Short Communication: Use of Infrared Thermometers for Cutaneous Temperature Recording: Agreement with the Rectal Temperature in Felis catus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal and Experimental Design
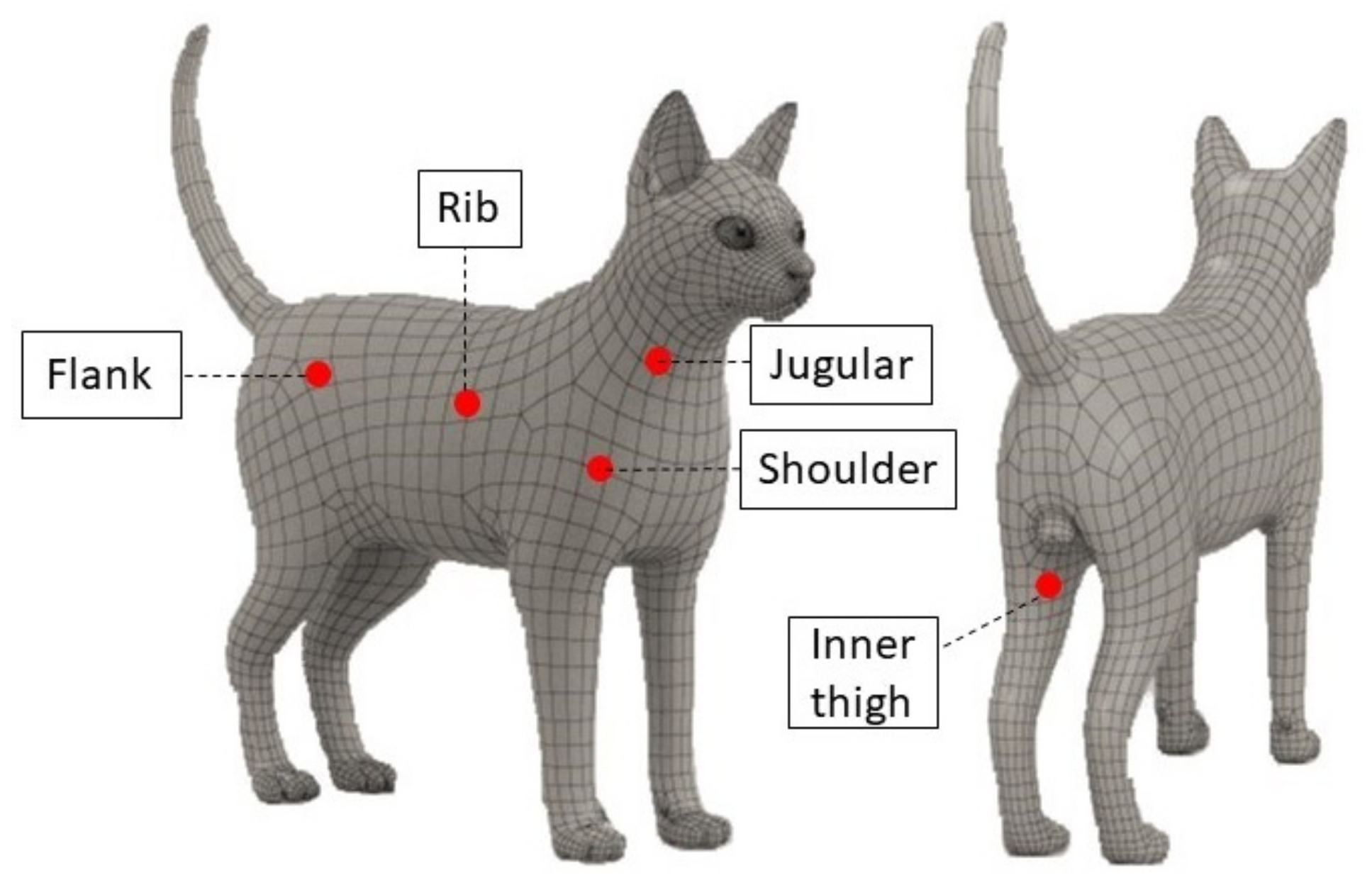
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.K.; Miki, K.; Sagawa, S.; Shiraki, K. Evaluation of mean skin temperature formulas by infrared thermography. Int. J. Biometeorol. 1997, 41, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Arfuso, F.; Giudice, E.; Gianesella, M.; Fazio, F.; Panzera, M.; Piccione, G. Infrared methodologies for the assessment of skin temperature daily rhythm in two domestic mammalian species. J. Therm. Biol. 2020, 92, 102677. [Google Scholar] [CrossRef] [PubMed]
- Southward, E.S.; Mann, F.A.; Dodam, J.; Wagner-Mann, C.C. A comparison of auricular, rectal, and pulmonary artery thermometry in dogs with anesthesia-induced hypothermia. J. Vet. Emerg. Crit. Care 2006, 16, 172–175. [Google Scholar] [CrossRef]
- Greer, R.J.; Cohn, L.A.; Dodam, J.R.; Wagner-Mann, C.C.; Mannet, F.A. Comparison of three methods of temperature measurement in hypothermic, euthermic and hyperthermic dogs. J. Am. Vet. Med. Assoc. 2007, 230, 1841–1848. [Google Scholar] [CrossRef]
- Nutt, K.R.; Levy, J.K.; Tucker, S.J. A comparison of non-contact infrared thermometry and rectal thermometry in cats. J. Feline Med. Surg. 2016, 18, 798–803. [Google Scholar] [CrossRef]
- Hall, E.J.; Fleming, A.; Carter, A.J. Investigating the use of non-contact infrared thermometers in cats and dogs. Vet. Nurse 2019, 10, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.A.; Lamb, V.; McBrearty, A.R. Comparison of axillary, tympanic membrane and rectal temperature measurement in cats. J. Feline Med. Surg. 2015, 17, 1028–1034. [Google Scholar] [CrossRef]
- Schmid, S.M.; Büscher, W.; Steinhoff-Wagner, J. Suitability of Different Thermometers for Measuring Body Core and Skin Temperatures in Suckling Piglets. Animals 2021, 11, 1004. [Google Scholar] [CrossRef]
- Stella, J.L.; Croney, C.C. Environmental aspects of domestic cat care and management: Implications for cat welfare. Sci. World J. 2016, 2016, 6296315. [Google Scholar] [CrossRef] [Green Version]
- Amat, M.; Camps, T.; Manteca, X. Stress in owned cats: Behavioural changes and welfare implications. J. Feline Med. Surg. 2016, 18, 577–586. [Google Scholar] [CrossRef]
- Horwitz, D.F.; Rodan, I. Behavioral awareness in the feline consultation: Understanding physical and emotional health. J. Feline Med. Surg. 2018, 20, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, F.C.; Mandese, W.W.; Reynolds, P.S. Evaluation of clinical examination location on stress in cats: A randomized crossover trial. J. Feline Med. Surg. 2021, 23, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Oka, T. Psychogenic fever: How psychological stress affects body temperature in the clinical population. Temperature 2015, 2, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herron, M.E.; Shreyer, T. The pet-friendly veterinary practice: A guide for practitioners. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, M.; Horst, C.; Levine, E.; Overall, K.; Radosta, L.; Rafter-Ritchie, M.; Yin, S. AAHA canine and feline behavior management guidelines. J. Am. Anim. Hosp. Assoc. 2015, 51, 205–221. [Google Scholar] [CrossRef]
- Soerensen, D.D.; Pedersen, L.J. Infrared skin temperature measurements for monitoring health in pigs: A review. Acta Vet. Scand. 2015, 57, 5. [Google Scholar] [CrossRef] [Green Version]
- Acri, G.; Tripepi, M.G.; Vermiglio, V.; Sansotta, C.; Testagrossa, B.; Causa, F.; Vermiglio, G. Telethemographic evidence of tattoos dermatological relevance. Acta Med. Mediterr. 2010, 26, 17–23. [Google Scholar]
- Stoop, R.; Hohenauer, E.; Aerenhouts, D.; Barel, A. Comparison of two skin temperature assessment methods after the application of topical revulsive products: Conductive iButton data logger system vs contact-free infrared thermometry. Skin Res. Technol. 2020, 26, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- Mot-Rojas, D.; Goncalves Titto, C.; Orihuela, A.; Mertinez-Burnes, J.; Gomez-Prado, J.; Torres-Bernal, F.; Flores-Pailla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and behavioral mechanisms of themoregulation in mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Aloweni, F.A.B.; Ang, S.Y.; Chang, Y.Y.; Ng, X.P.; Teo, K.Y.; Choh, A.C.L.; Goh, I.H.Q.; Lim, S.H. Evaluation of infrared technology to detect category I and suspected deep tissue injury in hospitalised patients. J. Wound Care 2019, 28 (Suppl. 12), S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Nitrini, A.G.C.; Cogliati, B.; Matera, J.M. Thermographic assessment of skin and soft tissue tumors in cats. J. Feline Med. Surg. 2020, 23, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Vainionpää, M.H.; Raekallio, M.R.; Junnila, J.J.; Hielm-Björkman, A.K.; Snellman, M.P.; Vainio, O.M. A comparison of thermographic imaging, physical examination and modified questionnaire as an instrument to assess painful conditions in cats. J. Feline Med. Surg. 2013, 15, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouzot-Nevoret, C.; Barthélemy, A.; Goy-Thollot, I.; Boselli, E.; Cambournac, M.; Guillaumin, J.; Bonnet-Garin, J.; Allaouchiche, J.-M. Infrared thermography: A rapid and accurate technique to detect feline aortic thromboembolism. J. Feline Med. Surg. 2018, 20, 780–785. [Google Scholar] [CrossRef]
- Teller, J.; Ragazzi, M.; Simonetti, G.D.; Lava, S.A.G. Accuracy of tympanic and forehead thermometers in private paediatric practice. Acta Paediatr. 2014, 103, E80–E83. [Google Scholar] [CrossRef]
- Sener, S.; Karcioglu, O.; Eken, C.; Yaylaci, S.; Ozsarac, M. Agreement between axillary, tympanic, and mid-forehead body temperature measurements in adult emergency department patients. Eur. J. Emerg. Med. 2012, 19, 252–256. [Google Scholar] [CrossRef]
- Fortuna, E.L.; Carney, M.M.; Macy, M.; Stanley, R.M.; Younger, J.G.; Bradin, S.A.; Arbor, A. Accuracy of Non-Contact Infrared Thermometry Versus Rectal Thermometry in Young Children Evaluated in The Emergency Department for Fever. J. Emerg. Nurs. 2010, 36, 101–104. [Google Scholar] [CrossRef]
- Chen, P.H.; White, C.E. Comparison of rectal, microchip transponder, and infrared thermometry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus). J. Am. Assoc. Lab. Anim. Sci. 2006, 45, 57–63. [Google Scholar]
- Brunell, M.K. Comparison of noncontact infrared thermometry and 3 commercial subcutaneous temperature transponding microchips with rectal thermometry in Rhesus Macaques (Macaca mulatta). J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 479–484. [Google Scholar]
- Stephens-DeValle, J.M. Comparison of tympanic, transponder, and noncontact infrared laser thermometry with rectal thermometry in strain 13 guinea pigs (Cavia porcellus). Contem. Top. Lab. Anim. Sci. 2005, 44, 35–38. [Google Scholar]
- Warn, P.A.; Brampton, M.W.; Sharp, A.; Morrissey, G.; Steel, N.; Denning, D.W.; Priest, T. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab. Anim. 2003, 37, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye surface infrared thermography usefulness as a noninvasive method of measuring stress response in sheep during shearing: Correlations with serum cortisol and rectal temperature values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, C.; Gianesella, M.; Arfuso, F.; Carcangiu, V.; Rizzo, M.; Fazio, F.; Abbate, F.; Piccione, G. Change of serum mitochondral uncpling protein 1 (ucp1) levels and daily rhythm of rectal and cutaneous temperatures in Equus caballus and Capra hyrcus. Biol. Rhythm Res. 2017, 48, 931–938. [Google Scholar] [CrossRef]
- Johnson, S.R.; Rao, S.; Hussey, S.B.; Morley, P.S.; Traub-Dargatz, J.L. Thermographic Eye Temperature as an Index to Body Temperature in Ponies. J. Equine Vet. Sci. 2016, 31, 63–66. [Google Scholar] [CrossRef]
- Salles, M.S.V.; da Silva, C.S.; Sallesa, F.A.; Roma, L.C.; El Faro, L.; Bustos Mac Lean, P.A.; de Oliveira, C.E.L.; Martello, L.S. Mapping the body surface temperature of cattle by infrared thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef]
- Ramey, D.; Bachmann, K.; Lee, M.L. A comparative study of non-contact infrared and digital rectal thermometer measurements of body temperature in the horse. J. Equine Vet. Sci. 2011, 31, 191–193. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C.; Marafioti, S.; Casella, S.; Assenza, A.; Fazio, F. Comparison of daily rhythm of rectal and auricular temperatures in horses kept under a natural photoperiod and constant darkness. J. Thermal. Biol. 2011, 36, 245–249. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C.; Fazio, F.; Giudice, E. Accuracy of auricular temperature determination as body temperature index and its daily rhythmicity in healthy dog. Biol. Rhythm Res. 2011, 42, 437–443. [Google Scholar] [CrossRef]
- Giannetto, C.; Fazio, F.; Panzera, M.; Alberghina, D.; Piccione, G. Comparison of rectal and vaginal temperature daily rhythm in dogs (canis familiaris) under different photoperiod. Biol. Rhythm. Res. 2015, 46, 113–119. [Google Scholar] [CrossRef]
- Giannetto, C.; di Pietro, S.; Falcone, A.; Pennisi, M.; Giudice, E.; Piccione, G.; Acri, G. Thermographic ocular temperature correlated with rectal temperature in cats. J. Thermal. Biol. 2021, 102, 103104. [Google Scholar] [CrossRef]
- Goic, J.B.; Reineke, E.L.; Drobatz, K.J. Comparison of rectal and axillary temperatures in dogs and cats. J. Am. Vet. Med. Assoc. 2014, 244, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Yanmaz, L.E.; Dogan, E.; Okumus, Z.; Senocak, M.G. Comparison of rectal, eye and ear temperatures in kangal breed dogs. Kafkas Univ. Vet. Fak. Derg. 2015, 21, 615–617. [Google Scholar]
- Zanghi, B.M. Eye and ear temperature using infrared thermography are related to rectal temperature in dogs at rest or with exercise. Front. Vet. Sci. 2016, 3, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cugman, B.; Susteric, P.; Gorenjec, N.R.; Plavec, T. Comparison between rectal and body surface temperature in dogs by the calibrate infrared thermometer. Vet. Anim. Sci. 2020, 9, 100120. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, G.A.; Nicklin, C.F.; Sullivan-Tamboe, D.L. Comparison of Body Temperature in Cats Using a Veterinary Infrared Thermometer and a Digital Rectal Thermometer. J. Am. Anim. Hosp. Assoc. 2004, 40, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.G.; Carareto, R.; Pereira-Junior, V.A.; Aquino, M.C. Agreement between auricular and rectal measurements of body temperature in healthy cats. J. Feline Med. Surg. 2013, 15, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.J. Tympanic infrared thermometry to determine cat body temperature. Contemp. Top. Lab. Anim. Sci. 1995, 34, 89–92. [Google Scholar]
- Girod, M.; Vandenheede, M.; Farnir, F.; Gommeren, K. Axillary temperature measurement: A less stressful alternative for hospitalised cats? Vet. Rec. 2016, 178, 192. [Google Scholar] [CrossRef] [Green Version]
- Piccione, G.; Marafioti, S.; Giannetto, C.; Panzera, M.; Fazio, F. Daily rhythm of total locomotor activity pattern in domestic cats (Felis silvestris catus) maintained in two different housing conditions. J. Vet. Behav. 2013, 8, 189–194. [Google Scholar] [CrossRef]
- Švejdová, K.; Šoch, M.; Šimková, A.; Zábranský, L.; Novák, P.; Brouček, J.; Cermak, B.; Palka, V.; Šimák-Líbalová, K. Measuring the body surface temperature of animals using a thermographic camera. Acta Univ. Cibiniensis Ser. E Food Technol. 2013, 17, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.; Arfuso, F.; Alberghina, D.; Giudice, E.; Gianesella, M. Monitoring changes in body surface temperature associated with treadmill exercise in dogs by use of infrared methodologies. J. Therm. Biol. 2017, 69, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Meisfjord Jørgensen, G.H.; Mejdell, C.M.; Bøe, K.E. Effects of hair coat characteristics on radiant surface temperature in horses. J. Therm. Biol. 2020, 87, 102474. [Google Scholar] [CrossRef] [PubMed]

| Minutes | Digital | IR1 | IR2 | IR3 |
|---|---|---|---|---|
| 0 | 42 | 42 | 42 | 41.8 |
| 1 | 40.8 | 40.7 | 40.6 | 40.7 |
| 2 | 40.1 | 40 | 40 | 39.8 |
| 3 | 39.2 | 39.1 | 39.2 | 38.8 |
| 4 | 38.2 | 38.4 | 38.4 | 38 |
| 5 | 37.5 | 37.2 | 37.3 | 37.1 |
| 6 | 36.4 | 36.1 | 36 | 35.9 |
| 7 | 35.6 | 35.2 | 35.3 | 35 |
| 8 | 34.8 | 34.5 | 34.6 | 34.5 |
| 9 | 34 | 33.8 | 33.7 | 33.6 |
| 10 | 32.9 | 33 | 32.8 | 32.8 |
| 11 | 32 | 31.6 | 31.7 | 31.8 |
| 12 | 29.8 | 29.7 | 29.8 | 29.8 |
| 13 | 29.3 | 28.9 | 29 | 29.1 |
| 14 | 28.5 | 28.2 | 28.4 | 28.3 |
| 15 | 27.3 | 27.2 | 27.3 | 27.2 |
| Thermometers | Jugular | Shoulder | Rib | Flank | Inner thigh | Rectal |
|---|---|---|---|---|---|---|
| IR1 | 36.05 ± 1.39 | 36.27 ± 1.64 | 35.77 ± 1.73 | 35.55 ± 1.46 | 37.21 ± 1.54 #° | |
| IR2 | 34.41 ± 1.25 b | 34.42 ± 1.70 b | 34.48 ± 0.87 b | 34.81 ± 0.89 | 35.17 ± 1.06 b | |
| IR3 | 31.51 ± 1.42 a | 32.76 ± 1.93 a# | 33.30 ± 1.948 b# | 33.87 ± 1.80 b# | 34.08 ± 1.93 a#* | |
| Digital | 38.59 ± 0.60 |
| Cutaneous Regions | Infrared Thermometers | IR2 | IR3 | IR3 | ||
|---|---|---|---|---|---|---|
| Jugular | Upper Limit | IR1 | 5.66 | 8.40 | IR2 | 6.96 |
| Bias | 1.63 | 4.53 | 2.89 | |||
| Lower Limit | −2.38 | 0.66 | −1.17 | |||
| Shoulder | Upper Limit | IR1 | 3.94 | 7.29 | IR2 | 5.10 |
| Bias | 1.85 | 3.50 | 1.65 | |||
| Lower Limit | −0.24 | −0.27 | −1.78 | |||
| Rib | Upper Limit | IR1 | 3.89 | 7.34 | IR2 | 4.84 |
| Bias | 1.29 | 2.47 | 1.18 | |||
| Lower Limit | −1.31 | −2.40 | −2.48 | |||
| Flank | Upper Limit | IR1 | 2.94 | 4.38 | IR2 | 3.57 |
| Bias | 0.73 | 1.67 | 0.94 | |||
| Lower Limit | −1.47 | −1.03 | −1.69 | |||
| Inner Thigh | Upper Limit | IR1 | 4.54 | 5.96 | IR2 | 3.83 |
| Bias | 2.03 | 3.13 | 1.09 | |||
| Lower Limit | 0.46 | 0.29 | −1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannetto, C.; Acri, G.; Pennisi, M.; Piccione, G.; Arfuso, F.; Falcone, A.; Giudice, E.; Di Pietro, S. Short Communication: Use of Infrared Thermometers for Cutaneous Temperature Recording: Agreement with the Rectal Temperature in Felis catus. Animals 2022, 12, 1275. https://doi.org/10.3390/ani12101275
Giannetto C, Acri G, Pennisi M, Piccione G, Arfuso F, Falcone A, Giudice E, Di Pietro S. Short Communication: Use of Infrared Thermometers for Cutaneous Temperature Recording: Agreement with the Rectal Temperature in Felis catus. Animals. 2022; 12(10):1275. https://doi.org/10.3390/ani12101275
Chicago/Turabian StyleGiannetto, Claudia, Giuseppe Acri, Melissa Pennisi, Giuseppe Piccione, Francesca Arfuso, Annastella Falcone, Elisabetta Giudice, and Simona Di Pietro. 2022. "Short Communication: Use of Infrared Thermometers for Cutaneous Temperature Recording: Agreement with the Rectal Temperature in Felis catus" Animals 12, no. 10: 1275. https://doi.org/10.3390/ani12101275






