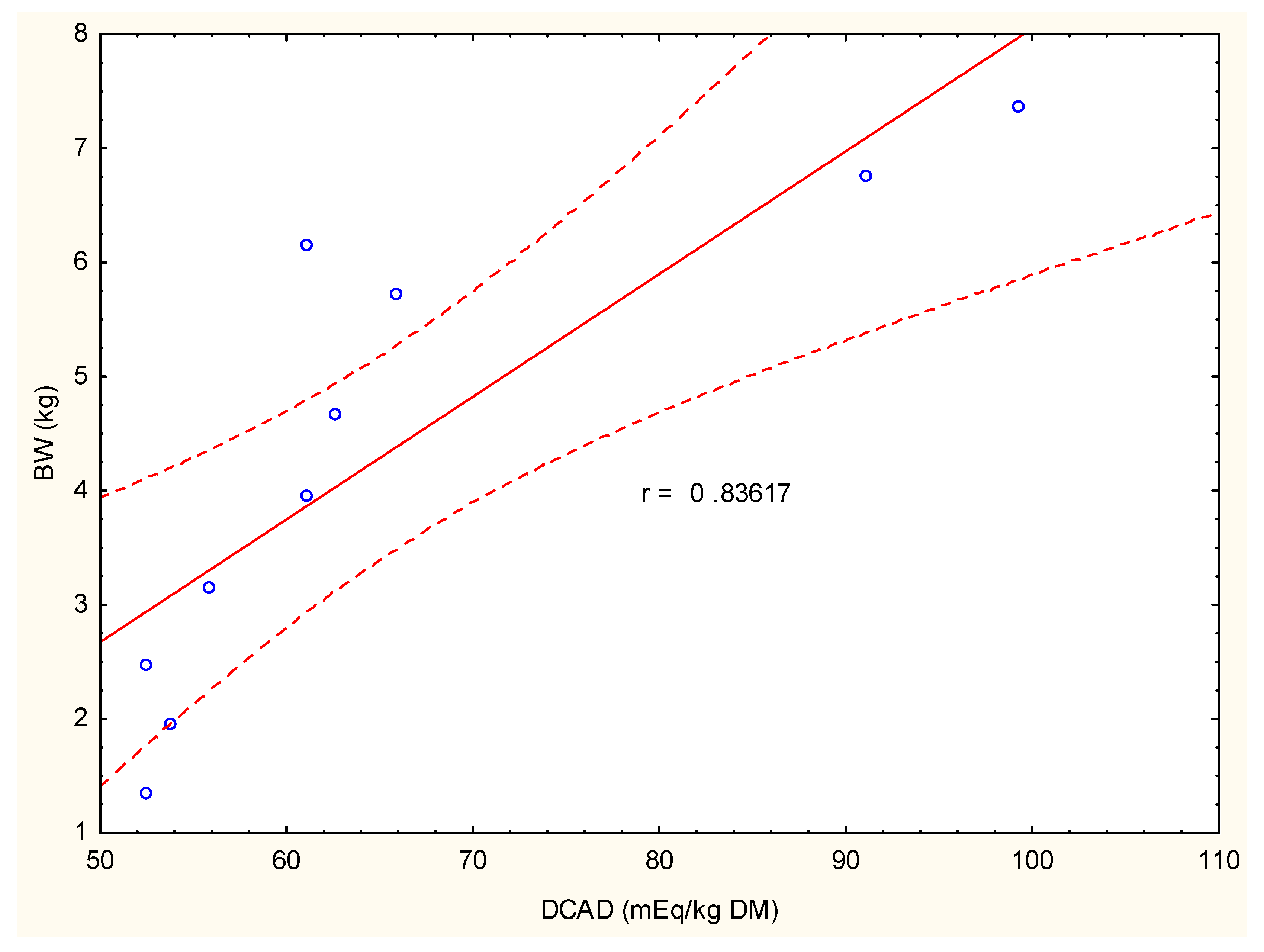
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gruszczyński, K.; Strobel, W.; Wójcik, M.; Kosior-Korzecka, U.; Wessely-Szponder, J.; Bobowiec, R. Growth rate and acid-base balance in turkeys fed a silage-containing diet modified by different dietary cation-anion difference. Med. Weter. 2017, 73, 786–791. [Google Scholar] [CrossRef][Green Version]
- Abbas, A.; Khan, M.J.; Naeem, M.; Ayaz, M.; Sufyan, A.; Somro, M.H. Cation anion balance in avian diet: A review. Agric. Sci. Res. J. 2012, 2, 302–307. [Google Scholar]
- Halley, J.T.; Nelson, L.K.; Shutze, J.V. The effect of dietary mineral on growth, leg abnormalities and blood base excess in broiler chicks. Poult. Sci. 1987, 66, 1684–1692. [Google Scholar] [CrossRef]
- Onyango, E.M.; Hester, P.Y.; Stoshine, R.; Adeola, O. Bone densitometry as an indicator of percentage tibia ash in broiler chicks fed varying dietary calcium and phosphorus levels. Poult. Sci. 2003, 82, 1787–1791. [Google Scholar] [CrossRef]
- Delport, M.; Mass, S.; van der Merve, S.W.; Laurens, J.B. Quantitation of hydroxyproline in bone by gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 804, 345–351. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, G. Roles of dietary glicine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef]
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol, E.N. Determnation of hydroxyproline in tissue and the evaluation of the collagen content of the tissue. J. Anal. Chem. 2007, 62, 51–57. [Google Scholar] [CrossRef]
- Wójcik, M.; Martelli, F.; Bobowiec, R.; Patkowski, K.; Chałabis-Mazurek, A.; Wałkuska, G. Influence of diet cation-anion difference (DCAD) on plasma acid-base status in pregnant sheep. Med. Wet. 2009, 65, 679–682. [Google Scholar]
- Kitowski, I.; Sujak, A.; Strobel, W.; Wiacek, D. Trace elements in eggshells of the Grey Heron (Ardea cinerea) from the colony in the Roztocze Hills (South East Poland). Zool. Ecol. 2013, 23, 240–244. [Google Scholar] [CrossRef]
- Monnier, V.M.; Vishwanath, V.; Frank, K.E.; Elments, C.A.; Dauchot, P.; Kohn, R.R. Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N. Eng. J. Med. 1986, 314, 403–408. [Google Scholar] [CrossRef]
- Borges, S.A.; Fischer da Silva, A.V.; Majorka, A.; Hooge, D.M.; Cummings, K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram). Poult. Sci. 2004, 83, 1551–1558. [Google Scholar] [CrossRef]
- Borges, S.A.; da Silva, A.V.F.; Ariki, J.; Hooge, D.M.; Cummings, K.R. Dietary electrolyte balance for broiler chickens exposed to thermoneutral or heat-stress environments. Poult. Sci. 2003, 82, 428–435. [Google Scholar] [CrossRef]
- Veldkamp, T.; Kwakkel, R.P.; Ferket, P.R.; Simson, P.C.; Noordhuizen, J.P.; Pijpers, A.B. Effect of ambient temperature, arginine-to lysine ratio, and electrolyte balance no performance, carcass, and blood parameters in commercial male turkeys. Poult. Sci. 2000, 79, 1608–1616. [Google Scholar] [CrossRef]
- Yen, I.T.; Pond, W.G.; Prior, R.L. Calcium chloride as a regulator of feed intake and weight gain in pigs. J. Anim. Sci. 1981, 52, 778–782. [Google Scholar] [CrossRef]
- Kim, H.W.; Han, I.K.; Choi, Y.J. Effects of lysine level and Na+ K-Cl ratio on lysine-arginine antagonism, blood pH, blood acid-base parameters and growth performance in broiler chicks. Asian Austral. J. Anim. Sci. 1989, 2, 7–16. [Google Scholar] [CrossRef]
- Adedokun, S.S.; Applegate, T.J. Dietary electrolyte balance influences ileal endogenous amino acid losses in broiler chicken. Poult. Sci. 2014, 93, 935–945. [Google Scholar] [CrossRef]
- Farahat, M.H.; Abdel-Razik, W.M.; Hassanein, E.I.; Noll, S.L. Effect of phytase supplementation to diets varying in chloride level on performance, litter moisture, foot pad score, and gait score of growing turkeys. Poult. Sci. 2013, 92, 1837–1847. [Google Scholar] [CrossRef]
- Gous, R.M. Nutritional limitations on growth and development in poultry. Livest. Sci. 2010, 130, 25–32. [Google Scholar] [CrossRef]
- Johnson, R.J.; Kurunajeewa, H. The effect of dietary minerals and electrolytes on the growth and physiology of the young chick. J. Nutr. 1985, 115, 1680–1690. [Google Scholar] [CrossRef]
- Kraut, J.A.; Mishler, D.R.; Singer, F.R.; Goodman, W.G. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int. 1986, 30, 694–700. [Google Scholar] [CrossRef]
- Brandao-Burch, A.; Utting, J.C.; Orriss, T.R.; Arnett, T.R. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. 2005, 77, 167–174. [Google Scholar] [CrossRef]
- Reaich, D.; Channon, S.M.; Scrimgeour, C.M.; Goodship, T.H. Ammonium chloride-induced acidosis increases protein breakdown and amino acid oxidation in humans. Am. J Physiol. 1992, 263, 735–739. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Piera, G.R.; Favus, M.R.; Coe, F.L. Response of serum 1,25(OH)2D3 to variation of ionized calcium during chronicacidosis. Am. J. Physiol. 1985, 249, F361–F365. [Google Scholar]
- Ramp, W.K.; Lenz, L.G.; Kaysinger, K.K. Medium pH modulates matrix, mineral, and energy metabolism in cultured chick bones and osteoblast-like cells. Bone Miner 1994, 24, 59–73. [Google Scholar] [CrossRef]

| Ingredients (%) | Grower I | Grower II | Finisher |
|---|---|---|---|
| Wheat | 29.208 | 22.045 | 23.605 |
| Soybean meal | 27 | 19.567 | 13.3 |
| Yellow corn | 20 | 25 | 25 |
| Triticale | 15 | 25 | 30 |
| Soybean oil | 3.1 | 3.233 | 3.233 |
| Hemoglobin | 1.5 | 1.5 | 1.5 |
| Fodder chalk | 1.433 | 1.167 | 0.967 |
| 1-Ca Phosphate | 0.933 | 0.667 | 0.517 |
| PRMX BR IND 0.5% * | 0.5 | 0.5 | 0.5 |
| L-Lysine HCl | 0.277 | 0.267 | 0.297 |
| L-Methionine | 0.243 | 0.203 | 0.197 |
| NaHCO3 | 0.2 | 0.2 | 0.2 |
| NaCl | 0.147 | 0.15 | 0.153 |
| Toxi-Tect ** | 0.1 | 0.1 | 0.1 |
| Noack AC BIL 1 Na *** | 0.1 | 0.1 | 0.1 |
| Choline chloride | 0.087 | 0.077 | 0.07 |
| L-Treonina | 0.067 | 0.083 | 0.077 |
| Arg90/Val10 **** | 0.06 | 0.097 | 0.15 |
| Avizyme | 0.025 | 0.025 | 0.025 |
| Axtra PHY, g/kg | 0.02 | 0.02 | 0.01 |
| Nutrients | |||
| MEpoultry, kcal/kg | 3025.24 | 3125.25 | 3199.47 |
| MEpoultry, MJ/kg | 12.66 | 13.08 | 13.39 |
| Humidity, g/kg | 119.51 | 119.27 | 119.51 |
| Crude protein, g/kg | 215.29 | 187 | 167.01 |
| Crude fat, g/kg | 50.41 | 52.6 | 52.24 |
| Crude ash, g/kg | 55.99 | 48.21 | 42.1 |
| Crude fiber, g/kg | 28.02 | 26.26 | 25.59 |
| P-inositol, g/kg | 2.4 | 2.25 | 2.12 |
| Calcium, g/kg | 10.86 | 9.5 | 8.04 |
| Phosphorus, g/kg | 5.87 | 5.02 | 4.49 |
| AvPhosph poultry, g/kg | 5.41 | 4.73 | 4.02 |
| Phosphorus Abs poultry, g/kg | 4.86 | 4.23 | 3.6 |
| Sodium, g/kg | 1.81 | 1.81 | 1.71 |
| Chlorine, g/kg | 2.34 | 2.4 | 2.55 |
| Lysine, g/kg | 12.99 | 11.07 | 9.81 |
| Lysine dig poultry, g/kg | 11.61 | 9.89 | 8.78 |
| Methionine, g/kg | 5.45 | 4.71 | 4.39 |
| Met dig poultry g/kg | 5.14 | 4.45 | 4.15 |
| Meth + cyst, g/kg | 8.95 | 7.86 | 7.29 |
| Meth + cyst dig poultry, g/kg | 8 | 7 | 6.51 |
| Threonine, g/kg | 8.26 | 7.31 | 6.35 |
| Threonine dig poultry, g/kg | 7 | 6.22 | 5.4 |
| Tryptophane, g/kg | 2.64 | 2.22 | 1.92 |
| Isoleucine, g/kg | 8.23 | 6.86 | 5.81 |
| Arginine, g/kg | 13.44 | 11.52 | 10.19 |
| Arginine dig poultry, g/kg | 12.1 | 10.3 | 0.07 |
| Choline added, mg/kg | 564.2 | 499.1 | 455.7 |
| Choline chloride added, mg/kg | 650 | 575 | 525 |
| Dry matter, g/kg | 880.49 | 880.73 | 880.49 |
| Sodium analitic, g/kg | 1.4 | 1.4 | 1.41 |
| Calcium analitic, g/kg | 8.87 | 7.51 | 6.36 |
| 6-Phyt EC 3.1.3.26, FTU/kg | 1000 | 1000 | 500 |
| Protease EC 3.4.21.62, U/kg | 4000 | 4000 | 4000 |
| Endo-1,4-betaxynalase, U/kg | 2300 | 2300 | 2300 |
| Alfa-amylase EC 3.2.1.1, U/kg | 400 | 400 | 400 |
| Lasalocid sodium, mg/kg | 90 | -------- | -------- |
| DM | 27% |
| CP% of DM | 8.6 |
| NDF% of DM | 43.0 |
| ADF% of DM | 26.1 |
| Lignin% of DM | 4.0 |
| Ether extract% of DM | 2.9 |
| Ash% of DM | 3.0 |
| Calcium% of DM | 0.33 |
| Phosphorous% of DM | 0.28 |
| Control and Experimental Groups | Grower I, mEq/kg DM | Grower II, mEq/kg DM | Finisher, mEq/kg DM |
|---|---|---|---|
| A | 127.30 ± 21.3 | 141.66 ± 8.23 | 139.3 ± 18.19 |
| B | 52.5 ± 4.19 * | 61.10 ± 12.37 * | 91.16 ± 3.14 * |
| C | −100.56 ± 7.18 * | −101.35 ± 17.21 * | −115.20 ± 34.58 * |
| D | 255.38 ± 39.76 * | 242.67 ± 4.18 | 214.02 ± 19.13 * |
| E | 315.68 ± 44. 6 * | 310.10 ± 29.14 * | 332.62 ± 15.9 * |
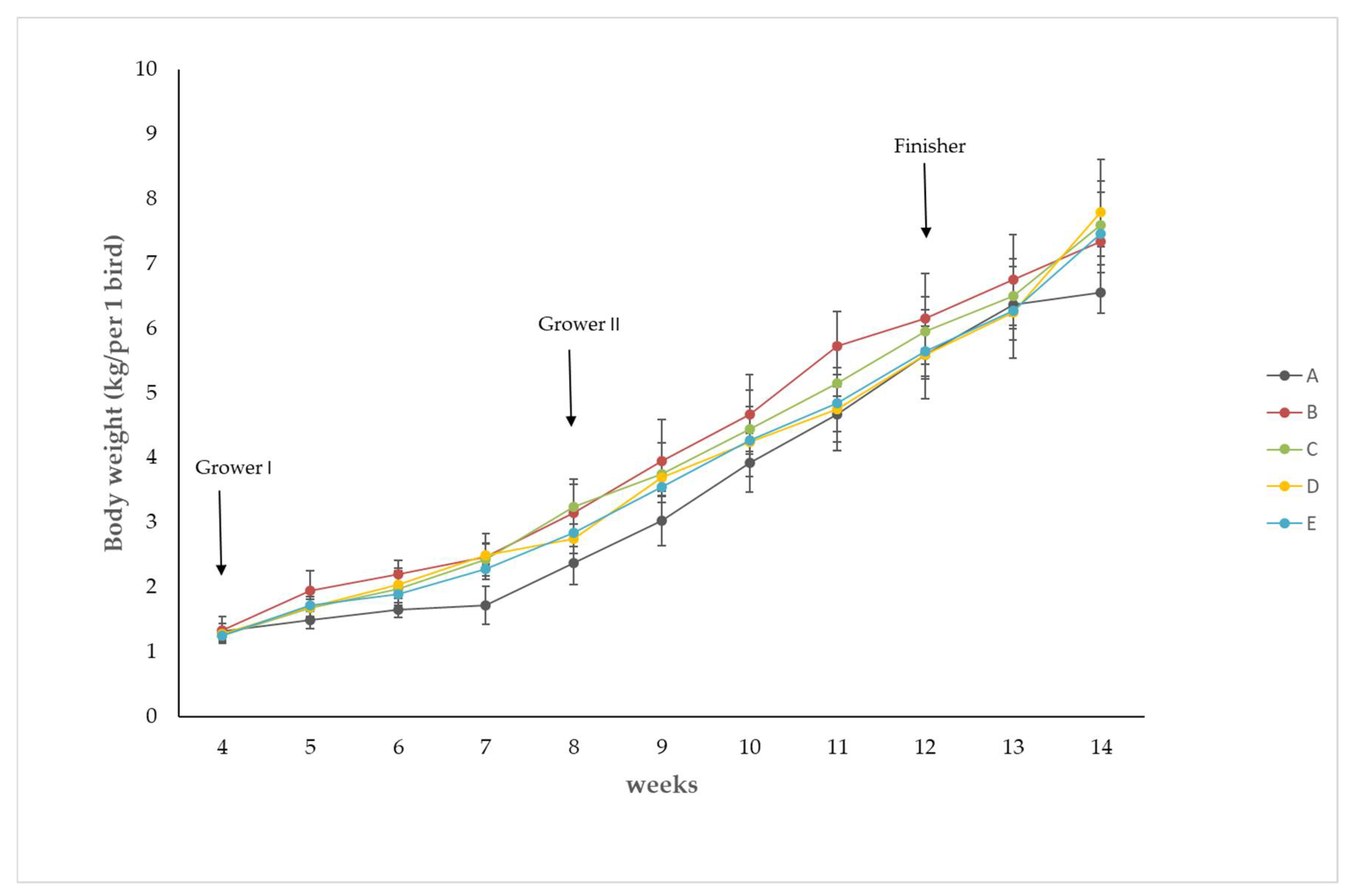
| 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | 13th | 14th | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1.32 | 1.5 | 1.65 | 1.72 | 2.38 | 3.03 | 3.93 | 4.67 | 5.6 | 6.38 | 6.55 |
| ±0.13 | ±0.14 | ±0.11 | ±0.29 | ±0.33 | ±0.38 | ±0.45 | ±0.43 | ±0.37 | ±0.39 | ±0.31 | |
| B | 1.34 | 1.95 * | 2.2 * | 2.48 * | 3.15 * | 3.95 * | 4.68 * | 5.73 * | 6.15 | 6.75 | 7.35 * |
| ±0.21 | ±0.31 | ±0.21 | ±0.36 | ±0.53 | ±0.64 | ±0.62 | ±0.54 | ±0.70 | ±0.70 | ±0.76 | |
| C | 1.25 | 1.68 | 1.98 | 2.43 | 3.25 * | 3.75 | 4.45 | 5.15 | 5.95 | 6.5 | 7.6 * |
| ±0.13 | ±0.24 | ±0.31 | ±0.26 | ±0.34 | ±0.48 | ±0.60 | ±0.62 | ±0.54 | ±0.57 | ±0.68 | |
| D | 1.28 | 1.7 | 2.04 | 2.49 * | 2.75 | 3.7 | 4.25 | 4.75 | 5.6 | 6.25 ±0.70 | 7.8 * |
| ±0.08 | ±0.15 | ±0.21 | ±0.17 | ±0.22 | ±0.22 | ±0.54 | ±0.64 | ±0.69 | ±0.82 | ||
| E | 1.26 | 1.73 | 1.89 | 2.28 | 2.85 | 3.55 | 4.28 | 4.85 | 5.65 | 6.28 | 7.46 * |
| ±0.08 | ±0.10 | ±0.17 | ±0.10 | ±0.13 | ±0.13 | ±0.17 | ±0.44 | ±0.39 | ±0.45 | ±0.35 |
| Control and Experimental Groups | Blood Collection Period | |||||
|---|---|---|---|---|---|---|
| Before Experiment | 6th Week | 8th Week | 10th Week | 12th Week | 14th Week | |
| A | 7.36 | 7.37 | 7.37 | 7.36 | 7.37 | 7.37 |
| ±0.24 | ± 0.55 | ±0.2 | ±0.2 | ±0.2 | ±0.96 | |
| B | 7.37 | 7.34 *,a | 7.35 *,a | 7.35 *,a | 7.36 | 7.35 |
| ±0.75 | ±0.77 | ±0.3 | ±0.1 | ±0.05 | ±1.04 | |
| C | 7.37 | 7.34 *,a | 7.34 *,a | 7.34 *,a | 7.34 *,a | 7.35 |
| ±0.02 | ±0.55 | ±0.21 | ±0.21 | ±0.21 | ±0.63 | |
| D | 7.37 | 7.38 | 7.39 *,a | 7.38 * | 7.39 *,a | 7.40 *,a |
| ±0.98 | ±0.49 | ±0.15 | ±0.15 | ±0.15 | ±0.40 * | |
| E | 7.36 | 7.39 a | 7.40 *,a | 7.42 *,a | 7.42 *,a | 7.44 *,a |
| ±0.50 | ±0.04 | ±0.26 | ±0.6 | ±0.8 | ±0.69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, M.; Stachal, K.; Burzec, M.; Gruszczyński, K.; Korga-Plewko, A. Growth Rate and Bone Hydroxyproline Concentration in Turkeys Fed with a Silage-Composed Diet Modified with Different Diet Cation–Anion Differences (DCADs). Animals 2022, 12, 66. https://doi.org/10.3390/ani12010066
Wójcik M, Stachal K, Burzec M, Gruszczyński K, Korga-Plewko A. Growth Rate and Bone Hydroxyproline Concentration in Turkeys Fed with a Silage-Composed Diet Modified with Different Diet Cation–Anion Differences (DCADs). Animals. 2022; 12(1):66. https://doi.org/10.3390/ani12010066
Chicago/Turabian StyleWójcik, Marta, Klaudia Stachal, Mateusz Burzec, Kamil Gruszczyński, and Agnieszka Korga-Plewko. 2022. "Growth Rate and Bone Hydroxyproline Concentration in Turkeys Fed with a Silage-Composed Diet Modified with Different Diet Cation–Anion Differences (DCADs)" Animals 12, no. 1: 66. https://doi.org/10.3390/ani12010066
APA StyleWójcik, M., Stachal, K., Burzec, M., Gruszczyński, K., & Korga-Plewko, A. (2022). Growth Rate and Bone Hydroxyproline Concentration in Turkeys Fed with a Silage-Composed Diet Modified with Different Diet Cation–Anion Differences (DCADs). Animals, 12(1), 66. https://doi.org/10.3390/ani12010066






