Effects of Implanting Exogenous Melatonin 40 Days before Lambing on Milk and Colostrum Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Milk Records
2.2. Milk and Colostrum Analyses
2.3. Statistical Analysis
3. Results
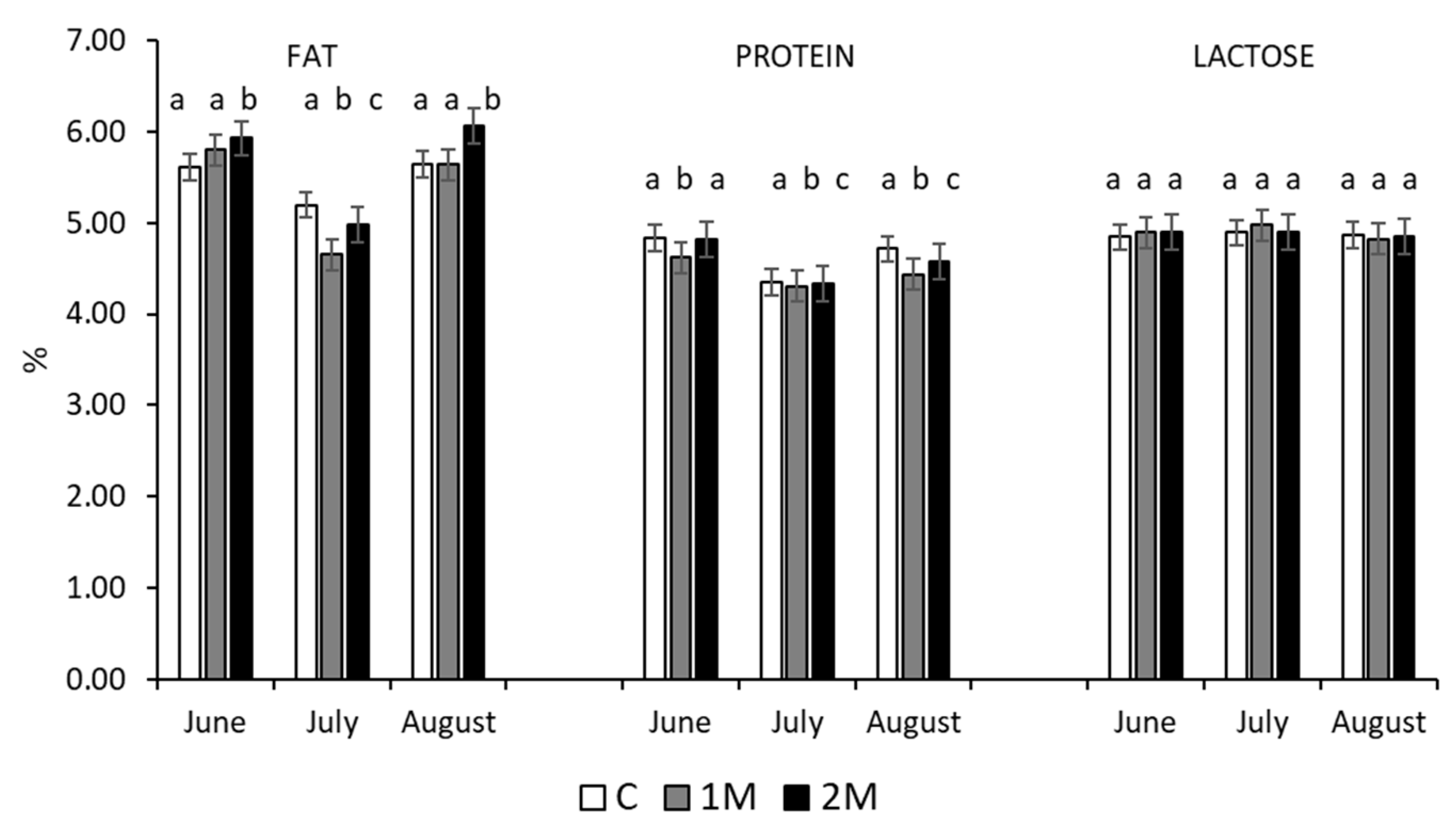
3.1. Milk Production and Quality
3.2. Colostrum Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molik, E.; Bonczar, G.; Misztal, T.; Żebrowska, A.; Zięba, D. Milk Protein; Hurley, W.L., Ed.; IntechOpen: London, UK, 2012. Available online: https://www.intechopen.com/books/milkprotein/the-effect-of-the-photoperiod-and-exogenous-melatonin-on-the-protein-content-in-sheep–milk (accessed on 20 March 2022).
- Barrington, G.M.; Parish, S.M. Bovine neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 463–476. [Google Scholar] [CrossRef]
- Lérias, J.R.; Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Castro, N.; Pourlis, A.; Almeida, A.M. The mammary gland in small ruminants: Major morphological and functional events underlying milk production—A review. J. Dairy Res. 2014, 81, 304–318. [Google Scholar] [CrossRef]
- Abecia, J.A.; Garrido, C.; Gave, M.; García, A.I.; López, D.; Luis, S.; Valares, J.A.; Mata, L. Exogenous melatonin and male foetuses improve the quality of sheep colostrum. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1305–1309. [Google Scholar] [CrossRef]
- Bittman, E.L.; Karsch, F.J.; Hopkins, J.W. Role of the pineal gland in ovine photoperiodism: Regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology 1983, 113, 229–336. [Google Scholar] [CrossRef]
- Palacin, I.; Forcada, F.; Abecia, J.A. Meta-analysis of the efficacy of melatonin implants for improving reproductive performance in sheep. Span. J. Agric. Res. 2011, 9, 730–743. [Google Scholar] [CrossRef]
- Abecia, J.A.; Forcada, F.; Casao, A.; Palacín, I. Effect of exogenous melatonin on the ovary, the embryo and the establishment of pregnancy in sheep. Animal 2008, 2, 399–404. [Google Scholar] [CrossRef][Green Version]
- Abecia, J.A.; Forcada, F.; Vázquez, M.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Pérez-Pe, R.; Casao, A. Role of melatonin on embryo viability in sheep. Reprod. Fertil. Dev. 2018, 31, 82–89. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Maestroni, G.J.M.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity: II. Melatonin enhances the antibody response via an opiatergic mechanism. Clin. Exp. Immunol. 1987, 68, 384–391. [Google Scholar]
- Demas, G.E.; Nelson, R.J. Photoperiod and temperature interact to affect immune parameters in adult male deer mice (Peromyscus maniculatus). J. Biol. Rhythm. 1996, 11, 94–102. [Google Scholar] [CrossRef]
- El Hadi, A. The Impact of Shearing and Hormonal Treatments (Melatonin or Cabergoline) in Lactating Dairy Ewes. Ph.D. Thesis, Universidad Autonoma de Barcelona, Barcelona, Spain, 2020. [Google Scholar]
- Abecia, J.A.; Forcada, F.; Valares, J.A.; Palacín, I.; Martín, S.; Martino, A.; Gómez, M.I.; Palacios, C. Does melatonin treatment during lactation influence milk production in Lacaune and Assaf ewes? Span. J. Agric. Res. 2005, 3, 396–401. [Google Scholar] [CrossRef]
- Cosso, G.; Mura, M.C.; Pulinas, L.; Curone, G.; Vigo, D.; Carcangiu, V.; Luridiana, S. Effects of melatonin treatment on milk traits, reproductive performance and immune response in Sarda dairy sheep. Ital. J. Anim. Sci. 2021, 20, 632–639. [Google Scholar] [CrossRef]
- Molik, E.; Błasiak, M.; Pustkowiak, H. Impact of photoperiod length and treatment with exogenous melatonin during pregnancy on chemical composition of sheep’s milk. Animals 2020, 10, 1721. [Google Scholar] [CrossRef]
- Bouroutzika, E.; Ciliberti, M.G.; Caroprese, M.; Theodosiadou, E.; Papadopoulos, S.; Makri, S.; Skaperda, Z.-V.; Kotsadam, G.; Michailidis, M.-L.; Valiakos, G.; et al. Association of melatonin administration in pregnant ewes with growth, redox status and immunity of their offspring. Animals 2021, 11, 3161. [Google Scholar] [CrossRef] [PubMed]
- Bouroutzika, E.; Kouretas, D.; Papadopoulos, S.; Veskoukis, A.S.; Theodosiadou, E.; Makri, S.; Paliouras, C.; Michailidis, M.L.; Caroprese, M.; Valasi, I. Effects of melatonin administration to pregnant ewes under heat-stress conditions, in redox status and reproductive outcome. Antioxidants 2020, 23, 266. [Google Scholar] [CrossRef]
- Forcada, F.; Abecia, J.A.; Zúñiga, O.; Lozano, J.M. Variation in the ability of melatonin implants inserted at two different times after the winter solstice to restore reproductive activity in reduced seasonality ewes. Aust. J. Agric. Res. 2002, 53, 167–173. [Google Scholar] [CrossRef]
- ICAR. International Agreement of Recording Practices; ICAR: Roma, Italy, 2016. [Google Scholar]
- Standard 021; Milk—Cream and Evaporated Milk. Determination of Total Solids Content. International Dairy Federation: Brussels, Belgium, 2010.
- Standard 105; Milk—Determination of fat content. Gerber butyrometers. International Dairy Federation: Brussels, Belgium, 2008.
- Standard 020-5; Milk—Determination of nitrogen content. Part 5: Determination of protein-nitrogen content. International Dairy Federation: Brussels, Belgium, 2002.
- Gonzalo, C.; Boixo, J.C.; Carriedo, J.A.; San Primitivo, F. Evaluation of rapid somatic cell counters under different analytical conditions in ovine milk. J. Dairy Sci. 2004, 87, 3623–3628. [Google Scholar] [CrossRef]
- Galan-Malo, P.; Valares, J.A.; Langa, V.; Razquín, P.; Mata, L. Determination of IgG levels in bulk ewe’s milk. Small Rumin. Res. 2014, 119, 156–160. [Google Scholar] [CrossRef]
- SPSS Statistics for Windows, Version 26.0; IBM Corp: Armonk, NY, USA, 2019.
- Davis, R.; Green, J.M.; Abecia, J.A. Field Trials of the Use of Melatonin Implants during Pregnancy as a Tool to Improve Lamb Performances. In Proceedings of the British Society of Animal Science (BSAS), Online, 12–15 April 2021. [Google Scholar]
- Flinn, T.; Gunn, J.R.; Kind, K.L.; Swinbourne, A.M.; Weaver, A.C.; Kelly, J.M.; Walker, S.K.; Gatford, K.L.; van Wettere, W.H.E.J.; Kleemann, D.O. Maternal melatonin implants improve twin merino lamb survival. J. Anim. Sci. 2020, 98, 344. [Google Scholar] [CrossRef]
- Flinn, T.; McCarthy, N.L.; Swinbourne, A.M.; Gatford, K.L.; Weaver, A.C.; McGrice, H.A.; Kelly, J.M.; Walker, S.K.; Kind, K.L.; Kleemann, D.O.; et al. Supplementing merino ewes with melatonin during the last half of pregnancy improves tolerance of prolonged parturition and survival of second-born twin lambs. J. Anim. Sci. 2020, 98, 372. [Google Scholar] [CrossRef]
- Sales, F.; Parraguez, V.H.; McCoard, S.; Cofré, E.; Peralta, O.A.; Subiabre, I. Fetal brown fat deposition is increased by melatonin implants in sheep. J. Anim. Sci. 2017, 95, 152–153. [Google Scholar] [CrossRef][Green Version]
- Avilés, R.; Delgadillo, J.A.; Flores, J.A.; Duarte, G.; Vielma, J.; Flores, M.J.; Petrovski, K.; Zarazaga, L.A.; Hernández, H. Melatonin administration during the dry period stimulates subsequent milk yield and weight gain of offspring in subtropical does kidding in summer. J. Dairy Sci. 2019, 102, 11536–11543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Chen, J.X.; Zhao, Y.X.; Zheng, Z.; Song, Y.L.; Wang, H.; Tong, D. The inhibitory effect of melatonin on mammary function of lactating dairy goats. Biol. Reprod. 2018, 100, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Luis, S.; Canto, F. Implanting melatonin at lambing enhances lamb growth and maintains high fat content in milk. Vet. Res. Commun. 2021, 45, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Molik, E.; Bonczar, G.; Żebrowska, A.; Misztal, T.; Pustkowiak, H.; Zieba, D. Effect of day length and exogenous melatonin on chemical composition of sheep milk. Arch. Anim. Breed. 2011, 54, 177–187. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Guo, W.; Xu, H.; Tang, K.; Zan, L.; Yang, W. Melatonin suppresses milk fat synthesis by inhibiting the mTOR signalling pathway via the MT1 receptor in bovine mammary epithelial cells. J. Pineal Res. 2019, 67, e12593. [Google Scholar] [CrossRef]
- Yang, W.C.; Tang, K.Q.; Wang, Y.N.; Zhang, Y.Y.; Zan, L.S. Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci. Rep. 2017, 7, 15080. [Google Scholar] [CrossRef]
- Jimenez, A.; Andres, S.; Sanchez, J. Effect of melatonin implants on somatic cell counts in dairy goats. Small Rumin. Res. 2009, 84, 116–120. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Pirisi, A.; de Crémoux, R.; Gonzalo, C. Somatic cells of goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin. Res. 2007, 68, 126–144. [Google Scholar] [CrossRef]
- Shin, I.S.; Shin, N.R.; Park, J.W.; Jeon, C.M.; Hong, J.M.; Kwon, O.K.; Kim, J.S.; Lee, I.C.; Kim, J.C.; Oh, S.R.; et al. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J. Pineal Res. 2015, 58, 50–60. [Google Scholar] [CrossRef]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Geraily, G.; Norouzi, F.; Heidari, M.; Rezapoor, S. The melatonin immunomodulory actions in radiotherapy. Biophys. Rev. 2017, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.M.; Kubota, H.; Okita, M.; Maeda, T. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS ONE 2017, 12, e0178525. [Google Scholar] [CrossRef] [PubMed]
| Group | Number of Ewes/Farm | Interval Implant–Lambing (Days) | Interval Implant–Milk Sampling (Days) | DIM (Days) | ||||
|---|---|---|---|---|---|---|---|---|
| F1 F2 F3 F4 F5 | June | July | August | June | July | August | ||
| C | 35 87 27 30 153 | -- | -- | -- | -- | 12.6 ± 0.4 | 54.7 ± 1.1 | 95.7 ± 1.0 |
| 1M | 28 91 33 23 41 | 44.6 ± 0.6 | 57.3 ± 0.8 | 100.3 ± 1.0 | 134.0 ± 0.8 | 15.9 ± 0.5 | 55.8 ± 0.9 | 89.5 ± 0.9 |
| 2M | 32 40 34 20 41 | 40.0 ± 0.7 | 48.1 ± 0.9 | 96.9 ± 1.7 | 133.0 ± 1.4 | 12.0 ± 0.7 | 57.4 ± 1.4 | 93.5 ± 1.3 |
| Group | DMY (kg) | SCC (×1000) | ||||
|---|---|---|---|---|---|---|
| June | July | August | June | July | August | |
| C | 2.98 ± 0.06 a | 2.99 ± 0.07 a | 2.55 ± 0.06 a | 1597 ± 213 a,b | 1420 ± 216 a | 1355 ± 173 a,b |
| 1M | 3.36 ± 0.07 b | 3.47 ± 0.07 b | 2.98 ± 0.06 b | 1491 ± 230 a | 884 ± 133 b | 1220 ± 183 a |
| 2M | 3.19 ± 0.08 c | 3.21 ± 0.07 c | 2.75 ± 0.08 c | 960 ± 202 c | 623 ± 159 b | 715 ± 187 c |
| Group | IgG (mg/mL) | °Brix (%) | Fat (%) | Protein (%) | Lactose (%) |
|---|---|---|---|---|---|
| C | 16.99 ± 1.13 a | 21.70 ± 0.49 a | 7.02 ± 0.34 a | 7.06 ± 0.11 a,x | 6.71 ± 0.11 a |
| 1M | 19.88 ± 1.39 b | 22.06 ± 0.38 a | 7.09 ± 0.28 a | 7.18 ± 0.13 a | 6.89 ± 0.13 a |
| 2M | 24.41 ± 1.46 c | 22.57 ± 0.42 a | 7.65 ± 0.29 a | 7.39 ± 0.16 a,y | 7.06 ± 0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canto, F.; González, E.; Abecia, J.A. Effects of Implanting Exogenous Melatonin 40 Days before Lambing on Milk and Colostrum Quality. Animals 2022, 12, 1257. https://doi.org/10.3390/ani12101257
Canto F, González E, Abecia JA. Effects of Implanting Exogenous Melatonin 40 Days before Lambing on Milk and Colostrum Quality. Animals. 2022; 12(10):1257. https://doi.org/10.3390/ani12101257
Chicago/Turabian StyleCanto, Francisco, Eloi González, and José Alfonso Abecia. 2022. "Effects of Implanting Exogenous Melatonin 40 Days before Lambing on Milk and Colostrum Quality" Animals 12, no. 10: 1257. https://doi.org/10.3390/ani12101257
APA StyleCanto, F., González, E., & Abecia, J. A. (2022). Effects of Implanting Exogenous Melatonin 40 Days before Lambing on Milk and Colostrum Quality. Animals, 12(10), 1257. https://doi.org/10.3390/ani12101257






