Efficacy of Serial Ultrasonographic Examinations in Predicting Return to Play in Agility Dogs with Shoulder Lameness
Abstract
:Simple Summary
Abstract
1. Introduction
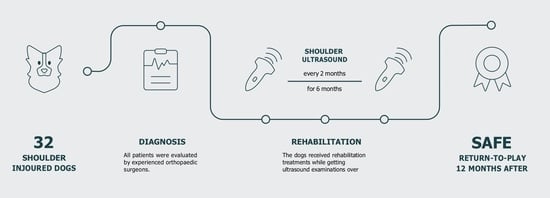
2. Materials and Methods
2.1. Dogs
2.2. Clinical Evaluation
2.3. Ultrasound Evaluation
2.4. Physiotherapeutic Protocol
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullen, K.L.; Dickey, J.P.; Bent, L.R.; Thomason, J.J.; Moëns, N.M. Internet-based survey of the nature and perceived causes of injury to dogs participating in agility training and competition events. J. Am. Vet. Med. Assoc. 2013, 243, 1010–1018. [Google Scholar] [CrossRef] [Green Version]
- Levy, I.; Trentacosta, N.; Percival, M. A preliminary retrospective survey of injuries occurring in dogs participating in canine agility. Vet. Comp. Orthop. Traumatol. 2009, 22, 321–324. [Google Scholar] [CrossRef] [Green Version]
- Cullen, K.L.; Dickey, J.P.; Bent, L.R.; Thomason, J.J.; Moëns, N.M. Survey-based analysis of risk factors for injury among dogs participating in agility training and competition events. J. Am. Vet. Med. Assoc. 2013, 243, 1019–1024. [Google Scholar] [CrossRef]
- Long, C.D.; Nyland, T.G. Ultrasonographic evaluation of the canine shoulder. Vet. Radiol. Ultrasound. 1999, 40, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.L.; Latimer, C.; Millis, D.L. Rehabilitation and physical therapy for selected orthopedic conditions in veterinary patients. Vet. Clin. North Am. Small Anim. Pract. 2015, 45, 91–121. [Google Scholar] [CrossRef]
- Tomilson, J.E.; Manfredi, J.M. Return to sport after injury: A web-based survey of owners and handlers of agility dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 473–478. [Google Scholar] [CrossRef]
- Heidorn, S.N.; Canapp, S.O., Jr.; Zink, C.M.; Leasure, C.S.; Can, B.J. Rate of return to agility competition for dogs with cranial cruciate ligament tears treated with tibial plateau levelling osteotomy. J. Am. Vet. Med. Assoc. 2018, 253, 1439–1444. [Google Scholar] [CrossRef]
- A Long and Complex Password Is Needed to Enter the Page. Available online: https://www.diagnosticmindset.com/courses/shoulder-us/ (accessed on 12 October 2021).
- Kramer, M.; Gerwing, M.; Sheppard, C.; Schimke, E. Ultrasonography for the diagnosis of diseases of the tendon and tendon sheath of the biceps brachii tendon muscle. Vet. Surg. 2001, 30, 64–71. [Google Scholar] [CrossRef]
- Cook, C.R. Ultrasound imaging of the musculoskeletal system. Vet. Clin. North Am. Small Anim. Pract. 2016, 46, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.L.; Dickey, J.P.; Brown, S.H.M.; Nykamp, S.G.; Bent, L.R.; Thomason, J.J.; Moens, N.M.M. The Magnitude of muscular activation of four canine forelimb muscles in dogs performing two agility specific tasks. BMC Vet. Res. 2017, 13, 68. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Joint Surg. 2005, 87, 187–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcellin-Little, D.J.; Levine, D.; Canapp, S.O., Jr. The Canine Shoulder: Selected disorders and their management with physical therapy. Clin. Tech. Small Anim. Pract. 2007, 22, 171–182. [Google Scholar] [CrossRef]
- Bansal, P.S.; Sobti, V.K.; Roy, K.S. Histomorphochemical effects of shortwave diathermy on healing of experimental muscular injury in dogs. Indian. J. Exp. Biol. 1990, 28, 766–770. [Google Scholar] [PubMed]
- Levine, D.; Millis, D.L.; Mynatt, T. Effects of 3.3 MHz ultrasound on a caudal thigh muscle temperature in dogs. Vet. Surg. 2001, 30, 170–174. [Google Scholar] [CrossRef]
- Marques, A.C.; Albertini, R.; Serra, A.J.; da Silva, E.A.; de Oliveira, V.L.; Silva, L.M.; Leal-Junior, E.C.; de Carvalho, P.T. Photobiomodulation therapy on collagen type I and III, vascular endothelial growth factor and metalloproteinase in experimentally induced tendinopathy in aged rats. Lasers Med. Sci. 2016, 31, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.; Millis, D.L.; Weng, H.Y. Exercise in canine physical rehabilitation: Range of motion of the forelimb during stair and ramp ascent. J. Small Anim. Pract. 2013, 54, 409–413. [Google Scholar] [CrossRef]
- Levine, D.; Marcellin-Little, D.J.; Millis, D.L.; Tragauer, V.; Osborne, J.A. Effects of partial immersion in water on vertical ground reaction forces and weight distribution in dogs. Am. J. Vet. Res. 2010, 71, 1413–1416. [Google Scholar] [CrossRef]
- Lamb, C.R.; Nelson, G.R. Diagnostic accuracy of tests based on radiologic measurements of dogs and cats: A systematic review. Vet. Radiol. Ultrasound. 2015, 56, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Carr, B.J.; Canapp, S.O.; Canapp, D.A.; Gamble, L.J.; Dycus, D.L. Adhesive Capsulitis in Eight Dogs: Diagnosis and Management. Front. Vet. Sci. 2016, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Voleti, P.B.; Buckley, M.R.; Soslowsky, L.J. Tendon healing: Repair and regeneration. Annu. Rev. Biomed. Eng. 2012, 14, 47–71. [Google Scholar] [CrossRef]
- Moyer, A.L.; Wagner, K.R. Regeneration versus fibrosis in skeletal muscle. Curr. Opin. Rheumatol. 2011, 23, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D. Towards understanding skeletal muscle regeneration. Pathol. Res. Pract. 1991, 187, 1–22. [Google Scholar] [CrossRef]
- Tidball, J.G. Mechanism of muscle injury, repair and regeneration. Compar. Physiol. 2011, 1, 2029–2062. [Google Scholar] [CrossRef]
- Dowling, B.A.; Dart, A.J.; Hodgson, D.R.; Smith, R.K.W. Superficial Digital Flexor tendonitis in the horse. Equine Vet. J. 2000, 32, 369–378. [Google Scholar] [CrossRef]
- Potenza, A.D. Tendon healing within the flexor digital sheath in the dog. J. Bone Joint. Surg. Am. 1962, 44, 49–64. [Google Scholar] [CrossRef]
- Millis, D.L.; Ciuperca, I.A. Evidence for canine rehabilitation and Physical therapy. Vet. Clin. North Am. Small Anim. Pract. 2015, 45, 1–27. [Google Scholar] [CrossRef] [PubMed]
- López-De-Celis, C.; Hidalgo-Garcìa, C.; Pérez-Bellmunt, A.; Fanlo-Mazas, P.; González-Pueda, V.; Tricás-Moreno, J.M.; Ortiz, S.; Rodriguez-Sanz, J. Thermal and non-thermal effects of capacitive-resistive electric transfer application on the Achilles tendon and musculotendineous junction of the gastrocnemius muscle: A cadaveric study. BMC Musculoskelet. Disord. 2020, 21, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-De Jesus, J.; Spadacci-Morena, D.D.; dos Anjos-Rabelo, N.D.; Pinfildi, C.E.; Fukuda, T.Y.; Plapler, H. Low Level Laser Therapy on tissue repair of partially injuried Achilles Tendon in rats. Photomed. Laser. Surg. 2014, 32, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Schollmeier, G.; Sarkar, K.; Fukuhara, K.; Uhthoff, H.K. Structural and functional changes in the canine shoulder after cessation of immobilization. Clin. Orthop. Relat. Res. 1996, 323, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.; Cutner, D. Aquatic therapy in the rehabilitation of athletic injuries. Clin. Sports Med. 1999, 18, 447–461. [Google Scholar] [CrossRef]
- Marcellin Little, D.J.; Levine, D.; Taylor, R. Rehabilitation and conditioning of sporting dogs. Vet. Clin. North Am. Small Anim. Pract. 2005, 35, 1427–1439. [Google Scholar] [CrossRef]
- Marr, C.M.; McMillian, I.; Boyd, J.S.; Wright, N.G.; Murray, M. Ultrasonographic and histopathological findings in equine superficial flexor tendon injury. Equine Vet. J. 1993, 25, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Zellers, J.A.; Cortes, D.H.; Pholig, R.T.; Silbernagel, K.G. Tendon morphology and mechanical properties assessed by ultrasound show change early in recovery and potential prognostic ability for six months outcome. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2831–2839. [Google Scholar] [CrossRef] [PubMed]
- Alzola, R.; Easter, C.; Riggs, C.; Gardener, D.S.; Freeman, S.L. Ultrasonographic-based predictive factors influencing successful return to racing after superficial digital flexor tendon injuries in racehorses: A retrospective cohort study in 469 Thoroughbred racehorses in Hong Kong. Equine Vet. J. 2018, 50, 602–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Barella, G.; Lodi, M.; Faverzani, S. Ultrasonographic findings of shoulder teno-muscular structures in symptomatic and asymptomatic dogs. J. Ultrasound. 2018, 21, 145–152. [Google Scholar] [CrossRef]
- Ülke, Ç.G.; Deniz, S.I.; Nureddin, Ç. Evaluation of return to races rates in racehorses after tendon injuries: Lesion-related parameters. J. Equine Vet. Sci. 2020, 87, 102931. [Google Scholar] [CrossRef]




| Score | Clinical Appearance |
|---|---|
| 0 | No lameness |
| 1 | Lameness not perceptible at walking but perceptible at trotting |
| 2 | Lameness perceptible at walking and apparent at trotting |
| 3 | Lameness apparent at walking and severe at trotting |
| 4 | No weight bearing |
| Score | Ultrasonographic Appearance |
|---|---|
| None | No lesions detected; the affected shoulder is identical to the non-affected shoulder |
| Minimal | The lesions were resolved but are still barely visible or |
| The lesions were completely resolved, but minimal degenerative changes are present | |
| Mild | Tendon + reduction diameter of less than 40% |
| Minimal or no signs of swelling of the surrounding tissues | |
| No more than two structures involved # | |
| Moderate | Tendon + reduction diameter of less than 40% Swelling of the surrounding tissues of an area of less than 5 mm Maximum of three structures involved # |
| Severe | Tendon + reduction diameter greater than 40% |
| Swelling of the surrounding tissues of an area larger than 5 mm Three or more structures involved # |
| Time | Lesions | CLS | ULS | p |
|---|---|---|---|---|
| T0 | With | 32/32; 100% | 32/32; 100% | ns |
| Without | 0/32; 0% | 0/32; 0% | ns | |
| T2 | With | 7/32; 22% | 32/32; 100% | <0.05 |
| Without | 25/32; 78% | 0/32; 0% | <0.05 | |
| T4 | With | 0/27; 0% | 12/27; 44% | <0.05 |
| Without | 7/27; 100% | 15/27; 56% | <0.05 | |
| T6 | With | 0/27; 0% | 1/27; 4% | ns |
| Without | 27/27; 100% | 26/27; 96% | ns |
| Time | Scoring System According to the CLS and the ULS | N | % | RR | 95% Confidence Interval | p |
|---|---|---|---|---|---|---|
| T2 | CLS | 32/32; 100% | 32/32; 100% | 23.8 | 3.45–166.18 | <0.05 |
| ULS | 0/32; 0% | 0/32; 0% | ||||
| T4 | CLS | 7/32; 22% | 32/32; 100% | 2.51 | 1.67–3.99 | <0.05 |
| ULS | 25/32; 78% | 0/32; 0% | ||||
| T6 | CLS | 0/27; 0% | 12/27; 44% | 1.02 | 0.15–4.19 | ns |
| ULS | 7/27; 100% | 15/27; 56% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Entani, M.G.; Franini, A.; Dragone, L.; Barella, G.; De Rensis, F.; Spattini, G. Efficacy of Serial Ultrasonographic Examinations in Predicting Return to Play in Agility Dogs with Shoulder Lameness. Animals 2022, 12, 78. https://doi.org/10.3390/ani12010078
Entani MG, Franini A, Dragone L, Barella G, De Rensis F, Spattini G. Efficacy of Serial Ultrasonographic Examinations in Predicting Return to Play in Agility Dogs with Shoulder Lameness. Animals. 2022; 12(1):78. https://doi.org/10.3390/ani12010078
Chicago/Turabian StyleEntani, Maria Grazia, Alessio Franini, Ludovica Dragone, Gabriele Barella, Fabio De Rensis, and Giliola Spattini. 2022. "Efficacy of Serial Ultrasonographic Examinations in Predicting Return to Play in Agility Dogs with Shoulder Lameness" Animals 12, no. 1: 78. https://doi.org/10.3390/ani12010078
APA StyleEntani, M. G., Franini, A., Dragone, L., Barella, G., De Rensis, F., & Spattini, G. (2022). Efficacy of Serial Ultrasonographic Examinations in Predicting Return to Play in Agility Dogs with Shoulder Lameness. Animals, 12(1), 78. https://doi.org/10.3390/ani12010078