Staphylococcus aureus and Methicillin-Resistant Coagulase-Negative Staphylococci in Nostrils and Buccal Mucosa of Healthy Camels Used for Recreational Purposes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
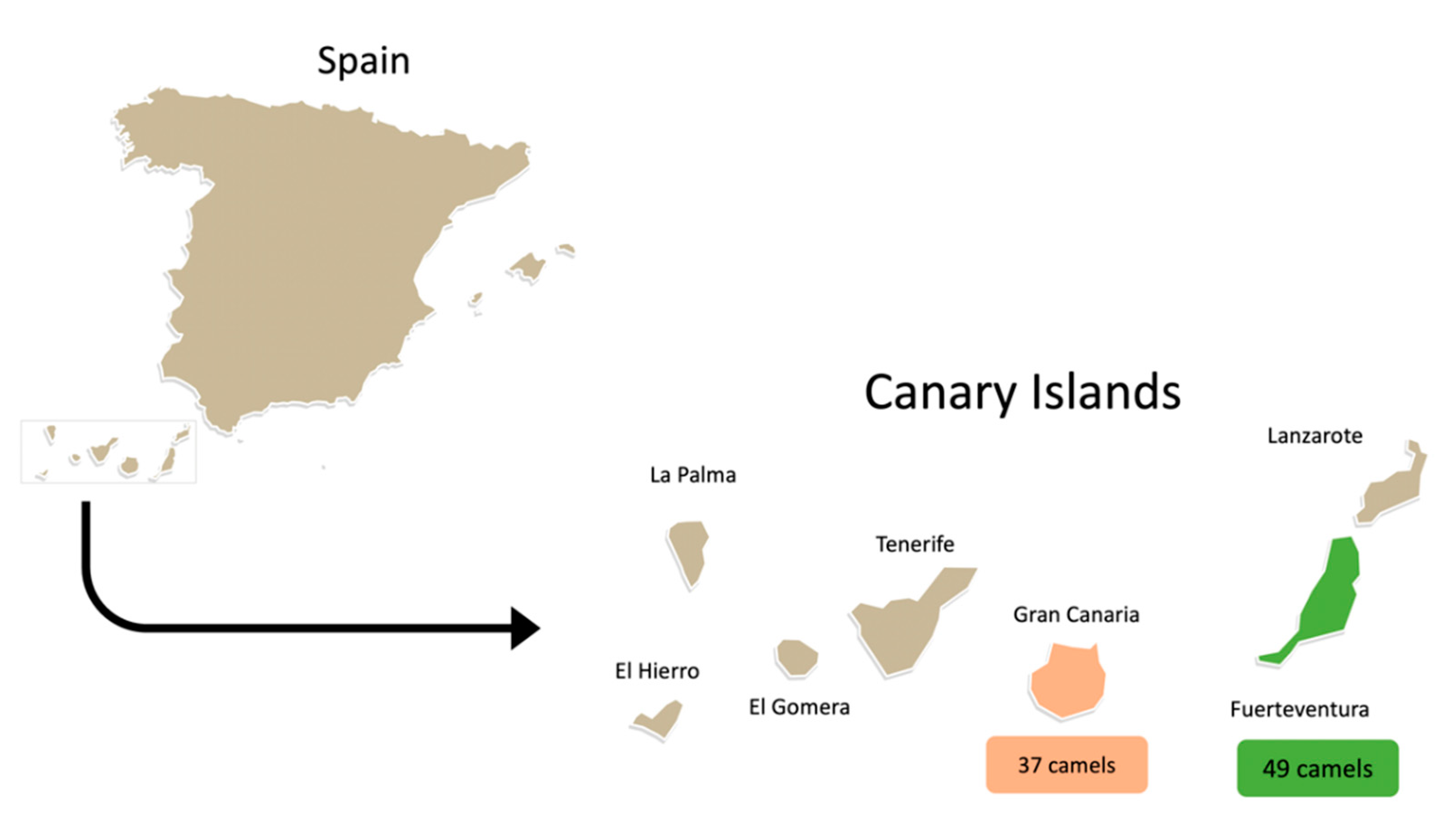
2.1. Animals and Bacterial Isolates
2.2. Phenotypic Antimicrobial Resistance
2.3. Antimicrobial Resistance and Virulence Genes
2.4. Molecular Typing
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, L.C.; Cawthorn, D. SPECIES OF MEAT ANIMALS|Game and Exotic Animals; Dikeman, M., Devine, C.B.T.-E., Second, E., Eds.; Academic Press: Oxford, UK, 2014; pp. 345–356. ISBN 978-0-12-384734-8. [Google Scholar]
- Suliman, G.M.; Alowaimer, A.N.; Hussein, E.O.; Ali, H.S.; Abdelnour, S.A.; El-Hack, M.E.A.; Swelum, A.A. Chemical Composition and Quality Characteristics of Meat in Three One-Humped Camel (Camelus dromedarius) Breeds as Affected by Muscle Type and Post-Mortem Storage Period. Animals 2019, 9, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatoon, H.; Najam, R. Chapter 29—Bioactive Components in Camel Milk: Their Nutritive Value and Therapeutic Application; Watson, R.R., Collier, R.J., Preedy, V.R.B.T.-N., Eds.; Academic Press: Oxford, UK, 2017; pp. 377–387. ISBN 978-0-12-809762-5. [Google Scholar]
- Ben Chehida, F.; Gharsa, H.; Tombari, W.; Selmi, R.; Khaldi, S.; Daaloul, M.; Ben Slama, K.; Messadi, L. First Report of Antimicrobial Susceptibility and Virulence Gene Characterization Associated with Staphylococcus aureus Carriage in Healthy Camels from Tunisia. Animals 2021, 11, 2754. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Navarro, S.; Cerdà-Cuéllar, M.; Ayats, T.; Jordá, J.; Marin, C.; Vega, S. Characterization of Salmonella Frintrop isolated from dromedary camels (Camelus dromedarius). Transbound. Emerg. Dis. 2021, 68, 742–746. [Google Scholar] [CrossRef]
- Wilson, R.T.; Gutierrez, C. The one-humped camel in the Canary Islands: History and present status. Tropicultura 2015, 33, 288–298. [Google Scholar]
- Sallam, A.M. Future opportunities for genetic improvement of the egyptian camels. Egypt. J. Anim. Prod. 2020, 57, 39–45. [Google Scholar]
- Iglesias Pastrana, C.; Navas González, F.J.; Ciani, E.; González Ariza, A.; Delgado Bermejo, J.V. A tool for functional selection of leisure camels: Behaviour breeding criteria may ensure long-term sustainability of a European unique breed. Res. Vet. Sci. 2021, 140, 142–152. [Google Scholar] [CrossRef]
- Elhariri, M.; Hamza, D.; Elhelw, R.; Dorgham, S.M. Extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in camel in Egypt: Potential human hazard. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Tejedor-Junco, M.T.; Gonzalez-Martin, M.; González, N.F.R.; Gutiérrez, C. Identification, antimicrobial susceptibility, and virulence factors of Enterococcus spp. strains isolated from Camels in Canary Islands, Spain. Vet. Ital. 2015, 5, 179–183. [Google Scholar]
- Carvalho, I.; Tejedor-Junco, M.T.; González-Martín, M.; Corbera, J.A.; Silva, V.; Igrejas, G.; Torres, C.; Poeta, P. Escherichia coli Producing Extended-Spectrum β-lactamases (ESBL) from Domestic Camels in the Canary Islands: A One Health Approach. Animals 2020, 10, 1295. [Google Scholar] [CrossRef]
- Abed, A.H.; Hegazy, E.F.; Omar, S.A.; El-Baky, R.M.A.; El-Beih, A.A.; Al-Emam, A.; Menshawy, A.M.S.; Khalifa, E. Carvacrol Essential Oil: A Natural Antibiotic against Zoonotic Multidrug-Resistant Staphylococcus Species Isolated from Diseased Livestock and Humans. Antibiotics 2021, 10, 1328. [Google Scholar] [CrossRef]
- Haag, A.F.; Fitzgerald, J.R.; Penadés, J.R. Staphylococcus aureus in Animals. Gram-Posit. Pathog. 2019, 7, 731–746. [Google Scholar]
- Alzohairy, M.A. Colonization and antibiotic susceptibility pattern of methicillin resistance Staphylococcus aureus (MRSA) among farm animals in Saudi Arabia. Afr. J. Bacteriol. Res. 2011, 3, 63–68. [Google Scholar]
- Agabou, A.; Ouchenane, Z.; Essebe, C.N.; Khemissi, S.; Chehboub, M.T.E.; Chehboub, I.B.; Sotto, A.; Dunyach-Remy, C.; Lavigne, J.-P. Emergence of Nasal Carriage of ST80 and ST152 PVL+ Staphylococcus aureus Isolates from Livestock in Algeria. Toxins 2017, 9, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestergaard, M.; Leng, B.; Haaber, J.K.; Bojer, M.S.; Vegge, C.S.; Ingmer, H. Genome-Wide Identification of Antimicrobial Intrinsic Resistance Determinants in Staphylococcus aureus. Front. Microbiol. 2016, 7, 2018. [Google Scholar] [CrossRef] [Green Version]
- WHO. World Health Organization Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; Geneva, Switzerland, 2017; Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 22 November 2021).
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-negative staphylococci pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef]
- Marincola, G.; Liong, O.; Schoen, C.; Abouelfetouh, A.; Hamdy, A.; Wencker, F.D.R.; Marciniak, T.; Becker, K.; Köck, R.; Ziebuhr, W. Antimicrobial resistance profiles of coagulase-negative staphylococci in community-based healthy individuals in Germany. Front. Public Health 2021, 9, 796. [Google Scholar] [CrossRef]
- Silva, V.; Ferreira, E.; Manageiro, V.; Reis, L.; Tejedor-Junco, M.T.; Sampaio, A.; Capelo, J.L.; Caniça, M.; Igrejas, G.; Poeta, P. Distribution and Clonal Diversity of Staphylococcus aureus and Other Staphylococci in Surface Waters: Detection of ST425-t742 and ST130-t843 mecC-Positive MRSA Strains. Antibiotics 2021, 10, 1416. [Google Scholar] [CrossRef]
- Silva, V.; Lopes, A.F.; Soeiro, V.; Caniça, M.; Manageiro, V.; Pereira, J.E.; Maltez, L.; Capelo, J.L.; Igrejas, G.; Poeta, P. Nocturnal Birds of Prey as Carriers of Staphylococcus aureus and Other Staphylococci: Diversity, Antimicrobial Resistance and Clonal Lineages. Antibiotics 2022, 11, 240. [Google Scholar] [CrossRef]
- Suepaul, S.; Georges, K.; Unakal, C.; Boyen, F.; Sookhoo, J.; Ashraph, K.; Yusuf, A.; Butaye, P. Determination of the frequency, species distribution and antimicrobial resistance of staphylococci isolated from dogs and their owners in Trinidad. PLoS ONE 2021, 16, e0254048. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, G.-B.; Yang, S.-J. Co-occurrence of cfr-mediated linezolid-resistance in ST398 LA-MRSA and non-aureus staphylococci isolated from a pig farm. Vet. Microbiol. 2022, 266, 109336. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, Q.; Cheng, Y.; Wen, G.; Luo, Q.; Shao, H.; Zhang, T. High concentration of coagulase-negative staphylococci carriage among bioaerosols of henhouses in Central China. BMC Microbiol. 2020, 20, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caroline, M.; dos, S.A.; Ruili, J.; Helena, A.G.; Manuela, A.; Jean-François, P. Complete Genome Sequences of the Potential Zoonotic Pathogens Staphylococcus felis and Staphylococcus kloosii. Genome Announc. 2022, 6, e00404-18. [Google Scholar] [CrossRef] [Green Version]
- Paul, N.C.; Bärgman, S.C.; Moodley, A.; Nielsen, S.S.; Guardabassi, L. Staphylococcus pseudintermedius colonization patterns and strain diversity in healthy dogs: A cross-sectional and longitudinal study. Vet. Microbiol. 2012, 160, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Pereira, J.E.; Maltez, L.; Ferreira, E.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Diversity of methicillin-resistant staphylococci among wild Lepus granatensis: First detection of mecA-MRSA in hares. FEMS Microbiol. Ecol. 2020, 96, fiz204. [Google Scholar] [CrossRef]
- Zhu, W.; Sieradzki, K.; Albrecht, V.; McAllister, S.; Lin, W.; Stuchlik, O.; Limbago, B.; Pohl, J.; Rasheed, J.K. Evaluation of the Biotyper MALDI-TOF MS system for identification of Staphylococcus species. J. Microbiol. Methods 2015, 117, 14–17. [Google Scholar] [CrossRef]
- CLSI Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- EUCAST European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 8.0. 2018. Available online: https://eucast.org/eucast_news/news_singleview/?tx_ttnews%5Btt_news%5D=248&cHash=91e3ef09a79b333746462d8854ee016d (accessed on 22 November 2021).
- Silva, V.; Oliveira, A.; Manageiro, V.; Caniça, M.; Contente, D.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Capelo, J.L.; Igrejas, G.; et al. Clonal Diversity and Antimicrobial Resistance of Methicillin-Resistant Staphylococcus pseudintermedius Isolated from Canine Pyoderma. Microorganisms 2021, 9, 482. [Google Scholar] [CrossRef]
- Harmsen, D.; Claus, H.H.H.H.; Witte, W.; Rothgänger, J.; Claus, H.H.H.H.; Turnwald, D.; Vogel, U. Typing of Methicillin-Resistant Staphylococcus aureus in a University Hospital Setting by Using Novel Software for spa Repeat Determination and Database Management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef] [Green Version]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Shopsin, B.; Mathema, B.; Alcabes, P.; Said-Salim, B.; Lina, G.; Matsuka, A.; Martinez, J.; Kreiswirth, B.N. Prevalence of agr Sprecificity Groups amoung Staphylococcus aureus Strains Colonizing Children and Their Guardians. J. Clin. Microbiol. 2003, 41, 456–459. [Google Scholar] [CrossRef] [Green Version]
- Damborg, P.; Broens, E.M.; Chomel, B.B.; Guenther, S.; Pasmans, F.; Wagenaar, J.A.; Weese, J.S.; Wieler, L.H.; Windahl, U.; Vanrompay, D.; et al. Bacterial Zoonoses Transmitted by Household Pets: State-of-the-Art and Future Perspectives for Targeted Research and Policy Actions. J. Comp. Pathol. 2016, 155, S27–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondeau, L.D.; Deutscher, M.; Rubin, J.E.; Deneer, H.; Kanthan, R.; Sanche, S.; Blondeau, J.M. Urinary tract infection in a human male patient with Staphylococcus pseudintermedius transmission from the family dog. J. Chemother. 2021, 34, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Randad, P.R.; Larsen, J.; Kaya, H.; Pisanic, N.; Ordak, C.; Price, L.B.; Aziz, M.; Nadimpalli, M.L.; Rhodes, S.; Stewart, J.R. Transmission of antimicrobial-resistant Staphylococcus aureus clonal complex 9 between pigs and humans, United States. Emerg. Infect. Dis. 2021, 27, 740. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. The canine and feline skin microbiome in health and disease. Vet. Dermatol. 2013, 24, 137–145.e31. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.C.; Worthing, K.A.; Ward, M.P.; Norris, J.M. Commensal Staphylococci Including Methicillin-Resistant Staphylococcus aureus from Dogs and Cats in Remote New South Wales, Australia. Microb. Ecol. 2020, 79, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.T.; Kwaga, J.K.P.; Okolocha, E.C.; Bello, M. Phenotypic occurrence of methicillin-resistant Staphylococcus aureus in camels slaughtered at Kano abattoir, Kano, Nigeria. Sokoto J. Vet. Sci. 2017, 15, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; El-Deen, N.E.; El-Hariri, M. Bacteriological examination of respiratory tract of apparently healthy camels in Egypt. Int. J. 2014, 5, 65–68. [Google Scholar]
- Al-Thani, R.F.; Al-Ali, F. Incidences and antimicrobial susceptibility profile of Staphylococcus species isolated from animals in different Qatari farms. Afr. J. Microbiol. Res. 2012, 6, 7454–7458. [Google Scholar]
- Kaneko, J.; Kamio, Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004, 68, 981–1003. [Google Scholar] [CrossRef] [Green Version]
- Fountain, K.; Blackett, T.; Butler, H.; Carchedi, C.; Schilling, A.-K.; Meredith, A.; Gibbon, M.J.; Lloyd, D.H.; Loeffler, A.; Feil, E.J. Fatal exudative dermatitis in island populations of red squirrels (Sciurus vulgaris): Spillover of a virulent Staphylococcus aureus clone (ST49) from reservoir hosts. Microb. Genom. 2021, 7, 565. [Google Scholar] [CrossRef]
- Silva, V.; Gabriel, S.I.; Borrego, S.B.; Tejedor-Junco, M.T.; Manageiro, V.; Ferreira, E.; Reis, L.; Caniça, M.; Capelo, J.L.; Igrejas, G.; et al. Antimicrobial Resistance and Genetic Lineages of Staphylococcus aureus from Wild Rodents: First Report of mecC-Positive Methicillin-Resistant S. aureus (MRSA) in Portugal. Animals 2021, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Sahin-Tóth, J.; Albert, E.; Juhász, A.; Ghidán, Á.; Juhász, J.; Horváth, A.; Steward, M.C.; Dobay, O. Prevalence of Staphylococcus aureus in wild hedgehogs (Erinaceus europaeus) and first report of mecC-MRSA in Hungary. Sci. Total Environ. 2022, 815, 152858. [Google Scholar] [CrossRef] [PubMed]
- Azzi, O.; Lai, F.; Tennah, S.; Menoueri, M.N.; Achek, R.; Azara, E.; Tola, S. Spa-typing and antimicrobial susceptibility of Staphylococcus aureus isolated from clinical sheep mastitis in Médéa province, Algeria. Small Rumin. Res. 2020, 192, 106168. [Google Scholar] [CrossRef]
- Gharsa, H.; Slama, K.B.; Gómez-Sanz, E.; Lozano, C.; Zarazaga, M.; Messadi, L.; Boudabous, A.; Torres, C. Molecular Characterization of Staphylococcus aureus from Nasal Samples of Healthy Farm Animals and Pets in Tunisia. Vector-Borne Zoonotic Dis. 2015, 15, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Gharsa, H.; Chairat, S.; Chaouachi, M.; Ben Yahia, H.; Boudabous, A.; Ben Slama, K. High diversity of genetic lineages and virulence genes of Staphylococcus aureus isolated from dairy products in Tunisia. Ann. Microbiol. 2019, 69, 73–78. [Google Scholar] [CrossRef]
- Azara, E.; Piras, M.G.; Parisi, A.; Tola, S. Antimicrobial susceptibility and genotyping of Staphylococcus aureus isolates collected between 1986 and 2015 from ovine mastitis. Vet. Microbiol. 2017, 205, 53–56. [Google Scholar] [CrossRef]
- Togashi, A.; Aung, M.S.; Yoto, Y.; Akane, Y.; Tsugawa, T.; Kawaguchiya, M.; Tsutsumi, H.; Kobayashi, N. First report of Panton–Valentine leukocidin-positive methicillin-susceptible Staphylococcus aureus ST88 harbouring ΦSa2usa isolated from refractory breast abscesses in Japan. N. Microbes N. Infect. 2016, 13, 62–64. [Google Scholar] [CrossRef] [Green Version]
- Kpeli, G.; Buultjens, A.H.; Giulieri, S.; Owusu-Mireku, E.; Aboagye, S.Y.; Baines, S.L.; Seemann, T.; Bulach, D.; da Silva, A.G.; Monk, I.R. Genomic analysis of ST88 community-acquired methicillin resistant Staphylococcus aureus in Ghana. PeerJ 2017, 5, e3047. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.O.; Mahmoud, H.Y.A.H. Epidemiological studies based on multi-locus sequence typing genotype of methicillin susceptible Staphylococcus aureus isolated from camel’s milk. Onderstepoort J. Vet. Res. 2017, 84, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Jans, C.; Merz, A.; Johler, S.; Younan, M.; Tanner, S.A.; Kaindi, D.W.M.; Wangoh, J.; Bonfoh, B.; Meile, L.; Tasara, T. East and West African milk products are reservoirs for human and livestock-associated Staphylococcus aureus. Food Microbiol. 2017, 65, 64–73. [Google Scholar] [CrossRef]
- De Martino, L.; Lucido, M.; Mallardo, K.; Facello, B.; Mallardo, M.; Iovane, G.; Pagnini, U.; Tufano, M.A.; Catalanotti, P. Methicillin-Resistant Staphylococci Isolated from Healthy Horses and Horse Personnel in Italy. J. Vet. Diagn. Investig. 2010, 22, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugwu, C.C.; Gomez-Sanz, E.; Agbo, I.C.; Torres, C.; Chah, K.F. Characterization of mannitol-fermenting methicillin-resistant staphylococci isolated from pigs in Nigeria. Braz. J. Microbiol. 2015, 46, 885–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Agidi, S.; LeJeune, J.T. Diversity of staphylococcal cassette chromosome in coagulase-negative staphylococci from animal sources. J. Appl. Microbiol. 2009, 107, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [Green Version]
- Nemeghaire, S.; Vanderhaeghen, W.; Argudín, M.A.; Haesebrouck, F.; Butaye, P. Characterization of methicillin-resistant Staphylococcus sciuri isolates from industrially raised pigs, cattle and broiler chickens. J. Antimicrob. Chemother. 2014, 69, 2928–2934. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, U.; Kriegeskorte, A.; Schubert, T.; Peters, G.; Rudack, C.; Pieper, D.H.; Wos-Oxley, M.; Becker, K. The culturome of the human nose habitats reveals individual bacterial fingerprint patterns. Environ. Microbiol. 2016, 18, 2130–2142. [Google Scholar] [CrossRef]
- Loncaric, I.; Tichy, A.; Handler, S.; Szostak, M.P.; Tickert, M.; Diab-Elschahawi, M.; Spergser, J.; Künzel, F. Prevalence of Methicillin-Resistant Staphylococcus sp. (MRS) in Different Companion Animals and Determination of Risk Factors for Colonization with MRS. Antibiotics 2019, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Tsubakishita, S.; Kuwahara-Arai, K.; Sasaki, T.; Hiramatsu, K. Origin and molecular evolution of the determinant of methicillin resistance in staphylococci. Antimicrob. Agents Chemother. 2010, 54, 4352–4359. [Google Scholar] [CrossRef] [Green Version]
- Nemeghaire, S.; Argudín, M.A.; Haesebrouck, F.; Butaye, P. Molecular epidemiology of methicillin-resistant Staphylococcus sciuri in healthy chickens. Vet. Microbiol. 2014, 171, 357–363. [Google Scholar] [CrossRef]
- França, A.; Gaio, V.; Lopes, N.; Melo, L. Virulence Factors in Coagulase-Negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef]
- Zhang, K.; Sparling, J.; Chow, B.L.; Elsayed, S.; Hussain, Z.; Church, D.L.; Gregson, D.B.; Louie, T.; Conly, J.M. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J. Clin. Microbiol. 2004, 42, 4947–4955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnellmann, C.; Gerber, V.; Rossano, A.; Jaquier, V.; Panchaud, Y.; Doherr, M.G.; Thomann, A.; Straub, R.; Perreten, V. Presence of New mecA and mph(C) Variants Conferring Antibiotic Resistance in Staphylococcus spp. Isolated from the Skin of Horses before and after Clinic Admission. J. Clin. Microbiol. 2006, 44, 4444–4454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutcliffe, J.; Grebe, T.; Tait-Kamradt, A.; Wondrack, L. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 1996, 40, 2562–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Sanz, E.; Torres, C.; Lozano, C.; Fernández-Pérez, R.; Aspiroz, C.; Ruiz-Larrea, F.; Zarazaga, M. Detection, Molecular Characterization, and Clonal Diversity of Methicillin-Resistant Staphylococcus aureus CC398 and CC97 in Spanish Slaughter Pigs of Different Age Groups. Foodborne Pathog. Dis. 2010, 7, 1269–1277. [Google Scholar] [CrossRef]
- Wondrack, L.; Massa, M.; Yang, B.V.; Sutcliffe, J. Clinical strain of Staphylococcus aureus inactivates and causes efflux of macrolides. Antimicrob. Agents Chemother. 1996, 40, 992–998. [Google Scholar] [CrossRef] [Green Version]
- Lina, G.; Quaglia, A.; Reverdy, M.E.; Leclercq, R.; Vandenesch, F.; Etienne, J. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob. Agents Chemother. 1999, 43, 1062–1066. [Google Scholar] [CrossRef] [Green Version]
- Bozdogan, B.; Berrezouga, L.; Kuo, M.S.; Yurek, D.A.; Farley, K.A.; Stockman, B.J.; Leclercq, R. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 1999, 43, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Lozano, C.; Aspiroz, C.; Rezusta, A.; Gómez-Sanz, E.; Simon, C.; Gómez, P.; Ortega, C.; Revillo, M.J.; Zarazaga, M.; Torres, C. Identification of novel vga (A)-carrying plasmids and a Tn5406-like transposon in meticillin-resistant Staphylococcus aureus and Staphylococcus epidermidis of human and animal origin. Int. J. Antimicrob. Agents 2012, 40, 306–312. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Jensen, L.B.; Aarestrup, F.M. Detection of the satA gene and transferability of virginiamycin resistance in Enterococcus faecium from food-animals. FEMS Microbiol. Lett. 1998, 168, 145–151. [Google Scholar] [CrossRef]
- McLaws, F.; Chopra, I.; O’Neill, A.J. High prevalence of resistance to fusidic acid in clinical isolates of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2008, 61, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-J.; Hung, W.-C.; Tseng, S.-P.; Tsai, J.-C.; Hsueh, P.-R.; Teng, L.-J. Fusidic acid resistance determinants in Staphylococcus aureus clinical isolates. Antimicrob. Agents Chemother. 2010, 54, 4985–4991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Etienne, J.; Vandenesch, F.; Nesme, X. Relationships between Staphylococcus aureus Genetic Background, Virulence Factors, agr Groups (Alleles), and Human Disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lina, G.; Piemont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.-O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine Leukocidin - Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- van Wamel, W.J.B.; Rooijakkers, S.H.M.; Ruyken, M.; van Kessel, K.P.M.; van Strijp, J.A.G. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Isolate | Antimicrobial Resistance | Virulence | Molecular Typing | |||
|---|---|---|---|---|---|---|
| Phenotype | Genotype | ST (CC) | spa | agr | ||
| VS3140 | Susceptible | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3141 | Susceptible | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3142 | Susceptible | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3143 | Susceptible | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3144 | CIP | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3145 | Susceptible | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3146 | Susceptible | - | hla, hlb, hld | 7345 | t1773 | III |
| VS3147 | Susceptible | - | hla, hlb, hld | 88 | t3221 | III |
| VS3148 | Susceptible | - | hla, hlb, hld | 88 | t3221 | III |
| VS3149 | Susceptible | - | hla, hlb, hld | 88 | t3221 | III |
| VS3150 | Susceptible | - | hla, hlb, hld | 8 (8) | t008 | I |
| Isolate Species | Antimicrobial Resistance | Virulence Factors | ||
|---|---|---|---|---|
| Phenotype | Genotype | |||
| VS3151 | chromogenes | PEN | mecA | |
| VS3152 | epidermidis | PEN | mecA | hla, hld |
| VS3153 | hominis | PEN | mecA | |
| VS3154 | lentus | PEN, ERY, CD | mecA, mphC | hla |
| VS3155 | lentus | PEN | mecA | |
| VS3156 | lentus | PEN, FD | mecA | |
| VS3157 | lentus | PEN | mecA | |
| VS3158 | lentus | PEN | mecA | |
| VS3159 | lentus | PEN | mecA | |
| VS3160 | lentus | PEN | mecA | |
| VS3161 | lentus | PEN | mecA | |
| VS3162 | lentus | PEN | mecA | |
| VS3163 | lentus | PEN, FD | mecA | hld |
| VS3164 | lentus | PEN, FD | mecA | |
| VS3165 | lentus | PEN | mecA | |
| VS3166 | lentus | PEN, CD | mecA, mphC | |
| VS3167 | sciuri | PEN | mecA | |
| VS3168 | sciuri | PEN | mecA | |
| VS3169 | sciuri | PEN | mecA | |
| VS3170 | sciuri | PEN | mecA | |
| VS3171 | sciuri | PEN | mecA | |
| VS3172 | sciuri | PEN | mecA | |
| VS3173 | sciuri | PEN | mecA | hld |
| VS3174 | sciuri | PEN | mecA | |
| VS3175 | sciuri | PEN | mecA | |
| VS3176 | sciuri | PEN | mecA | hld |
| VS3177 | sciuri | PEN | mecA | hld |
| VS3178 | sciuri | PEN, CD, FD | mecA, mphC | |
| VS3179 | xylosus | PEN | mecA | |
| VS3180 | xylosus | PEN | mecA | |
| VS3181 | xylosus | PEN | mecA | hld |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.; Caniça, M.; Manageiro, V.; Verbisck, N.; Tejedor-Junco, M.T.; González-Martin, M.; Corbera, J.A.; Poeta, P.; Igrejas, G. Staphylococcus aureus and Methicillin-Resistant Coagulase-Negative Staphylococci in Nostrils and Buccal Mucosa of Healthy Camels Used for Recreational Purposes. Animals 2022, 12, 1255. https://doi.org/10.3390/ani12101255
Silva V, Caniça M, Manageiro V, Verbisck N, Tejedor-Junco MT, González-Martin M, Corbera JA, Poeta P, Igrejas G. Staphylococcus aureus and Methicillin-Resistant Coagulase-Negative Staphylococci in Nostrils and Buccal Mucosa of Healthy Camels Used for Recreational Purposes. Animals. 2022; 12(10):1255. https://doi.org/10.3390/ani12101255
Chicago/Turabian StyleSilva, Vanessa, Manuela Caniça, Vera Manageiro, Newton Verbisck, María Teresa Tejedor-Junco, Margarita González-Martin, Juan Alberto Corbera, Patrícia Poeta, and Gilberto Igrejas. 2022. "Staphylococcus aureus and Methicillin-Resistant Coagulase-Negative Staphylococci in Nostrils and Buccal Mucosa of Healthy Camels Used for Recreational Purposes" Animals 12, no. 10: 1255. https://doi.org/10.3390/ani12101255
APA StyleSilva, V., Caniça, M., Manageiro, V., Verbisck, N., Tejedor-Junco, M. T., González-Martin, M., Corbera, J. A., Poeta, P., & Igrejas, G. (2022). Staphylococcus aureus and Methicillin-Resistant Coagulase-Negative Staphylococci in Nostrils and Buccal Mucosa of Healthy Camels Used for Recreational Purposes. Animals, 12(10), 1255. https://doi.org/10.3390/ani12101255












