Expansion of Canine Heartworm in Spain
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Climatology
2.2. Samples and Assays
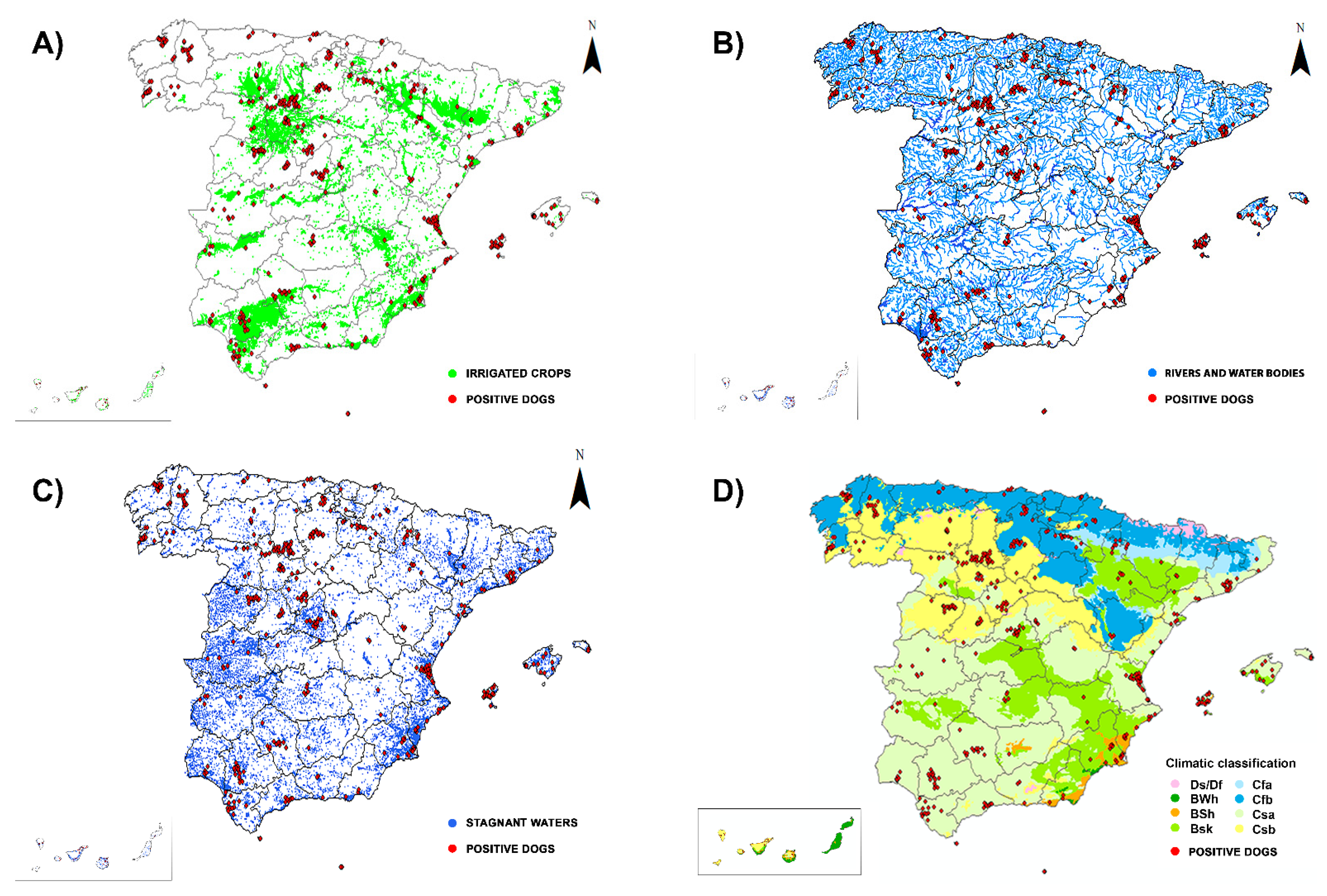
2.3. Geographic Information System (GIS) Mapping
2.4. Statistical Analysis
3. Results
| Autonomous Community | Autonomous Community | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Province | Climate | n | + | % | Province | Climate | n | + | % |
| Galicia | 559 | 42 | 7.51 | 29. Soria | Csb/Cfb | 143 | 1 | 0.70 | |
| 1. A Coruña | Cfb/Csb | 187 | 16 | 8.56 | 30. Segovia | Csa/Csb | 280 | 16 | 5.71 |
| 2. Lugo | Cfb/Csb | 154 | 8 | 5.19 | 31. Ávila | Csa/Csb | 143 | 6 | 4.20 |
| 3. Ourense | Csb | 107 | 4 | 3.74 | Madrid | 647 | 17 | 2.63 | |
| 4. Pontevedra | Cfb/Csb | 111 | 14 | 12.61 | 32. Madrid | Bsk/Csa/Csb | 647 | 17 | 2.63 |
| Asturias | 152 | 3 | 1.97 | Extremadura | 250 | 22 | 8.80 | ||
| 5. Asturias | Cfb | 152 | 3 | 1.97 | 33. Cáceres | Csa | 163 | 15 | 9.20 |
| Cantabria | 161 | 3 | 1.86 | 34. Badajoz | Bsk/Csa | 87 | 7 | 8.05 | |
| 6. Santander | Cfb | 161 | 3 | 1.86 | Castilla-La Mancha | 523 | 22 | 4.21 | |
| Basque Country | 294 | 5 | 1.70 | 35. Toledo | Bsk/Csa | 135 | 10 | 7.41 | |
| 7. Araba | Cfb | 74 | 1 | 1.35 | 36. Guadalajara | Csa/Csb | 104 | 5 | 4.81 |
| 8. Bizkaia | Cfb | 106 | 2 | 1.89 | 37. Cuenca | Csa | 74 | 2 | 2.70 |
| 9. Gipuzkoa | Cfb | 114 | 2 | 1.75 | 38. Ciudad Real | Bsk/Csa | 129 | 3 | 2.33 |
| Navarra | 147 | 5 | 3.40 | 39. Albacete | Bsk/Csa | 81 | 2 | 2.47 | |
| 10. Navarra | Cfa/Cfb/Bsk | 147 | 5 | 3.40 | Andalusia | 1154 | 86 | 7.45 | |
| La Rioja | 164 | 12 | 7.32 | 40. Huelva | Csa | 108 | 12 | 11.11 | |
| 11. La Rioja | Cfa/Cfb | 164 | 12 | 7.32 | 41. Sevilla | Csa | 305 | 29 | 9.51 |
| Aragon | 366 | 19 | 5.19 | 42. Cádiz | Csa | 95 | 13 | 13.68 | |
| 12. Huesca | Ds/Cfa/Cfb/Bsk | 101 | 3 | 2.97 | 43. Córdoba | Csa | 183 | 12 | 6.56 |
| 13. Zaragoza | Cfa/Cfb/Bsk | 177 | 12 | 6.78 | 44. Málaga | Bsk/Csa/Csb/Df | 158 | 12 | 7.59 |
| 14. Teruel | Cfb/Csb/Bsk | 88 | 4 | 4.55 | 45. Jaén | BSh/Csa/Csb | 81 | 1 | 1.23 |
| Catalonia | 768 | 36 | 4.69 | 46. Granada | Bsk/Csa/Csb/Df | 116 | 4 | 3.45 | |
| 15. Lleida | Ds/Cfa/Cfb/Bsk/Csa | 111 | 4 | 3.60 | 47. Almería | BSh/Bsk/BW/Csa/Csb | 108 | 3 | 2.78 |
| 16. Girona | Csa/Cfa/Cfb | 105 | 2 | 1.90 | Canary Islands * | 967 | 112 | 11.58 | |
| 17. Barcelona | Csa/Cfa/Cfb | 385 | 17 | 4.42 | 48. La Palma | BSh/Csa/Csb | 115 | 18 | 15.65 |
| 18. Tarragona | Csa/Bsk | 167 | 13 | 7.78 | 49. El Hierro | BW/BSh/Csa/Csb | 67 | 0 | 0.00 |
| Valencian Community | 771 | 51 | 6.61 | 50. La Gomera | BW/BSh/Csa/Csb | 78 | 9 | 11.54 | |
| 19. Castellón | Csa/Csb | 94 | 6 | 6.38 | 51. Tenerife | BW/BSh/Csa/Csb | 254 | 44 | 17.32 |
| 20. Valencia | Csa/Bsk/BSh | 375 | 28 | 7.47 | 52. Gran Canaria | BW/BSh/Csa/Csb | 237 | 38 | 16.03 |
| 21. Alicante | Csa/Csb/BSh | 302 | 17 | 5.63 | 53. Fuerteventura | BW/BSh | 115 | 2 | 1.74 |
| Murcia | 264 | 26 | 9.85 | 54. Lanzarote | BW/BSh | 101 | 1 | 0.99 | |
| 22. Murcia | Csa/Csb/BSh/BW | 264 | 26 | 9.85 | Balearic Islands * | 414 | 45 | 10.87 | |
| Castilla y León | 1831 | 108 | 5.90 | 55. Formentera | Bk/Csa | 27 | 3 | 11.11 | |
| 23. León | Df/Csb | 235 | 8 | 3.40 | 56. Ibiza | BSh/Bsk/Csa | 117 | 20 | 17.09 |
| 24. Zamora | Csa/Csb/Bsk | 140 | 8 | 5.71 | 57. Mallorca | Bsk/Csa/Csb | 169 | 19 | 11.24 |
| 25. Salamanca | Csa/Csb | 258 | 18 | 6.98 | 58. Menorca | Csa | 101 | 3 | 2.97 |
| 26. Valladolid | Csa/Csb/Bsk | 251 | 22 | 8.76 | Autonomous cities * | ||||
| 27. Palencia | Csb | 134 | 11 | 8.21 | 59. Ceuta | Csa | 58 | 1 | 1.72 |
| 28. Burgos | Csb/Cfb | 247 | 18 | 7.29 | 60. Melilla | Csa | 53 | 2 | 3.77 |
| TOTAL | 9543 | 617 | 6.47 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morchón, R.; Carretón, E.; González-Miguel, J.; Mellado-Hernández, I. Heartworm Disease (Dirofilaria immitis) and Their Vectors in Europe—New Distribution Trends. Front. Physiol. 2012, 3, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noack, S.; Harrington, J.; Carithers, D.S.; Kaminsky, R.; Selzer, P.M. Heartworm disease-Overview, intervention, and industry perspective. Intern. J. Parasitol. Drugs Drug Res. 2021, 16, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E. Treatment of dogs with severe heartworm disease. Vet. Parasitol. 2020, 283, 109131. [Google Scholar] [CrossRef]
- Genchi, C.; Kramer, L.H. The prevalence of Dirofilaria immitis and D. repens in the Old World. Vet. Parasitol. 2020, 280, 108995. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F.; Brianti, E.; Traversa, D.; Petrić, D.; Genchi, C.; Capelli, G. Vector-borne helminths of dogs and humans in Europe. Parasit. Vectors 2013, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Solano-Gallego, L.; Llull, J.; Osso, M.; Hegarty, B.; Breitschwerdt, E. A serological study of exposure to arthropod-borne pathogens in dogs from northeastern Spain. Vet. Res. 2006, 37, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Miró, G.; Montoya, A.; Roura, X.; Gálvez, R.; Sainz, A. Seropositivity rates for agents of canine vector-borne diseases in Spain: A multicentre study. Parasit. Vectors 2013, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7, 564429. [Google Scholar] [CrossRef]
- Simón, L.; Afonin, A.; López-Díez, L.I.; González-Miguel, J.; Morchón, R.; Carretón, E.; Montoya-Alonso, J.A.; Kartashev, V.; Simón, F. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops. The case of Spain. Vet. Parasitol. 2014, 200, 257–264. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Simón, L.; González-Miguel, J.; García-Guasch, L.; Morchón, R.; Simón, F. Prevalence of Dirofilaria immitis in dogs from Barcelona: Validation of a geospatial prediction model. Vet. Parasitol. 2015, 212, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Carretón, E.; Morchón, R.; Silveira-Viera, L.; Falcón, Y.; Simón, F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet. Parasitol. 2016, 216, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; Falcón-Cordón, Y.; Falcón-Cordón, S.; Simón, F.; Carretón, E. Prevalence of heartworm in dogs and cats of Madrid, Spain. Parasit. Vectors 2017, 10, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morchón, R.; Bargues, M.D.; Latorre, J.M.; Melero-Alcíbar, R.; Pou-Barreto, C.; Mas-Coma, S.; Simón, F. Haplotype H1 of Culex pipiens implicated as natural vector of Dirofilaria immitis in an endemic area of Western Spain. Vector-Borne Zoonotic Dis. 2007, 7, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Barriga, D.; Parreira, R.; Almeida, A.P.; Calado, M.; Blanco-Ciudad, J.; Serrano-Aguilera, F.J.; Pérez-Martín, J.E.; Sánchez-Peinado, J.; Pinto, J.; Reina, D.; et al. Culex pipiens as a potential vector for transmission of Dirofilaria immitis and other unclassified Filarioidea in Southwest Spain. Vet. Parasitol. 2016, 223, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Morchón, R.; Bargues, M.D.; Latorre-Estivalis, J.M.; Pou-Barreto, C.; Melero-Alcibar, R.; Moreno, M.; Valladares, B.; Molina, R.; Montoya-Alonso, J.A.; Mas-Coma, S.; et al. Molecular Characterization of Culex theileri from Canary Islands, Spain, a potential vector of Dirofilaria immitis. Clin. Exp. Pathol. 2011, S3, 1. [Google Scholar]
- Agencia Estatal de Meteorología, Ministerio de Agricultura, Alimentación y Medio Ambiente. In Iberian Climate Atlas. Air Temperature and Precipitation (1971–2000). 2011. Available online: https://www.aemet.es/documentos/es/conocermas/publicaciones/Atlas-climatologico/Atlas.pdf (accessed on 20 March 2022).
- Climate Shifts. In Worldmaps of Köppen-Geiger Climate Classification. 2019. Available online: http://koeppen-geiger.vu-wien.ac.at/shifts.html (accessed on 18 March 2022).
- Pérez Pérez, P.; Rodríguez-Escolar, I.; Carretón, E.; Sánchez Agudo, J.Á.; Lorenzo-Morales, J.; Montoya-Alonso, J.A.; Morchón, R. Serological Survey of Canine Vector-Borne Infections in North-Center Spain. Front. Vet. Sci. 2021, 8, 784331. [Google Scholar] [CrossRef]
- Simón, F.; Morchón, R.; González-Miguel, M.; Rodes-Moltó, D. Dirofilariosis canina en La Coruña. Galicia. Argos 2009, 106, 10–12. [Google Scholar]
- Morchón, R.; Mellado, I.; González-Miguel, J.; Vicente, M.; Vicente, L.; Simón, F. Prevalencia de la dirofilariosis cardiopulmononar canina. Argos 2011, 126, 30. [Google Scholar]
- Díaz-Regañón, D.; Roura, X.; Suárez, M.L.; León, M.; Sainz, Á. Serological evaluation of selected vector-borne pathogens in owned dogs from northern Spain based on a multicenter study using a commercial test. Parasit. Vectors 2020, 13, 301. [Google Scholar] [CrossRef]
- Rodes, D. Últimos datos epidemiológicos sobre filariosis canina. Argos 2006, 79, 52. [Google Scholar]
- Guerrero, J.; Rojo, F.; Ródenas, A. Estudio de la incidencia de la enfermedad del gusano del corazón en la población canina española. Med. Vet. 1989, 6, 217–220. [Google Scholar]
- Ortega-Mora, L.M.; Gómez, M.; Rojo-Vázquez, F.A.; Ródenas, A.; Guerrero, J. A survey of the prevalence of canine filariasis in Spain. Vet. Med. 1991, 11, 63–68. [Google Scholar] [CrossRef]
- Genchi, C.; Rinaldi, L.; Cascone, C.; Mortarino, M.; Cringoli, G. Is heartworm disease really spreading in Europe? Vet. Parasitol. 2005, 133, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Carretón, E.; Juste, M.C.; Mellado, I.; Morchón, R.; Simón, F. Epidemiological survey of canine heartworm disease on the island of Gran Canaria (Canary Islands-Spain) between 2000 and 2008. Vet. Parasitol. 2010, 173, 165–168. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Mellado, I.; Carretón, E.; Cabrera-Pedrero, E.D.; Morchón, R.; Simón, F. Canine dirofilariosis caused by Dirofilaria immitis is a risk factor for the human population on the island of Gran Canaria, Canary Islands, Spain. Parasitol. Res. 2010, 107, 1265–1269. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; Corbera, J.A.; Juste, M.C.; Mellado, I.; Morchón, R.; Simón, F. Current prevalence of Dirofilaria immitis in dogs, cats and humans from the island of Gran Canaria, Spain. Vet. Parasitol. 2011, 176, 291–294. [Google Scholar] [CrossRef]
- Morchón, R.; Moya, I.; González-Miguel, J.; Montoya, M.N.; Simón, F. Zoonotic Dirofilaria immitis infections in a province of Northern Spain. Epidemiol. Infect. 2010, 138, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, E.D.; Carretón, E.; Morchón, R.; Falcón-Cordón, Y.; Falcón-Cordón, S.; Simón, F.; Montoya-Alonso, J.A. The Canary Islands as a model of risk of pulmonary dirofilariasis in a hyperendemic area. Parasitol. Res. 2018, 117, 933–936. [Google Scholar] [CrossRef]
- Simón, F.; Muro, A.; Cordero, M.; Martin, J. A seroepidemiologic survey of human dirofilariosis in Western Spain. Trop. Med. Parasitol. 1991, 42, 106–108. [Google Scholar]
- Espinoza, E.; Cordero, M.; Muro, A.; Lorente, F.; Simón, F. Anti-Dirofilaria immitis IgE: Seroepidemiology and seasonal variation in an exposed human population. Trop. Med. Parasitol. 1993, 44, 172–176. [Google Scholar] [PubMed]
- Cordero, M.; Muro, A.; Simón, F.; Tapia, J.I.; Espinoza, E. Are transient pulmonary solitary nodules a common event in human dirofilariosis? Clin. Investig. 1992, 70, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.; Muñoz, M.R.; Muro, A.; Simón, F. Transient solitary pulmonary nodule caused by Dirofilaria immitis. Eur. Respir. J. 1990, 3, 1070–1071. [Google Scholar] [PubMed]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Collantes, F.; Delacour, S.; Alarcón-Elbal, P.M.; Ruiz-Arrondo, I.; Delgado, J.A.; Torrell-Sorio, A.; Bengoa, M.; Eritja, R.; Miranda, M.Á.; Molina, R.; et al. Review of ten-years presence of Aedes albopictus in Spain 2004–2014: Known distribution and public health concerns. Parasit. Vectors 2015, 8, 655. [Google Scholar] [CrossRef] [Green Version]
- Diniz, D.F.A.; de Albuquerque, C.M.R.; Oliva, L.O.; de Melo-Santos, M.A.V.; Ayres, C.F.J. Diapause and quiescence: Dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasit. Vectors 2017, 10, 310. [Google Scholar] [CrossRef]
- Cancrini, G.; Frangipane di Regalbono, A.; Ricci, I.; Tessarin, C.; Gabrielli, S.; Pietrobelli, M. Aedes albopictus is a natural vector of Dirofilaria immitis in Italy. Vet. Parasitol. 2003, 118, 195–202. [Google Scholar] [CrossRef]
- Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Peña, R.; Montalvo, T.; Bueno-Marí, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Env. Res. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Montoya-Alonso, J.A.; Carretón, E.; García-Guasch, L.; Expósito, J.; Armario, B.; Morchón, R.; Simón, F. First epidemiological report of feline heartworm infection in the Barcelona metropolitan area (Spain). Parasit. Vectors 2014, 7, 506. [Google Scholar] [CrossRef]


| BSh | Bsk | BWh | Cfa | Cfb | Csa | Csb | Total | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | + | % | n | + | % | n | + | % | n | + | % | n | + | % | n | + | % | n | + | % | n | + | % | ||
| Sex | Female | 157 | 10 | 6.37 | 234 | 14 | 5.98 | 196 | 9 | 4.59 | 67 | 4 | 5.97 | 336 | 24 | 7.14 | 2678 | 153 | 5.71 | 1304 | 97 | 7.44 | 4972 | 311 | 6.26 |
| Male | 145 | 10 | 6.90 | 203 | 11 | 5.42 | 168 | 8 | 4.76 | 57 | 4 | 7.02 | 401 | 27 | 6.73 | 2451 | 148 | 6.04 | 1146 | 98 | 8.55 | 4571 | 306 | 6.69 | |
| Age | <1 year | 5 | 1 | 20.00 | 12 | 1 | 8.33 | 15 | 0 | 0.00 | 1 | 0 | 0.00 | 21 | 1 | 4.76 | 193 | 5 | 2.59 | 51 | 3 | 5.88 | 298 | 11 | 3.69 |
| 1–4 years | 72 | 5 | 6.94 | 135 | 5 | 3.70 | 104 | 4 | 3.85 | 16 | 1 | 6.25 | 198 | 14 | 7.07 | 1994 | 87 | 4.36 | 785 | 42 | 5.35 | 3304 | 158 | 4.78 | |
| 5–10 years | 113 | 10 | 8.85 | 167 | 12 | 7.19 | 173 | 7 | 4.05 | 71 | 6 | 8.45 | 391 | 29 | 7.42 | 2443 | 162 | 6.63 | 1262 | 121 | 9.59 | 4620 | 347 | 7.51 | |
| 11–15 years | 109 | 4 | 3.67 | 109 | 6 | 5.50 | 69 | 5 | 7.25 | 35 | 1 | 2.86 | 89 | 5 | 5.62 | 316 | 39 | 12.34 | 321 | 27 | 8.41 | 1048 | 87 | 8.30 | |
| >15 years | 3 | 0 | 0.00 | 14 | 1 | 7.14 | 3 | 1 | 33.33 | 1 | 0 | 0.00 | 38 | 2 | 5.26 | 183 | 8 | 4.37 | 31 | 2 | 6.45 | 273 | 14 | 5.13 | |
| Habitat | Outdoors | 185 | 16 | 8.65 | 269 | 21 | 7.81 | 154 | 10 | 6.49 | 92 | 7 | 7.61 | 308 | 27 | 8.77 | 1985 | 163 | 8.21 | 1244 | 129 | 10.37 | 4237 | 373 | 8.80 |
| Indoors | 94 | 2 | 2.13 | 157 | 3 | 1.91 | 46 | 1 | 2.17 | 21 | 0 | 0.00 | 185 | 6 | 3.24 | 1281 | 22 | 1.72 | 391 | 11 | 2.81 | 2175 | 45 | 2.07 | |
| Indoors/Outdoors | 23 | 2 | 8.70 | 11 | 1 | 9.09 | 164 | 6 | 3.66 | 11 | 1 | 9.09 | 244 | 18 | 7.38 | 1863 | 116 | 6.23 | 815 | 55 | 6.75 | 3131 | 199 | 6.36 | |
| 302 | 20 | 6.62 | 437 | 25 | 5.72 | 364 | 17 | 4.67 | 124 | 8 | 6.45 | 737 | 51 | 6.92 | 5129 | 301 | 5.87 | 2450 | 195 | 7.96 | 9543 | 617 | 6.47 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya-Alonso, J.A.; Morchón, R.; García-Rodríguez, S.N.; Falcón-Cordón, Y.; Costa-Rodríguez, N.; Matos, J.I.; Rodríguez Escolar, I.; Carretón, E. Expansion of Canine Heartworm in Spain. Animals 2022, 12, 1268. https://doi.org/10.3390/ani12101268
Montoya-Alonso JA, Morchón R, García-Rodríguez SN, Falcón-Cordón Y, Costa-Rodríguez N, Matos JI, Rodríguez Escolar I, Carretón E. Expansion of Canine Heartworm in Spain. Animals. 2022; 12(10):1268. https://doi.org/10.3390/ani12101268
Chicago/Turabian StyleMontoya-Alonso, José Alberto, Rodrigo Morchón, Sara Nieves García-Rodríguez, Yaiza Falcón-Cordón, Noelia Costa-Rodríguez, Jorge Isidoro Matos, Iván Rodríguez Escolar, and Elena Carretón. 2022. "Expansion of Canine Heartworm in Spain" Animals 12, no. 10: 1268. https://doi.org/10.3390/ani12101268
APA StyleMontoya-Alonso, J. A., Morchón, R., García-Rodríguez, S. N., Falcón-Cordón, Y., Costa-Rodríguez, N., Matos, J. I., Rodríguez Escolar, I., & Carretón, E. (2022). Expansion of Canine Heartworm in Spain. Animals, 12(10), 1268. https://doi.org/10.3390/ani12101268










