Performance, Blood Lipid Profile, and the Expression of Growth Hormone Receptor (GHR) and Insulin-like Growth Factor-1 (IGF-1) Genes in Purebred and Crossbred Quail Lines
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Management and Experimental Design
2.2. Body Weight, Feed Intake, and Carcass Traits
2.3. Blood Sampling and Lipid Profiles
2.4. Analysis of mRNA Expression of GHR and IGF-1 Genes
2.5. Statistical Analyses
3. Results
3.1. Growh Performance and Carcass Traits
3.2. Blood Lipid Profile
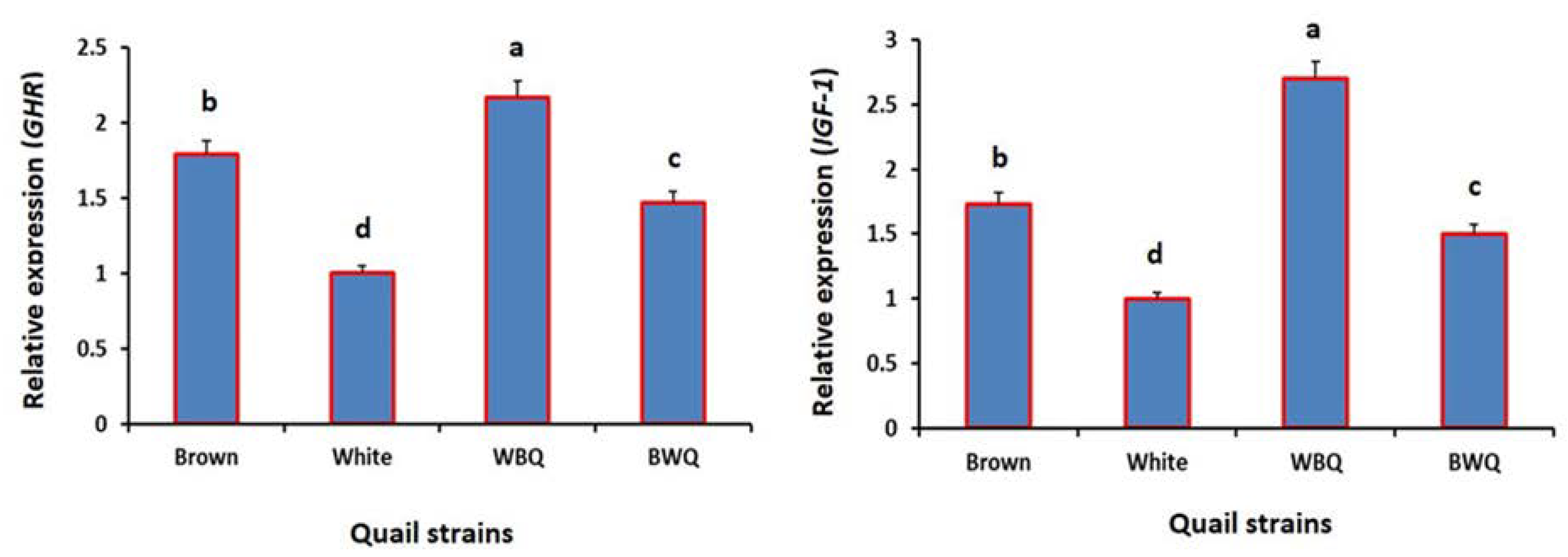
3.3. Relative Gene Expressions
3.4. Heterosis Percentages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Türkmut, L.; Altan, Ö.; Oğuz, İ.; Yalçin, S. Effects of selection for four week body weight on slaughter, carcass, and abdominal fat and some organ weights and blood serum parameters in Japanese quail. Turk. J. Vet. Anim. Sci. 1999, 23, 63–68. [Google Scholar]
- Vali, N. Growth, feed consumption and carcass composition of Coturnix japonica, Coturnix ypsilophorus and their reciprocal crosses. Asian J. Poult. Sci. 2009, 3, 132–137. [Google Scholar] [CrossRef]
- Özsoy, A.N.; Aktan, S. Estimation of genetic parameters for body weight and egg weight traits in Japanese quails. Trends Anim. Vet. Sci. 2011, 2, 17–20. [Google Scholar]
- Ojedapo, L.; Amao, S. Sexual dimorphism on carcass characteristics of Japanese quail (Coturnix Coturnix japonica) reared in derived Savanna zone of Nigeria. Int. J. Environ. Sci. Technol. 2014, 3, 250–257. [Google Scholar]
- Saatci, M.; Omed, H.; Ap Dewi, I. Genetic parameters from univariate and bivariate analyses of egg and weight traits in Japanese quail. Poult. Sci. 2006, 85, 185–190. [Google Scholar] [CrossRef]
- Narinc, D.; Karaman, E.; Aksoy, T.; Firat, M.Z. Genetic parameter estimates of growth curve and reproduction traits in Japanese quail. Poult. Sci. 2014, 93, 24–30. [Google Scholar] [CrossRef]
- Li, Y.; van der Werf, J.H.; Kinghorn, B.P. Optimization of a crossing system using mate selection. Genet. Sel. Evol. 2006, 38, 147–165. [Google Scholar] [CrossRef]
- Dickerson, G. Manual for evaluation of breeds and crosses of domestic animals. In Food and Agriculture Organization of the United Nations; United Nations: Rome, Italy, 1992; p. 47. [Google Scholar]
- Ferdous, C. Study on Body Weight and Growth in Japanese Quail. Master’s Thesis, Bangladesh Agricultural University, Mymensingh, Bangladesh, 2013. [Google Scholar]
- Abasht, B.; Lamont, S. Genome-wide association analysis reveals cryptic alleles as an important factor in heterosis for fatness in chicken F2 population. Anim. Genet. 2007, 38, 491–498. [Google Scholar] [CrossRef]
- Kaeppler, S. Heterosis: Many genes, many mechanisms—End the search for an undiscovered unifying theory. Int. Sch. Res. Not. 2012, 2012, 682824. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.; Price, S.; Siegel, P. Heterosis of growth and reproductive traits in fowl. Poult. Sci. 2002, 81, 1109–1112. [Google Scholar] [CrossRef]
- Wang, W.; Ouyang, K.; Ouyang, J.; Li, H.; Lin, S.; Sun, H. Polymorphism of insulin-like growth factor I gene in six chicken breeds and its relationship with growth traits. Asian-Australas. J. Anim. Sci. 2004, 17, 301–304. [Google Scholar] [CrossRef]
- Sun, D.-X.; Wang, D.; Yu, Y.; Zhang, Y. Cloning and characterization of liver cDNAs that are differentially expressed between chicken hybrids and their parents. Asian-Australas. J. Anim. Sci. 2005, 18, 1684–1690. [Google Scholar]
- Kita, K.; Nagao, K.; Okumura, J. Nutritional and tissue specificity of IGF-I and IGFBP-2 gene expression in growing chickens-A review. Asian-Australas. J. Anim. Sci. 2005, 18, 747–754. [Google Scholar] [CrossRef]
- Su, Y.; Shu, J.; Zhang, M.; Zhang, X.; Shan, Y.; Li, G.; Yin, J.; Song, W.; Li, H.; Zhao, G. Association of chicken growth hormone polymorphisms with egg production. Genet. Mol. Res. 2014, 13, 4893–4903. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.T.L.; Kunhareang, S.; Duangjinda, M. Association of chicken growth hormones and insulin-like growth factor gene polymorphisms with growth performance and carcass traits in Thai broilers. Asian-Australas. J. Anim. Sci. 2015, 28, 1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurtry, J.; Francis, G.; Upton, Z. Insulin-like growth factors in poultry. Domest. Anim. Endocrinol. 1997, 14, 199–229. [Google Scholar] [CrossRef]
- Boschiero, C.; Jorge, E.C.; Ninov, K.; Nones, K.; do Rosário, M.F.; Coutinho, L.L.; Ledur, M.C.; Burt, D.W.; Moura, A.S.A. Association of IGF1 and KDM5A polymorphisms with performance, fatness and carcass traits in chickens. J. Appl. Genet. 2013, 54, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Brake, J.; Havenstein, G.; Scheideler, S.; Ferket, P.; Rives, D. Relationship of sex, age, and body weight to broiler carcass yield and offal production. Poult. Sci. 1993, 72, 1137–1145. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Bauer, J. Clinical Laboratory Methods, 9th ed.; CV Mosby Company: St. Louis, MO, USA, 1982; Volume 33, p. 555. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gasparino, E.; Del Vesco, A.; Voltolini, D.; Nascimento, C.D.; Batista, E.; Khatlab, A.; Grieser, D.; Zancanela, V.; GuimarÃEs, S. The effect of heat stress on GHR, IGF-I, ANT, UCP and COXIII mRNA expression in the liver and muscle of high and low feed efficiency female quail. Br. Poult. Sci. 2014, 55, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, G. Experimental approaches to utilizing breed resources. Anim. Breed. Abstr. 1969, 37, 191–202. [Google Scholar]
- Abou Khadiga, G.; Mahmoud, B.Y.; El-Full, E. Genetic evaluation of a crossbreeding experiment included two selected lines of Japanese quail and their crosses for some growth and maturity-related traits. Egypt. Poult. Sci. 2014, 34, 831–848. [Google Scholar]
- Devi, K.S.; Gupta, B.R.; Prakash, M.G.; Qudratullah, S.; Reddy, A.R. Genetic studies on growth and production traits in two strains of Japanese quails. Tamilnadu J. Vet. Anim. Sci. 2010, 6, 223–230. [Google Scholar]
- Kumari, B.P.; Gupta, B.R.; Prakash, M.G.; Reddy, A.R.; Reddy, K.S. Genetic studies on productive and reproductive traits of Japanese quails. Indian J. Anim. Res. 2009, 43, 267–270. [Google Scholar]
- Moritsu, Y.; Nestor, K.; Noble, D.; Anthony, N.; Bacon, W. Divergent selection for body weight and yolk precursor in Coturnix Coturnix japonica. 12. Heterosis in reciprocal crosses between divergently selected lines. Poult. Sci. 1997, 76, 437–444. [Google Scholar] [CrossRef]
- Varkoohi, S.; Babak, M.M.S.; Pakdel, A.; Javaremi, A.N.; Zaghari, M.; Kause, A. Response to selection for feed conversion ratio in Japanese quail. Poult. Sci. 2010, 89, 1590–1598. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Mehmood, S.; Hussain, J.; Ishaq, H.M.; Abbas, Y.; Akram, M. Comparison of six-week growth performance in four different strains of Japanese quail (Coturnix Coturnix japonica). Sarhad J. Agric. 2015, 31, 59–64. [Google Scholar]
- Akram, M.; Rehman, Z.; Mahmood, A.; Javed, K.; Sahota, A.; Jaspal, M. Comparative productive performance of Japanese quail from different local and imported flocks. In Proceedings of the 33rd World Poultry Congress, Brisbane, Australia, 30 June–4 July 2008. [Google Scholar]
- Portillo Loera, J.J.; Carmona Medero, M.; Castro Tamayo, C.B.; Estrada Angulo, A.; Francisco Obregon, J.; Rios Rincon, F.G. Productive traits and reproductive performance of pure or crossbreed Japanese quail strains. Rev. Cient. Fac. Cienc. Vet. Univ. Zulia 2011, 21, 64–71. [Google Scholar]
- Ali, A.; Dewanti, R.; Wati, A.; Nuhriawangsa, A.; Cahyadi, M. Characteristics of carcass and non-carcass in F1 population crossbred brown and black Japanese quails. IOP Conf. Ser. Earth Environ. Sci. 2019, 387, 012045. [Google Scholar] [CrossRef]
- Dewanti, R.; Harminanti, I.; Widyas, N.; Cahyadi, M. The effects of plumage color lines and sex on slaughter weight and carcass parts of Japanese quail. IOP Conf. Ser. Mater. Sci. Eng. 2019, 633, 012024. [Google Scholar] [CrossRef] [Green Version]
- Jatoi, A.; Sahota, A.; Akram, M.; Javed, K.; Hussain, J.; Mehmood, S.; Jaspal, M. Response of different body weights on blood serum chemistry values in four close-bred flocks of adult Japanese quails (Coturnix Coturnix japonica). JAPS J. Anim. Plant Sci. 2013, 23, 35–39. [Google Scholar]
- Farahat, G.; Mahmoud, B.; El-Komy, E.; El-Full, E. Alterations in plasma constituents, growth and egg production traits due to selection in three genotypes of Japanese quail. J. Agric. Sci. 2018, 156, 118–126. [Google Scholar] [CrossRef]
- Rezvannejad, E. Productive, reproductive performance and biochemical parameters of short-term divergently selected Japanese quail lines and their reciprocal crosses. J. Livest. Sci. Technol. 2014, 2, 35–42. [Google Scholar]
- Gasparino, E.; Voltolini, D.M.; Del Vesco, A.P.; Guimarães, S.E.F.; do Nascimento, C.S.; de Oliveira Neto, A.R. IGF-I, GHR and UCP mRNA expression in the liver and muscle of high-and low-feed-efficiency laying Japanese quail at different environmental temperatures. Livest. Sci. 2013, 157, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Del Vesco, A.P.; Gasparino, E.; Grieser, D.O.; Zancanela, V.; Voltolini, D.M.; Khatlab, A.S.; Guimarães, S.E.F.; Soares, M.A.M.; Neto, A.R.O. Effects of methionine supplementation on the expression of protein deposition-related genes in acute heat stress-exposed broilers. PLoS ONE 2015, 10, e0115821. [Google Scholar] [CrossRef] [Green Version]
- El Sabry, M.I.; Eshak, M.G.; Stino, F.K.; Bondioli, K.R. Comparing growth, immune and pigmentation related gene expression in three lines of Japanese and wild European quail. Anim. Sci. Pap. Rep. 2017, 35, 407–418. [Google Scholar]
- Jia, J.; Ahmed, I.; Liu, L.; Liu, Y.; Xu, Z.; Duan, X.; Li, Q.; Dou, T.; Gu, D.; Rong, H.; et al. Selection for growth rate and body size have altered the expression profiles of somatotropic axis genes in chickens. PLoS ONE 2018, 13, e0195378. [Google Scholar] [CrossRef]
- Scanes, C.G. Perspectives on the endocrinology of poultry growth and metabolism. Gen. Comp. Endocrinol. 2009, 163, 24–32. [Google Scholar] [CrossRef]
- Tesseraud, S.; Métayer-Coustard, S.; Boussaid, S.; Crochet, S.; Audouin, E.; Derouet, M.; Seiliez, I. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cells. Biochem. Biophys. Res. Commun. 2007, 357, 181–186. [Google Scholar] [CrossRef]
- Goddard, C.; Wilkie, R.; Dunn, I. The relationship between insulin-like growth factor-1, growth hormone, thyroid hormones and insulin in chickens selected for growth. Domest. Anim. Endocrinol. 1988, 5, 165–176. [Google Scholar] [CrossRef]
- Beccavin, C.; Chevalier, B.; Cogburn, L.; Simon, J.; Duclos, M.J. Insulin-like growth factors and body growth in chickens divergently selected for high or low growth rate. J. Endocrinol. 2001, 168, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, J.; Okamoto, S.; Kobayashi, S.; Wada, Y.; Maeda, Y. Study of heterosis effects on productive traits of Japanese quail, 1: Heterosis effects on the crosses between large line and randombred population. J. Poult. Sci. 2002, 39, 139–146. [Google Scholar]
- Marks, H.L. Divergent Selection for Growth in Japanese Quail Under Split and Complete Nutritional Environments: 6. Differential Body Weights in Reciprocal Crosses. Poult. Sci. 1993, 72, 1847–1854. [Google Scholar] [CrossRef]
- Sato, K.; Fukuda, H.; Hedianto, Y.E.; Ino, T. Heterosis for Reproductive Traits in Reciprocal Crosses of Highly Inbred Lines of Japanese Quail. Jpn. Poult. Sci. 1989, 26, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Aboul-Seoud, D. Crossbreeding Effects among Two Selected Lines for Increased Feed and Water Consumption on Some Productive and Reproductive Traits in Japanese quail. Middle East J. 2016, 5, 752–760. [Google Scholar]
- McCann-Levorse, L.M.; Radecki, S.V.; Donoghue, D.J.; Malamed, S.; Foster, D.N.; Scanes, C.G. Ontogeny of pituitary growth hormone and growth hormone mRNA in the chicken. Exp. Biol. Med. 1993, 202, 109–113.51. [Google Scholar] [CrossRef]

| Item | g/kg DM of Feed |
|---|---|
| Ingredients | |
| Yellow corn | 556.0 |
| Soybean meal (44%CP) | 288.0 |
| Corn gluten meal (60% CP) | 105.0 |
| Vita. and Min. mix.† | 3.0 |
| DL-Methionine | 1.0 |
| L-lysine | 4.0 |
| Wheat bran | 20.0 |
| Limestone | 19.0 |
| Salt (NaCl) | 4.0 |
| Calculated chemical composition (%) | |
| ME (kcal/kg) | 2902.4 |
| CF | 3.87 |
| CP | 24.01 |
| Na | 0.17 |
| Ca | 0.82 |
| Available phosphorus | 0.41 |
| Methionine | 0.56 |
| Lysine | 1.39 |
| Gene | Primer Sequences (5′-3′) | Reference | Cycle Profile |
|---|---|---|---|
| GHR | F: AACACAGATACCCAACAGCC R: AGAAGTCAGTGTTTGTCAGGG | Gasparino et al. [24] | 95 °C for 10 min, 40 cycles 95 °C for 15 s (denaturation), 60 °C for 1 min (annealing and extension) |
| IGF-1 | F: CACCTAAATCTGCACGCT R: CTTGTGGATGGCATGATCT | ||
| β-actin | F: ACCCCAAAGCCAACAGA R: CCAGAGTCCATCACAATACC |
| Parameters | Quail Lines | |||||
|---|---|---|---|---|---|---|
| Brown | White | 1 WBQ | 2 BWQ | 3 SEM | p-Value | |
| Final BW (g) | 198 b | 229 a | 204 b | 205 b | 2.318 | <0.001 |
| 4 ADG (g/day) | 4.56 b | 5.28 a | 4.69 b | 4.73 b | 0.055 | <0.001 |
| 5 ADFI (g/day) | 17.95 b | 20.78 a | 17.23 c | 16.52 d | 1.112 | <0.001 |
| 6 FCR | 3.95 a | 3.94 a | 3.69 b | 3.58 b | 0.052 | 0.001 |
| Percentages | Quail Lines | |||||
|---|---|---|---|---|---|---|
| Brown | White | 1 WBQ | 2 BWQ | 3 SEM | p-Value | |
| Dressing | 65.82 b | 68.09 b | 73.62 a | 72.83 a | 2.145 | 0.04 |
| Gizzard | 3.03 a | 2.80 a | 2.35 b | 2.40 b | 0.108 | 0.01 |
| Heart | 0.83 | 0.78 | 0.85 | 0.86 | 0.055 | 0.85 |
| Intestine | 4.50 | 4.90 | 4.20 | 4.10 | 0.218 | 0.20 |
| Liver | 2.30 | 2.55 | 2.10 | 2.30 | 0.243 | 0.83 |
| Spleen | 0.06 | 0.09 | 0.10 | 0.07 | 0.012 | 0.06 |
| Parameters | Quail Lines | |||||
|---|---|---|---|---|---|---|
| Brown | White | 1 WBQ | 2 BWQ | 3 SEM | p-Value | |
| Cholesterol (mg/dL) | 238.3 | 174.0 | 199.8 | 185.3 | 16.38 | 0.11 |
| Triglycerides (mg/dL) | 345 a | 240 b | 293 ab | 237 b | 21.2 | 0.05 |
| 4 HDL (mg/dL) | 83.28 | 58.78 | 86.53 | 75.31 | 9.332 | 0.25 |
| 5 LDL (mg/dL) | 86.32 | 67.14 | 54.12 | 62.53 | 8.505 | 0.59 |
| 6 VLDL (mg/dL) | 69.24 a | 48.36 b | 58.62 ab | 47.97 b | 4.725 | 0.05 |
| Items | Crossbred Quails | |
|---|---|---|
| 1 WBQ | 2 BWQ | |
| Final BW | −4.39 | −3.90 |
| 3 ADG | −4.67 | −3.86 |
| 4 ADFI | −10.87 | −14.59 |
| 5 FCR | −6.46 | −9.25 |
| Dressing % | 7.54 | 6.38 |
| Gizzard % | −19.52 | −17.80 |
| Heart % | 4.94 | 6.17 |
| Intestine % | −10.64 | −12.77 |
| Liver % | −13.58 | −5.35 |
| Spleen % | 33.34 | −6.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafik, B.M.; Kamel, E.R.; Mamdouh, M.; Elrafaay, S.; Nassan, M.A.; El-Bahy, S.M.; El-Tarabany, M.S.; Manaa, E.A. Performance, Blood Lipid Profile, and the Expression of Growth Hormone Receptor (GHR) and Insulin-like Growth Factor-1 (IGF-1) Genes in Purebred and Crossbred Quail Lines. Animals 2022, 12, 1245. https://doi.org/10.3390/ani12101245
Shafik BM, Kamel ER, Mamdouh M, Elrafaay S, Nassan MA, El-Bahy SM, El-Tarabany MS, Manaa EA. Performance, Blood Lipid Profile, and the Expression of Growth Hormone Receptor (GHR) and Insulin-like Growth Factor-1 (IGF-1) Genes in Purebred and Crossbred Quail Lines. Animals. 2022; 12(10):1245. https://doi.org/10.3390/ani12101245
Chicago/Turabian StyleShafik, Basant M., Eman R. Kamel, Maha Mamdouh, Shimaa Elrafaay, Mohamed A. Nassan, Salah M. El-Bahy, Mahmoud S. El-Tarabany, and Eman A. Manaa. 2022. "Performance, Blood Lipid Profile, and the Expression of Growth Hormone Receptor (GHR) and Insulin-like Growth Factor-1 (IGF-1) Genes in Purebred and Crossbred Quail Lines" Animals 12, no. 10: 1245. https://doi.org/10.3390/ani12101245






