In Vivo Bacteriophages’ Application for the Prevention and Therapy of Aquaculture Animals–Chosen Aspects
Abstract
:Simple Summary
Abstract
1. Introduction
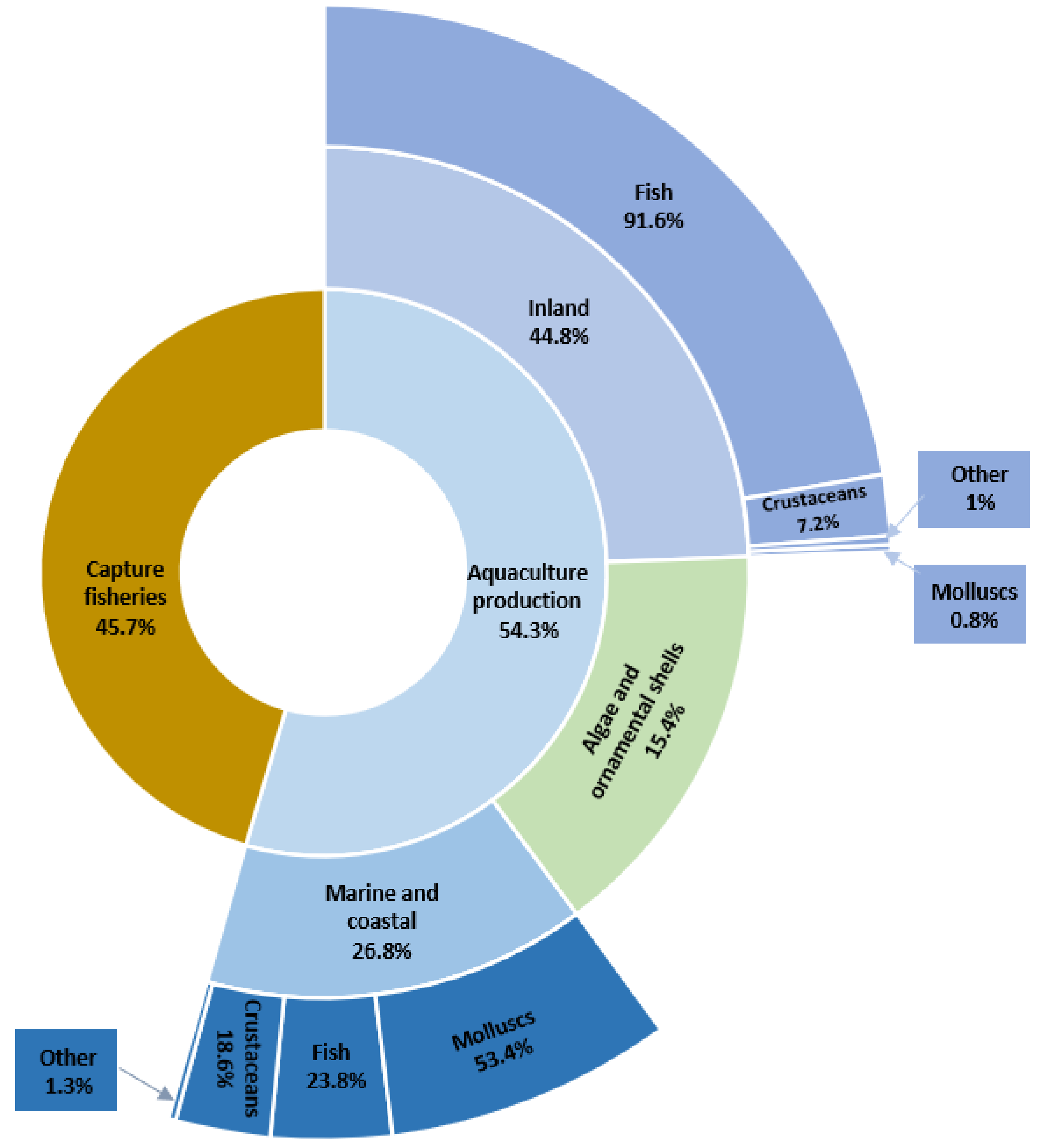
2. Antibiotic Resistance in Aquaculture
3. Bacteriophages
3.1. The Life Cycle of Bacteriophages
3.1.1. Lytic Cycle
3.1.2. Lysogenic Cycle
3.1.3. Pseudolysogenic Cycle
3.1.4. Chronic Infection
3.1.5. Abortive Infection
3.2. Bacteriophage Therapy
3.2.1. Methods of Administering Bacteriophages
3.2.2. In Vivo Use of Bacteriophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; de Miguel, T.; Sánchez, S.; Sánchez-Perez, A.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Crumlish, M. Bacterial diagnosis and control in fish and shellfish. In Diagnosis and Control of Diseases of Fish and Shellfish; Wiley: Hoboken, NJ, USA, 2017; pp. 5–18. [Google Scholar]
- Haenen, O. Major bacterial diseases affecting aquaculture. In Proceedings of the Aquatic AMR Workshop 1, Mangalore, India, 10–11 April 2017. [Google Scholar]
- Gui, L.; Zhang, Q.-Y. Disease Prevention and Control. In Aquaculture in China: Success Stories and Modern Trends; Gui, J.-F., Tang, Q., Li, Z., Liu, J., De Silva, S.S., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; Section 7; pp. 577–598. [Google Scholar]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018; Trends from 2010 to 2018. Tenth ESVAC Report; European Medicines Agency: Amsterdam, The Netherlands, 2020; p. 21. [Google Scholar]
- US Food and Drug Administration. 2019 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; FDA Report; US Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Carlson, K. Evolution of antibiotic occurrence in a river through pristine, urban and agricultural landscapes. Water Res. 2003, 37, 4645–4656. [Google Scholar] [CrossRef]
- Batt, A.L.; Snow, D.D.; Aga, D.S. Occurrence of sulfonamide antimicrobials in private water wells in Washington County, Idaho, USA. Chemosphere 2006, 64, 1963–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- MARD. List of veterinary drugs banned from use. In Promulgated Together with Circular No. 2016/TT-BNN dated 2016 of the Minister of Agriculture and Rural Development; Department of Animal Health Board: Hanoi, Vietnam, 2016. [Google Scholar]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Śnieszko, S.F.; Bullock, G.L. Treatment of sulfonamide resistant furunculosis in trout and determination of drug sensitivity. Fish. Bull. 1957, 125, 555–564. [Google Scholar]
- Watts, J.E.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preena, P.G.; Swaminathan, T.R.; Kumar, V.J.R.; Singh, I.S.B. Antimicrobial resistance in aquaculture: A crisis for concern. Biologia 2020, 75, 1497–1517. [Google Scholar] [CrossRef]
- Yano, Y.; Hamano, K.; Tsutsui, I.; Aue-Umneoy, D.; Ban, M.; Satomi, M. Occurrence, molecular characterization, and antimicrobial susceptibility of Aeromonas spp. in marine species of shrimps cultured at inland low salinity ponds. Food Microbiol. 2015, 47, 21–27. [Google Scholar] [CrossRef]
- McPhearson, R.M.; DePaola, A.; Zywno, S.R.; Motes, M.L.; Guarino, A.M. Antibiotic resistance in Gram-negative bacteria from cultured catfish and aquaculture ponds. Aquaculture 1991, 99, 203–211. [Google Scholar] [CrossRef]
- Dixon, B.A.; Issvoran, G. Antibacterial drug resistance in Aeromonas spp. isolated from domestic goldfish and koi from California. J. World Aquac. Soc. 1993, 24, 102–104. [Google Scholar] [CrossRef]
- Schmidt, A.S.; Bruun, M.S.; Dalsgaard, I.; Pedersen, K.; Larsen, J.L. Occurrence of antimicrobial resistance in fish-pathogenic and environmental bacteria associated with four Danish rainbow trout farms. Appl. Environ. Microb. 2000, 66, 4908–4915. [Google Scholar] [CrossRef] [Green Version]
- Akinbowale, A.L.; Peng, H.; Grant, P.; Barton, M.D. Antibiotic and heavy metal resistance in motile aeromonads and pseudomonads from rainbow trout (Oncorhynchus mykiss) farms in Australia. Int. J. Antimicrob. Agents 2007, 30, 177–182. [Google Scholar] [CrossRef]
- Vega-Sánchez, V.; Latif-Eugenín, F.; Soriano-Vargas, E.; Beaz-Hidalgo, R.; Figueras, M.J.; Aguilera-Arreola, M.G.; Castro-Escarpulli, G. Re-identification of Aeromonas isolates from rainbow trout and incidence of class 1 integron and β-lactamase genes. Vet. Microbiol. 2014, 172, 528–533. [Google Scholar] [CrossRef]
- John, N.; Hatha, A.A.M. Prevalence, distribution and drug resistance of motile aeromonads in freshwater ornamental fishes. Indian J. Fish. 2012, 59, 161–164. [Google Scholar]
- Chenia, H.Y. Prevalence and characterization of plasmid-mediated quinolone resistance genes in Aeromonas spp. isolated from South African freshwater fish. Int. J. Food Microbiol. 2016, 231, 26–32. [Google Scholar] [CrossRef]
- Dobiasova, H.; Kutilova, I.; Piackova, V.; Vesely, T.; Cizek, A.; Dolejska, M. Ornamental fish as a source of plasmid-mediated quinolone resistance genes and antibiotic resistance plasmids. Vet. Microbiol. 2014, 171, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.E.; Cho, M.Y.; Kim, J.W.; Kang, H.Y. Large antibiotic-resistance plasmid of Edwardsiella tarda contributes to virulence in fish. Microb. Pathog. 2012, 52, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Q.; Liu, Q.; Wang, X.; Liu, H.; Zhang, Y. Isolation and identification of fish pathogen Edwardsiella tarda from mariculture in China. Aquac. Res. 2008, 40, 13–17. [Google Scholar] [CrossRef]
- Sousa, M.; Torres, C.; Barros, J.; Somalo, S.; Igrejas, G.; Poeta, P. Gilthead seabream (Sparus aurata) as carriers of SHV-12 and TEM-52 extended-spectrum beta-lactamases-containing Escherichia coli isolates. Foodborne Pathog. Dis. 2011, 8, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Ruzauskas, M.; Klimiene, I.; Armalyte, J.; Bartkiene, E.; Siugzdiniene, R.; Skerniskyte, J.; Krasauskas, R.; Suziedeliene, E. Composition and antimicrobial resistance profile of Gram-negative microbiota prevalent in aquacultured fish. J. Food Saf. 2018, 38, e12447. [Google Scholar] [CrossRef]
- Shah, S.Q.; Nilsen, H.; Bottolfsen, K.; Colquhoun, D.J.; Sørum, H. DNA gyrase and topoisomerase IV mutations in quinolone-resistant Flavobacterium psychrophilum isolated from diseased salmonids in Norway. Microb. Drug Resist. 2012, 18, 207–214. [Google Scholar] [CrossRef]
- Kim, M.J.; Hirono, I.; Kurokawa, K.; Maki, T.; Hawke, J.; Kondo, H.; Santos, M.D.; Aoki, T. Complete DNA sequence and analysis of the transferable multiple-drug resistance plasmids (R plasmids) from Photobacterium damselae subsp. piscicida isolates collected in Japan and the United States. Antimicrob. Agents Chemother. 2008, 52, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Lamari, F.; Chakroun, I.; Rtimi, S. Assessment of the correlation among antibiotic resistance, adherence to abiotic and biotic surfaces, invasion and cytotoxicity of Pseudomonas aeruginosa isolated from diseased gilthead sea bream. Colloid Surf. B 2017, 158, 229–236. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Nguyen, H.T.; Tsai, M.A.; Byadgi, O.; Wang, P.C.; Yoshida, T.; Chen, S.C. Genetic diversity, virulence genes, and antimicrobial resistance of Streptococcus dysgalactiae isolates from different aquatic animal sources. Aquaculture 2017, 479, 256–264. [Google Scholar] [CrossRef]
- Abraham, T.J. Pathogenicity and antibiotic sensitivity of luminous Vibrio harveyi isolated from diseased penaeid shrimp. J. Aquac. Trop. 1997, 12, 1–8. [Google Scholar]
- Tendencia, E.A.; de la Peña, L.D. Antibiotic resistance of bacteria from shrimp ponds. Aquaculture 2001, 195, 193–204. [Google Scholar] [CrossRef]
- Nonaka, L.; Suzuki, S. New Mg2+-dependent oxytetracycline resistance determinant Tet 34 in Vibrio isolates from marine fish intestinal contents. Antimicrob. Agents Chemother. 2002, 46, 1550–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Anitimicrobial Resistance. In Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- WHO. No Time to Wait: Securing the Future from Drug-Resistant Infections; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniesa Perez, M.T.; Navarro Risueño, F. Bacteriophages in clinical samples can interfere with microbiological diagnostic tools. Sci. Rep. 2016, 6, 33000. [Google Scholar]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef] [Green Version]
- d’Herelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. CR Acad. Sci. Paris 1917, 165, 373–375. [Google Scholar]
- Simmonds, P.; Aiewsakun, P. Virus classification–where do you draw the line? Arch. Virol. 2018, 163, 2037–2046. [Google Scholar] [CrossRef] [Green Version]
- Pal, S. Phage Therapy an alternate disease control in Aquaculture: A review on recent advancements. IOSR J. Agric. Vet. Sci. 2015, 8, 68–81. [Google Scholar]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B. Bacteriophages and phage-derived proteins–application approaches. Curr. Med. Chem. 2015, 22, 1757–1773. [Google Scholar] [CrossRef]
- Seed, K.D. Battling phages: How bacteria defend against viral attack. PLoS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef] [Green Version]
- Maszewska, A. Phage associated polysaccharide depolymerases—Characteristics and application. Postepy Hig. Med. Dosw. 2015, 69, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Cenens, W.; Makumi, A.; Mebrhatu, M.T.; Lavigne, R.; Aertsen, A. Phage–host interactions during pseudolysogeny: Lessons from the Pid/dgo interaction. Bacteriophage 2013, 3, e1003269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, Z.; Abedon, S.T. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. FEMS Microbiol. Lett. 2016, 363, fnw047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasman, L.M.; Porter, L.D. Bacteriophages. StatPearls [Internet]—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493185/?msclkid=41765f02b40411ec87dc1f40ad8c6220 (accessed on 20 March 2020).
- Łoś, M.; Węgrzyn, G. Pseudolysogeny. Adv. Virus Res. 2012, 82, 339–349. [Google Scholar] [CrossRef]
- Chopin, M.C.; Chopin, A.; Bidnenko, E. Phage abortive infection in lactococci: Variations on a theme. Curr. Opin. Microbiol. 2005, 8, 473–479. [Google Scholar] [CrossRef]
- Lopatina, A.; Tal, N.; Sorek, R. Abortive infection: Bacterial suicide as an antiviral immune strategy. Annu. Rev. Virol. 2020, 7, 371–384. [Google Scholar] [CrossRef]
- Prasad, Y.; Kumar, D.; Sharma, A.K. Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn) from columnaris disease. J. Environ. Biol. 2011, 32, 161–168. [Google Scholar]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 2013, 416, 289–295. [Google Scholar] [CrossRef]
- Cao, Y.; Li, S.; Han, S.; Wang, D.; Zhao, J.; Xu, L.; Liu, H.; Lu, T. Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 2020, 523, 735193. [Google Scholar] [CrossRef]
- Donati, V.L.; Dalsgaard, I.; Sundell, K.; Castillo, D.; Er-Rafik, M.; Clark, J.; Wilkund, T.; Middelboe, M.; Madsen, L. Phage-Mediated Control of Flavobacterium psychrophilum in Aquaculture: In vivo Experiments to Compare Delivery Methods. Front. Microbiol. 2021, 12, 628309. [Google Scholar] [CrossRef]
- Kim, J.H.; Choresca, C.H.; Shin, S.P.; Han, J.E.; Jun, J.W.; Park, S.C. Biological Control of Aeromonas salmonicida subsp. salmonicida Infection in Rainbow Trout (Oncorhynchus mykiss) Using Aeromonas Phage PAS-1. Transbound. Emerg. Dis. 2015, 62, 81–86. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, X.; Wang, X.; Cao, Z.; Wang, L.; Xu, Y. Efficiency of a bacteriophage in controlling vibrio infection in the juvenile sea cucumber Apostichopus japonicus. Aquaculture 2016, 451, 345–352. [Google Scholar] [CrossRef]
- Luo, X.; Liao, G.; Liu, C.; Jiang, X.; Lin, M.; Zhao, C.; Tao, J.; Huang, Z. Characterization of bacteriophage HN48 and its protective effects in Nile tilapia Oreochromis niloticus against Streptococcus agalactiae infections. J. Fish Dis. 2018, 41, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, K.; Raut, M.P.; Chandekar, R.H.; Sanmukh, S.G.; Paunikar, W.N. Novel bacteriophage therapy for controlling metallo-beta-lactamase producing Pseudomonas aeruginosa infection in catfish. BMC Vet. Res. 2013, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laanto, E.; Bamford, J.K.; Ravantti, J.J.; Sundberg, L.R. The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front. Microbiol. 2015, 6, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akmal, M.; Rahimi-Midani, A.; Hafeez-ur-Rehman, M.; Hussain, A.; Choi, T.J. Isolation, characterization, and application of a bacteriophage infecting the fish pathogen Aeromonas hydrophila. Pathogens 2020, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Onarinde, B.A.; Dixon, R.A. Prospects for Biocontrol of Vibrio parahaemolyticus Contamination in Blue Mussels (Mytilus edulus)—A Year-Long Study. Front. Microbiol. 2018, 9, 1043. [Google Scholar] [CrossRef]
- Makarov, R.; Lomelí-Ortega, C.O.; Zermeño-Cervantes, L.A.; García-Álvarez, E.; Gutiérrez-Rivera, J.N.; Cardona-Félix, C.S.; Martínez-Díaz, S.F. Evaluation of a cocktail of phages for the control of presumptive Vibrio parahaemolyticus strains associated to acute hepatopancreatic necrosis disease. Aquac. Res. 2019, 50, 3107–3116. [Google Scholar] [CrossRef]
- Le, T.S.; Southgate, P.C.; O’Connor, W.; Vu, S.V.; Kurtböke, D.İ. Application of bacteriophages to control Vibrio alginolyticus contamination in oyster (Saccostrea glomerata) larvae. Antibiotics 2020, 9, 415. [Google Scholar] [CrossRef]
- Veyrand-Quirós, B.; Gómez-Gil, B.; Lomeli-Ortega, C.O.; Escobedo-Fregoso, C.; Millard, A.D.; Tovar-Ramírez, D.; Balcazar, J.L.; Quiroz-Guzmán, E. Use of bacteriophage vB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of longfin yellowtail (Seriola rivoliana) eggs. J. Appl. Microbiol. 2020, 129, 1497–1510. [Google Scholar] [CrossRef]
- Górski, A.; Ważna, E.; Dąbrowska, B.W.; Dąbrowska, K.; Świtała-Jeleń, K.; Międzybrodzki, R. Bacteriophage translocation. FEMS Immunol. Med. Mic. 2006, 46, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; Wiley Blackwell: Ames, IA, USA, 2010. [Google Scholar]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture 2019, 513, 734423. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, T.G.; Nagaraju, V.T.; Gita, S.; Paria, A.; Parhi, J. Advances in bacteriophage research for bacterial disease control in aquaculture. Rev. Fish. Sci. Aquac. 2017, 25, 113–125. [Google Scholar] [CrossRef]
- Cairns, B.J.; Payne, R.J. Bacteriophage therapy and the mutant selection window. Antimicrob. Agents Chemother. 2008, 52, 4344–4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunttu, H.M.; Runtuvuori-Salmela, A.; Middelboe, M.; Clark, J.; Sundberg, L.R. Comparison of Delivery Methods in Phage Therapy against Flavobacterium columnare Infections in Rainbow Trout. Antibiotics 2021, 10, 914. [Google Scholar] [CrossRef]
- Romero, J.; Feijoó, C.G.; Navarrete, P. Antibiotics in aquaculture—Use, abuse and alternatives. In Health and Environment in Aquaculture; Carvalho, E.D., David, G.S., DaSilva, R.J., Eds.; IntechOpen: London, UK, 2012; pp. 159–198. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef]
- Wu, J.L.; Lin, H.M.; Jan, L.; Hsu, Y.L.; Chang, L.H. Biological control of fish bacterial pathogen, Aeromonas hydrophila, by bacteriophage AH 1. Fish Pathol. 1981, 15, 271–276. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Feng, C.; Chi, T.; Qi, Y.; Abbas Raza, S.H.; Gao, N.; Jia, K.; Zhang, Y.; Fan, R.; et al. A phage cocktail in controlling phage resistance development in multidrug resistant Aeromonas hydrophila with great therapeutic potential. Microb. Pathog. 2022, 162, 105374. [Google Scholar] [CrossRef]
- El-Araby, D.A.; El-Didamony, G.; Megahed, M. New approach to use phage therapy against Aeromonas hydrophila induced motile Aeromonas septicemia in Nile tilapia. J. Mar. Sci. Res. Dev. 2016, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Dien, L.T.; Ky, L.B.; Huy, B.T.; Mursalim, M.F.; Kayansamruaj, P.; Senapin, S.; Rodkhum, C.; Dong, H.T. Characterization and protective effects of lytic bacteriophage pAh6.2TG against a pathogenic multidrug-resistant Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtböke, D.İ. Protective Effects of Bacteriophages against Aeromonas hydrophila Species Causing Motile Aeromonas Septicemia (MAS) in Striped Catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, T.H.O.; Xuan, T.T.; Duyen, L.T.; Le, N.P.; Hoang, H.A. Protective efficacy of phage PVN02 against haemorrhagic septicaemia in striped catfish Pangasianodon hypophthalmus via oral administration. J. Fish Dis. 2021, 44, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, M.; Dananjaya, S.; Park, S.C.; Lee, J.; Shin, H.; De Zoysa, M. Characterization of Bacteriophage PAh-1 and Its Protective Effects on Experimental Infection of Aeromonas hydrophila in Zebrafish (Danio rerio). J. Fish Dis. 2017, 40, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Robak, S.; Dastych, J.; Siwicki, A.K. Influence of bacteriophages cocktail on European eel (Anguilla anguilla) immunity and survival after experimental challenge. Fish Shellfish Immunol. 2019, 84, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Pajdak-Czaus, J.; Robak, S.; Dastych, J.; Siwicki, A.K. Bacteriophage-based cocktail modulates selected immunological parameters and post-challenge survival of rainbow trout (Oncorhynchus mykiss). J. Fish Dis. 2019, 42, 1151–1160. [Google Scholar] [CrossRef]
- Imbeault, S.; Parent, S.; Lagacé, M.; Uhland, C.F.; Blais, J.F. Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J. Aquat. Anim. Health 2006, 18, 203–214. [Google Scholar] [CrossRef]
- Silva, Y.J.; Moreirinha, C.; Pereira, C.; Costa, L.; Rocha, R.J.; Cunha, Â.; Gomez, N.C.M.; Calado, R.; Almeida, A. Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS-A. Aquaculture 2016, 450, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Verner–Jeffreys, D.W.; Algoet, M.; Pond, M.J.; Virdee, H.K.; Bagwell, N.J.; Roberts, E.G. Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 2007, 270, 475–484. [Google Scholar] [CrossRef]
- Jia, K.; Yang, N.; Zhang, X.; Cai, R.; Zhang, Y.; Tian, J.; Raza, S.H.A.; Kang, Y.; Qian, A.; Li, Y.; et al. Genomic, Morphological and Functional Characterization of Virulent Bacteriophage IME-JL8 Targeting Citrobacter freundii. Front. Microbiol. 2020, 11, 585261. [Google Scholar] [CrossRef]
- Royam, M.M.; Nachimuthu, R. Isolation, characterization, and efficacy of bacteriophages isolated against Citrobacter spp. an in vivo approach in a zebrafish model (Danio rerio). Res. Microbiol. 2020, 171, 341–350. [Google Scholar] [CrossRef]
- Cui, H.; Xu, Y.; Cong, C.; Li, C.; Li, X.; Li, S.; Li, J.; Wang, L. Evaluation of the preventive effect of phage cocktails on turbot ascites and its influence on main physiological indicators. Aquaculture 2022, 547, 737539. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Chandrarathna, H.P.S.U.; Dananjaya, S.H.S.; De Zoysa, M.; Lee, J. Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 2020, 63, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Higuera, G.; Villa, M.; Middelboe, M.; Dalsgaard, I.; Madsen, L.; Espejo, R.T. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J. Fish Dis. 2012, 35, 193–201. [Google Scholar] [CrossRef]
- Sundell, K.; Landor, L.; Castillo, D.; Middelboe, M.; Wiklund, T. Bacteriophages as Biocontrol Agents for Flavobacterium psychrophilum Biofilms and Rainbow Trout Infections. Phage 2020, 1, 198–204. [Google Scholar] [CrossRef]
- Nakai, T.; Sugimoto, R.; Park, K.H.; Matsuoka, S.; Mori, K.I.; Nishioka, T.; Maruyama, K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis. Aquat. Org. 1999, 37, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, S.M.; Bouzari, M.; Emtiazi, G. Preliminary characterization of Lactococcus garvieae bacteriophage isolated from wastewater as a potential agent for biological control of lactococcosis in aquaculture. Aquac. Int. 2014, 22, 1469–1480. [Google Scholar] [CrossRef]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microb. 2000, 66, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Park, S.C.; Nakai, T. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Organ. 2003, 53, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, S.; Hashizume, T.; Kanzaki, H.; Iwamoto, E.; Park, S.C.; Yoshida, T.; Nakai, T. Phage therapy against beta-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus. Fish Pathol. 2007, 42, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Kwon, A.S.; Kang, B.J.; Jun, S.Y.; Yoon, S.J.; Lee, J.H.; Kang, S.H. Evaluating the effectiveness of Streptococcus parauberis bacteriophage Str-PAP-1 as an environmentally friendly alternative to antibiotics for aquaculture. Aquaculture 2017, 468, 464–470. [Google Scholar] [CrossRef]
- Rørbo, N.; Rønneseth, A.; Kalatzis, P.G.; Rasmussen, B.B.; Engell-Sørensen, K.; Kleppen, H.P.; Wergeland, H.I.; Gram, L.; Middelboe, M. Exploring the effect of phage therapy in preventing Vibrio anguillarum infections in cod and turbot larvae. Antibiotics 2018, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 2013, 392, 128–133. [Google Scholar] [CrossRef]
- Kalatzis, P.G.; Bastías, R.; Kokkari, C.; Katharios, P. Isolation and Characterization of Two Lytic Bacteriophages, ΦSt2 and ΦGrn1; Phage Therapy Application for Biological Control of Vibrio alginolyticus in Aquaculture Live Feeds. PLoS ONE 2016, 11, e0151101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Cao, Z.; Li, Z.; Wang, L.; Li, H.; Wu, F.; Jin, L.; Li, X.; Li, S.; Xu, Y. Effect of bacteriophages on Vibrio alginolyticus infection in the sea cucumber, Apostichopus japonicus (Selenka). J. World Aquac. Soc. 2015, 46, 149–158. [Google Scholar] [CrossRef]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, A.; Calado, R.; Gomez, N.C.M.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef] [PubMed]
- Lomelí-Ortega, C.O.; Martínez-Sández, A.; Barajas-Sandoval, D.R.; Reyes, A.G.; Magallón-Barajas, F.; Veyrand-Quíros, B.; Gannon, L.; Harrison, C.; Michniewski, S.; Millard, A. Isolation and Characterization of Vibriophage VB_Vc_SrVc9: An Effective Agent in Preventing Vibrio campbellii Infections in Brine Shrimp Nauplii (Artemia franciscana). J. Appl. Microbiol. 2021, 131, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; et al. Application of the bacteriophage pVco-14 to prevent Vibrio coralliilyticus infection in Pacific oyster (Crassostrea gigas) larvae. J. Invertebr. Pathol. 2019, 167, 107244. [Google Scholar] [CrossRef]
- Quiroz-Guzmán, E.; Peña-Rodriguez, A.; Vázquez-Juárez, R.; Barajas-Sandoval, D.R.; Balcázar, J.L.; Martínez-Díaz, S.F. Bacteriophage cocktails as an environmentally-friendly approach to prevent Vibrio parahaemolyticus and Vibrio harveyi infections in brine shrimp (Artemia franciscana) production. Aquaculture 2018, 492, 273–279. [Google Scholar] [CrossRef]
- Misol, G.N.; Kokkari, C.; Katharios, P. Biological and Genomic Characterization of a Novel Jumbo Bacteriophage, vB_VhaM_pir03 with Broad Host Lytic Activity against Vibrio harveyi. Pathogens 2020, 9, 1051. [Google Scholar] [CrossRef]
- Stalin, N.; Srinivasan, P. Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet. Microbiol. 2017, 207, 83–96. [Google Scholar] [CrossRef]
- Karunasagar, I.; Shivu, M.M.; Girisha, S.K.; Krohne, G.; Karunasagar, I. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 2007, 268, 288–292. [Google Scholar] [CrossRef]
- Vinod, M.G.; Shivu, M.M.; Umesha, K.R.; Rajeeva, B.C.; Krohne, G.; Karunasagar, I.; Karunasagar, I. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 2006, 255, 117–124. [Google Scholar] [CrossRef]
- Patil, J.R.; Desai, S.N.; Roy, P.; Durgaiah, M.; Saravanan, R.S.; Vipra, A. Simulated Hatchery System to Assess Bacteriophage Efficacy against Vibrio harveyi. Dis. Aquat. Org. 2014, 112, 113–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Barton, M.; Elliott, L.; Li, X.; Abraham, S.; O’Dea, M.; Munro, J. Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 2017, 473, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Cong, C.; Wang, L.; Li, X.; Li, J.; Yang, H.; Li, S.; Xu, Y. Protective effectiveness of feeding phage cocktails in controlling Vibrio harveyi infection of turbot Scophthalmus maximus. Aquaculture 2021, 535, 736390. [Google Scholar] [CrossRef]
- Wu, L.; Tian, Y.; Pang, M.; Yang, Z.; Bao, H.; Zhou, Y.; Sun, L.; Wang, R.; Zhang, H. A novel vibriophage vB_VhaS_PcB-1G capable of inhibiting virulent Vibrio harveyi pathogen. Aquaculture 2021, 542, 736854. [Google Scholar] [CrossRef]
- Alagappan, K.; Karuppiah, V.; Deivasigamani, B. Protective effect of phages on experimental V. parahaemolyticus infection and immune response in shrimp (Fabricius, 1798). Aquaculture 2016, 453, 86–92. [Google Scholar] [CrossRef]
- Lomelí-Ortega, C.O.; Martínez-Díaz, S.F. Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae. Aquaculture 2014, 434, 208–211. [Google Scholar] [CrossRef]
- Jun, J.W.; Han, J.E.; Giri, S.S.; Tang, K.F.; Zhou, X.; Aranguren, L.F.; Kim, H.J.; Saekil, Y.; Kim, S.G.; Park, S.C. Phage application for the protection from acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2018, 58, 114–117. [Google Scholar] [CrossRef]
- Ding, T.; Sun, H.; Pan, Q.; Zhao, F.; Zhang, Z.; Ren, H. Isolation and characterization of Vibrio parahaemolyticus bacteriophage vB_VpaS_PG07. Virus Res. 2020, 286, 198080. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, L.; Cao, Z.; Xu, Y. Use of phages to control Vibrio splendidus infection in the juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2016, 54, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Parasion, S.; Kwiatek, M.; Gryko, R.; Mizak, L.; Malm, A. Bacteriophages as an alternative strategy for fighting biofilm development. Pol. J. Microbiol. 2014, 63, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Application of bacteriophages for detection of foodborne pathogens. Bacteriophage 2014, 4, e28137. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, L.D. Designing phage therapeutics. Curr. Pharm. Biotechnol. 2010, 11, 15–27. [Google Scholar] [CrossRef] [PubMed]

| Pathogen | Species | Location | Ineffective Antimicrobials | References |
|---|---|---|---|---|
| Aeromonas aquariorum | Pacific whiteleg shrimp (Litopenaeus vannamei), Tiger prawn (Penaeus monodon) | Thailand | ampicillin, ampicillin + sulbactam, cephalothin, cefotaxime, erythromycin, tetracycline, clindamycin, nalidixic acid, norfloxacin, trimethoprim-sulfamethoxazole | [20] |
| Aeromonas hydrophila | Channel catfish (Ictalurus punctatus) | United States | ampicillin, chloramphenicol, kanamycin, nitrofurantoin, oxytetracycline, tetracycline | [21] |
| Aeromonas spp. | Goldfish (Carassius auratus) | United States | ampicillin, furadantoin, sulfadiazine, sulfadimethoxine + ormetoprim, tetracycline, others | [22] |
| Rainbow trout (Oncorhynchus mykiss) | Denmark | amoxicillin, oxolinic acid, oxytetracycline, sulfadiazine + trimethoprim, | [23] | |
| Rainbow trout (Oncorhynchus mykiss) | Australia | amoxicillin, cephalothin, ceftiofur, chloramphenicol, florfenicol, nitrofurantoin, streptomycin, sulfamethoxazole, tetracycline, ticarcillin, trimethoprim | [24] | |
| Rainbow trout (Onchorynchus mykiss) | Mexico | β-lactams | [25] | |
| Ornamental fish | India | amoxicillin, cephalothin, cefpodoxime, carbenicillin, nalidixic acid, streptomycin, tetracycline, trimethoprim | [26] | |
| Mozambique tilapia (Oreochromis mossambicus), Rainbow trout (Oncorhynchus mykiss), Carp (Cyprinus carpio) | South Africa | ciprofloxacin, nalidixic acid, ofloxacin | [27] | |
| Aeromonas sobria | Koi carp (Cyprinus carpio koi) | Czech Republic | quinolones, sulfonamides, tetracycline | [28] |
| Aeromonas veronii | Pacific whiteleg shrimp (Litopenaeus vannamei), Tiger prawn (Penaeus monodon) | Thailand | ampicillin, ampicillin + sulbactam, cephalothin, erythromycin, imipenem, clindamycin, nalidixic acid, norfloxacin, tetracycline, trimethoprim + sulfamethoxazole | [20] |
| Edwardsiella tarda | Olive flounder (Paralichthys olivaceus) | South Korea | kanamycin, streptomycin, tetracycline | [29] |
| Turbot (Scophthalmus maximus) | China | chloramphenicol | [30] | |
| Escherichia coli | Gilt-head bream (Sparus aurata) | Portugal | β-lactams | [31] |
| Enterobacteriacae | Carp (Cyprinus carpio), Rainbow trout (Salmo gairdneri), Bighead carp (Hypophthalmichthys nobilis) | Lithuania | ampicillin, β-lactams, second-generation cephalosporins, carbapenems | [32] |
| Flavobacterium psychrophilum | Rainbow trout (Oncorhynchus mykiss) | Denmark | amoxicillin, oxolinic acid, oxytetracycline, sulfadiazine + trimethoprim | [23] |
| Rainbow trout (Oncorhynchus mykiss), Atlantic salmon (Salmo salar), Trout (Salmo trutta) | Norway | quinolones | [33] | |
| Plesiomonas shigelloides | Channel catfish (Ictalurus punctatus) | United States | ampicillin, chloramphenicol, kanamycin, nitrofurantoin, oxytetracycline, tetracycline | [21] |
| Photobacterium damselae | Yellowtail (Seriola quinqueradiata) | Japan | chloramphenicol, kanamycin, sulfonamide, tetracycline | [34] |
| Palmetto bas (Morone saxatilis × M. chrysops) | United States | |||
| Pseudomonas aeruginosa | Gilt-head bream (Sparus aurata) | Tunisia | ampicillin, chloramphenicol, erythromycin, tetracycline | [35] |
| Pseudomonas spp. | Carp (Cyprinus carpio), Rainbow trout (Salmo gairdneri), Bighead carp (Hypophthalmichthys nobilis) | Lithuania | β-lactams | [32] |
| Rainbow trout (Oncorhynchus mykiss) | Australia | amoxicillin, cephalothin, ceftiofur, ticarcillin, chloramphenicol, florfenicol, streptomycin, nitrofurantoin, and trimethoprim | [24] | |
| Streptococcus dysgalactiae | Mullet (Mugil cephalus), Cobia (Rachycentron canadum), Golden pompano (Trachinotus blochii), Amberjack (Seriola dumerili), Yellowtail (Seriola quinqueradiata), others | Taiwan and Japan | erythromycin and tetracycline | [36] |
| Vibrio harveyi | Penaeidae | India | ampicillin, ceprofloxacin, chlortetracycline, erythromycin, furazolidone, gentamicin, nalidixic acid, neomycin, novobiocin, oxytetracycline, penicillin G, polymyxin B, rifampicin, streptomycin | [37] |
| Tiger shrimp (Penaeus monodon) | Philippines | chloramphenicol, furazolidone, oxolinic acid, oxytetracycline | [38] | |
| Vibrio sp. | Yellowtail (Seriola quinqueradiata) | Japan | oxytetracycline | [39] |
| Yersinia ruckeri | Rainbow trout (Oncorhynchus mykiss) | Denmark | oxolinic acid | [23] |
| Pathogen | Species | Application | Outcome | References |
|---|---|---|---|---|
| Aeromonas hydrophila | Carp (Cyprinus carpio) | Intraperitoneal injection | Reduction in mortality by 100%, 60% or 50% depending on the bacteriophage or cocktail used. | [80] |
| Cyprinid loach (Misgurnus anguillicaudatus) | 1. Mortality drop from 39% to 0%; 2. A decrease in mortality from 100% to 43% or 17% depending on the used bacteriophage. | [58] | ||
| No mortality after 7 days compared to control group (65%) | [79] | |||
| Feed | 1. A decrease in mortality from 39% to 17% or 11% depending on the used bacteriophage; 2. A decrease in mortality from 96% to 47% or 27% depending on the used bacteriophage. | [58] | ||
| Bath | A 47% decrease in mortality; most surviving fish showed no signs of disease. | [66] | ||
| Nile tilapia (Oreochromis niloticus) | Intraperitoneal injection | A 50% decrease in mortality. | [81] | |
| Immersion | Reduction in mortality by 37.5–55% depending on bacteriophage dose. | [82] | ||
| Rainbow trout (Oncorhynchus mykiss) | Intraperitoneal injection | Reduction in mortality by 40% after prophylactic administration. | [59] | |
| Feed | Reduction in mortality by 70% after prophylactic administration. | |||
| Bath | Reduction in mortality by 80% after prophylactic administration. | |||
| Striped Catfish (Pangasianodon hypophthalmus) | Intraperitoneal injection | Reduction in mortality by 82%, 37% or 14% depending on bacteriophage dose. | [83] | |
| Feed | Reduction in mortality by 51.6–60% depending on bacteriophage dose. | [84] | ||
| Zebrafish (Danio rerio) | Immersion | Reduction of mortality by 43.3%. | [85] | |
| Aeromonas hydrophila and Pseudomonas fluorescens | European eel (Anguilla anguilla) | Bath | Reduction in mortality by 40%, 25% or 15% depending on time of initiation of therapy; reduction in mortality by 60% with prophylactic use. | [86] |
| Rainbow trout (Oncorhynchus mykiss) | Bath | Reduction in mortality by 25%, 15% or 10% depending on time of initiation of therapy; reduction in mortality by 36% with prophylactic use. | [87] | |
| Aeromonas salmonicida | Brook trout (Salvelinus fontinalis) | Immersion | Delayed disease onset by 7 days and reduced mortality from 100% to 10% | [88] |
| Rainbow trout (Oncorhynchus mykiss) | Intramuscular injection | Reduction in mortality from 100% to 70%. | [61] | |
| Senegalese sole (Solea senegalensis) | Immersion | No mortality compared to the control group (36%). | [89] | |
| Aeromonas salmonicida subsp. salmonicida | Atlantic salmon (Salmo salar) | Intraperitoneal injection | Delayed mortality; final mortality did not differ between groups. | [90] |
| Feed | ||||
| Bath | ||||
| Rainbow trout (Oncorhynchus mykiss) | Intramuscular injection | Reduction in mortality by 26.7%, no symptoms up to 14 days after bacteriophage administration. | [61] | |
| Citrobacter freundii | Carp (Cyprinus carpio) | Intraperitoneal injection | Reduction in mortality by 100%, 45% and 0% depending on time of bacteriophage administration. | [91] |
| Citrobacter spp. | Zebrafish (Danio rerio) | Bath | Reduction in mortality by 17%, 23% and 26% depending on the bacteriophage or cocktail used | [92] |
| Edwardsiella tarda | Turbot (Scophthalmus maximus) | Feed | Reduction in mortality by 53%, 76% or 80% depending on bacteriophage dose. | [93] |
| Zebrafish (Danio rerio) | Bath | Reduction in mortality by 50%. | [94] | |
| Flavobacterium columnare | Rainbow trout (Oncorhynchus mykiss) | Bath | Reduction of mortality by 33–42% depending on the number of bacteriophages. | [65] |
| Walking catfish (Clarias batrachu) | Intramuscular injection | No symptoms and 100% survival. | [57] | |
| Bath | ||||
| Feed | ||||
| Zebrafish (Danio rerio) | Immersion | Reduction in mortality by 60%. | [65] | |
| Flavobacterium psychrophilum | Atlantic salmon (Salmo salar) | Intraperitoneal injection | Mortality decreased from 45% to 18% and from 13% to 6% depending on the bacteriophage used. | [95] |
| Rainbow trout (Oncorhynchus mykiss) | Mortality decreased from 47% to 20% and from 80% to 47% depending on the bacteriophage used. | |||
| 23% reduction in mortality by phage administration 3 days after infection. | [60] | |||
| Cocktail reduced mortality by 17–54% depending on the bacteriophage/bacterial ratio. | [96] | |||
| Feed | No significant differences in final mortality. | [60] | ||
| Bath | ||||
| Lactococcus garvieae | Japanese amberjack (Seriola quinqueradiata) | Intraperitoneal injection | Mortality decreased from 90% to 0–50% depending on the timing of bacteriophage administration. | [97] |
| Feed | Mortality reduced from 65% to 10%. | |||
| Rainbow trout (Oncorhynchus mykiss) | Reduction in mortality from 100% to 70% after 2 weeks. | [98] | ||
| Photobacterium damselae subsp. Damselae | Longfin yellowtail (Seriola rivoliana) | Immersion | Increased egg hatch rate from 50% to 80%. | [70] |
| Pseudomonas aeruginosa | African catfish (Clarias gariepinus) | Locally on skin lesions | A seven-fold reduction in the size of the lesions. | [64] |
| Pseudomonas plecoglossicida | Aju sweetfish (Plecoglossus altivelis) | Feed | Reduction of mortality by 42.5% when bacteriophages were administered at the time of infection in 10 g fish; in 2.4 g fish by 78% and 67% depending on time of administration. | [99] |
| 1. Reduction in mortality by 40% and 73% with the cocktail; 2. Mortality reduced by 73% and 63% depending on the bacteriophage used; 3. Field infection- reduction in mortality from 18 kg per day to 6 kg after 3 applications of bacteriophage. | [100] | |||
| Streptococcus agalactiae | Nile tilapia (Oreochromis niloticus) | Intraperitoneal injection | A 3-day delay and 40% reduction in mortality. | [63] |
| Streptococcus iniae | Japanese flounder (Paralichthys olivaceus) | Intraperitoneal injection | The decrease in mortality by 28–90% depending on the dose and time of administration. | [101] |
| Streptococcus parauberis | Japanese flounder (Paralichthys olivaceus) | Feed | Improved fish growth, reduced bacterial detection and improved breeding survival. | [102] |
| Vibrio alginolyticus | Atlantic cod (Gadus morhua) | Immersion | Mortality delay; no statistically significant differences at the end of the experiment. | [103] |
| Atlantic salmon (Salmo salar) | 1. Reduction in mortality from 93% to 0–30% depending on the dose of bacteriophages under experimental conditions; 2. Reduction in mortality from 40% to 0% in breeding conditions. | [104] | ||
| Artemia salina | The total load of bacteria decreased by 93%. | [105] | ||
| Japanese sea cucumber (Apostichopus japonicus) | Feed | 70%, 47% and 44% reduction in mortality after using a cocktail depending on the dose; no difference in survival compared to the use of antibiotics. | [106] | |
| New Zealand rock oyster (Saccostrea glomerata) | Immersion | Reduction of larvae mortality by 50% after using a cocktail. | [69] | |
| Turbot (Scophthalmus maximus) | Mortality delay; no statistically significant differences at the end of the experiment. | [103] | ||
| Vibrio anguillarum | Zebrafish (Danio rerio) | Immersion | Mortality reduced from 17% to 3%. | [107] |
| Vibrio campbellii | Artemia franciscana | Immersion | Survival of nauplii increased by 24%. | [108] |
| Vibrio coralliilyticus | Pacyfic oyster (Crassostrea gigas) | Immersion | Reduction in larvae mortality after prophylactic use. | [109] |
| Vibro cyclitrophicus | Japanese sea cucumber (Apostichopus japonicus) | Feed | Mortality reduced from 81% to 18%. | [62] |
| Injection into the body cavity | Mortality reduced from 58% to 18%. | |||
| Immersion | Mortality reduced from 63% to 18%. | |||
| Vibrio harveyi | Brine shrimp (Artemia franciscana) | Immersion | Bacteriophage cocktails enhanced hatching success (100%, control groups had a hatching success of around 50%) and survival rate (85–89%, control groups survival rate was 40–50%). | [110] |
| Artemia salina | Larval mortality decreased 24 h post-infection. | [111] | ||
| Giant tiger prawn (Penaeus monodon) | Immersion | Larval mortality decreased by ∽43%. | [112] | |
| Reduction in mortality of larvae by 20% compared with antibiotic therapy. | [113] | |||
| 1. In experimental infection, larvae mortality decreased by 55%; 2. With a natural outbreak, larvae mortality decreased by 69% compared to the untreated control, by 46% compared to the antibiotic treatment group. | [114] | |||
| Reduction of larvae mortality by 50%. | [115] | |||
| Greenlip abalone (Haliotis laevigata) | Bath | Reduction in mortality of 70% compared with the control group. | [116] | |
| Turbot (Scophthalmus maximus) | Feed | Reduction in mortality by 58–28% depending on bacteriophage dose. | [117] | |
| Zebrafish (Danio rerio) | Intraperitoneal injection | 1. Reduction in mortality by 27.7–33.3% depending on infectious dose with prophylactic bacteriophage application; 2. Reduction in mortality by 13.3–26.7% depending on infectious dose with therapeutic bacteriophage application. | [118] | |
| Vibrio parahaemolyticus | Artemia franciscana | Immersion | Increase in breeding success and larval survival both when using a single bacteriophage and a cocktail. | [110] |
| Depending on the bacterial strain, larval mortality decreased by 35% or to a level comparable to the uninfected control. | [68] | |||
| Blue mussel (Mytilus edulus) | Immersion | Reduction of the bacteria number to undetectable levels in the tissues. | [67] | |
| Giant tiger prawn (Penaeus monodon) | Feed | Reduction in mortality of 40–45% by single bacteriophage and 50% by a cocktail. | [119] | |
| Whiteleg shrimp (Litopenaeus vannamei) | Immersion | Reduction in mortality of larvae in 18–21% depending on the bacteriophage; delayed therapy resulted in decreased larval survival. | [120] | |
| Reduction in mortality of 75 and 50% depending on time after prophylactic use; no effect of therapeutic use. | [121] | |||
| Feed | Reduction in mortality of 50% for prophylactic use, no effect of therapeutic use. | |||
| 20–40% dose-dependent reduction in mortality. | [122] | |||
| Vibrio splendidus | Japanese sea cucumber (Apostichopus japonicus) | Feed | Reduction in mortality of 32–47% by a single bacteriophage, and 64% by a cocktail. | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, P.; Pajdak-Czaus, J.; Siwicki, A.K. In Vivo Bacteriophages’ Application for the Prevention and Therapy of Aquaculture Animals–Chosen Aspects. Animals 2022, 12, 1233. https://doi.org/10.3390/ani12101233
Schulz P, Pajdak-Czaus J, Siwicki AK. In Vivo Bacteriophages’ Application for the Prevention and Therapy of Aquaculture Animals–Chosen Aspects. Animals. 2022; 12(10):1233. https://doi.org/10.3390/ani12101233
Chicago/Turabian StyleSchulz, Patrycja, Joanna Pajdak-Czaus, and Andrzej Krzysztof Siwicki. 2022. "In Vivo Bacteriophages’ Application for the Prevention and Therapy of Aquaculture Animals–Chosen Aspects" Animals 12, no. 10: 1233. https://doi.org/10.3390/ani12101233
APA StyleSchulz, P., Pajdak-Czaus, J., & Siwicki, A. K. (2022). In Vivo Bacteriophages’ Application for the Prevention and Therapy of Aquaculture Animals–Chosen Aspects. Animals, 12(10), 1233. https://doi.org/10.3390/ani12101233






