Neonatal Piglet Temperature Changes: Effect of Intraperitoneal Warm Saline Injection
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing and Management
2.2. Statistics
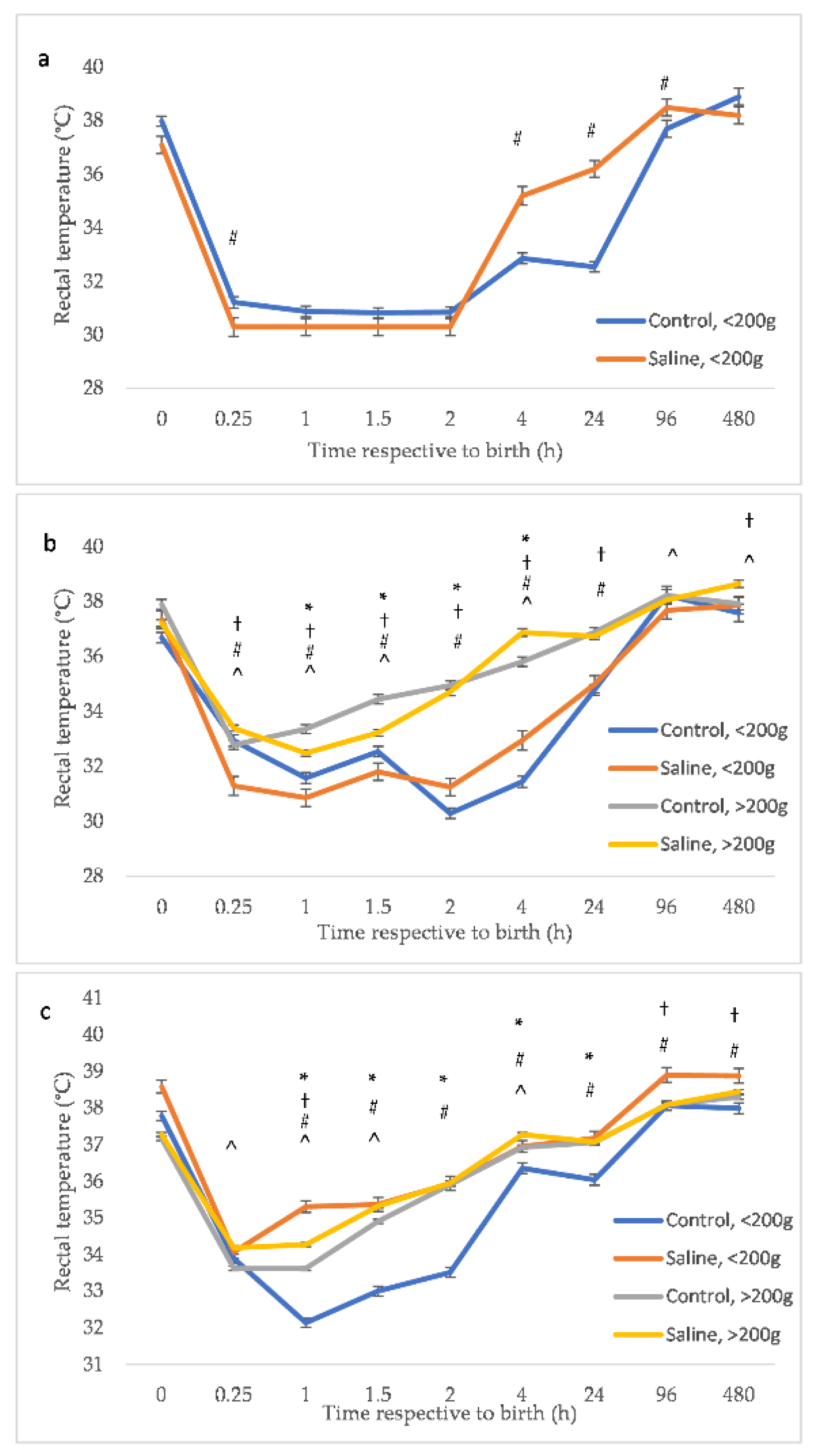
3. Results
3.1. Temperature
3.2. Colostrum Intake
3.3. Suckling Weight Gain
3.4. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herpin, P.; Damon, M.; Le Dividich, J. Development of thermoregulation and neonatal survival in pigs. Livest. Prod. Sci. 2002, 78, 25–45. [Google Scholar] [CrossRef]
- Villanueva-Garcíaa, D.; Mota-Rojasb, D.; Martínez-Burnesc, J.; Mora-Medinae, P.; Salmerónf, C.; Gómezb, J.; Boscatob, L.; Gutiérrez-Pérezf, O.; Cruzf, V.; Reyesb, B.; et al. Hypothermia in newly born piglets: Mechanisms of thermoregulation and pathophysiology of death. J. Anim. Behav. Biometerol. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Caldara, F.R.; Dos Santos, L.S.; Machado, S.T.; Moi, M.; de Alencar Naas, I.; Foppa, L.; Garcia, R.G.; de Kassia Silva Dos Santos, R. Piglets’ surface temperature change at different weights at birth. Asian Austr. J. Anim. Sci. 2014, 27, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vande Pol, K.D.; Cooper, N.; Tolosa, A.; Ellis, M.; Shull, C.M.; Brown, K.; Alencar, S. Effect of method of drying piglets at birth on rectal temperature over the first 24 hours after birth. Transl. Anim. Sci. 2020, 97, 4–5. [Google Scholar] [CrossRef]
- Edwards, S.A. Perinatal mortality in the pig: Environmental or physiological solutions? Livest. Prod. Sci. 2002, 78, 3–12. [Google Scholar] [CrossRef]
- Pandolfi, F.; Edwards, S.A.; Robert, F.; Kyriazakis, I. Risk factors associated with the different categories of piglet perinatal mortality in French farms. Prev. Vet. Med. 2017, 137, 1–12. [Google Scholar] [CrossRef]
- Cooper, N.; Vande Pol, K.D.; Ellis, M.; Xiong, Y.; Gates, R. Effect of piglet birth weight and drying on post-natal changes in rectal temperature. J. Anim. Sci. 2019, 97, 4. [Google Scholar] [CrossRef]
- Muns, R.; Malmkvist, J.; Larsen, M.L.V.; Sørensen, D.; Pedersen, L.J. High environmental temperature around farrowing induced heat stress in crated sows. J. Anim. Sci. 2016, 94, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.A.; Noblet, J.; Oliveira, R.F.; Donzele, J.L.; Primot, Y.; Renaudeau, D. Effects of dietary protein concentration and amino acid supplementation on the feeding behavior of multiparous lactating sows in a tropical humid climate. J. Anim. Sci. 2009, 87, 2104–2112. [Google Scholar] [CrossRef] [Green Version]
- Farmer, C.; Robert, S.; Choinière, Y. Reducing ambient temperature in farrowing houses with a new controlled-environment system. Can. J. Anim. Sci. 1998, 78, 23–28. [Google Scholar] [CrossRef]
- Declerck, I.; Dewulf, J.; Decaluwé, R.; Maes, D. Effects of energy supplementation to neonatal (very) low birth weight piglets on mortality, weaning weight, daily weight gain and colostrum intake. Livest. Sci. 2016, 183, 48–53. [Google Scholar] [CrossRef]
- Moreira, R.H.R.; Perez Palencia, J.Y.; Moita, V.H.C.; Caputo, L.S.S.; Saraiva, A.; Andretta, I.; Ferreira, R.A.; de Abreu, M.L.T. Variability of piglet birth weights: A systematic review and meta-analysis. J. Anim. Physiol. Anim. Nutr. 2020, 104, 657–666. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Effect of oral supplementation with different energy boosters in newborn piglets on pre-weaning mortality, growth and serological levels of IGF-I and IgG. J. Anim. Sci. 2017, 95, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Andersen, I.L.; Haukvik, I.A.; Bøe, K.E. Drying and warming immediately after birth may reduce piglet mortality in loose-housed sows. Animal 2009, 3, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Vande Pol, K.; Tolosa, A.; Shull, C.; Brown, C.; Alencar, S.; Ellis, M. Effect of drying and warming piglets at birth on pre-weaning mortality. Transl. Anim. Sci. 2021, 5, txab016. [Google Scholar] [CrossRef]
- Oshvandi, K.; Shiri, F.H.; Fazel, M.R.; Safari, M.; Ravari, A. The effect of pre-warmed intravenous fluids on prevention of intraoperative hypothermia in cesarean section. Iran. J. Nurs. Midwifery Res. 2014, 19, 64–69. [Google Scholar]
- Sheaff, C.M.; Fildes, J.J.; Keogh, P.; Smith, R.F.; Barrett, J.A. Safety of 65 °C intravenous fluid for the treatment of hypothermia. Am. J. Surg. 1996, 172, 52–55. [Google Scholar] [CrossRef]
- Fildes, J.; Sheaff, C.; Barrett, J. Very hot intravenous fluid in the treatment of hypothermia. J. Trauma 1993, 35, 683–686. [Google Scholar] [CrossRef]
- Campbell, G.; Alderson, P.; Smith, A.F.; Warttig, S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst. Rev. 2015, 4, CD009891. [Google Scholar] [CrossRef]
- Kammersgaard, T.S.; Pedersen, L.J.; Jørgensen, E. Hypothermia in neonatal piglets: Interactions and causes of individual differences. J. Anim. Sci. 2011, 89, 2073–2085. [Google Scholar] [CrossRef]
- Andersen, H.M.; Pedersen, L.J. Effect of radiant heat at the birth site in farrowing crates on hypothermia and behaviour in neonatal piglets. Animal 2016, 10, 128–134. [Google Scholar] [CrossRef]
- Kirkwood, R.; Aherne, F. Increasing the predictability of cloprostenol-induced farrowing in sows. J. Swine Health Prod. 1998, 6, 57–59. [Google Scholar]
- Devillers, N.; Farmer, C.; Le Dividich, J.; Prunier, A. Variability of colostrum yield and colostrum intake in pigs. Animal 2007, 1, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Hue, D.T.; Williams, J.L.; Petrovski, K.; Bottema, C.D.K. Predicting colostrum and calf blood components based on refractometry. J. Dairy Res. 2021, 88, 194–200. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Sakkas, P.; Wellock, I.; Almond, K.; Kyriazakis, I. Once small always small? To what extent morphometric characteristics and post-weaning starter regime affect pig lifetime growth performance. Porc. Health Manag. 2018, 4, 21–35. [Google Scholar] [CrossRef]
- Tucker, B.S.; Petrovski, K.R.; Craig, J.R.; Morrison, R.S.; Smits, R.J.; Kirkwood, R.N. Piglet Morphology: Indicators of Neonatal Viability? Animals 2022, 12, 658. [Google Scholar] [CrossRef]
- Decaluwé, R.; Maes, D.; Wuyts, B.; Cools, A.; Piepers, S.; Janssens, G.P.J. Piglets’ colostrum intake associates with daily weight gain and survival until weaning. Livest. Sci. 2014, 162, 185–192. [Google Scholar] [CrossRef]
- Devillers, N.; Le Dividich, J.; Prunier, A. Influence of colostrum intake on piglet survival and immunity. Animal 2011, 5, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Andersen, I.L.; Tajet, G.M.; Haukvik, I.A.; Kongsrud, S.; Bøe, K.E. Relationship between postnatal piglet mortality, environmental factors and management around farrowing in herds with loose-housed, lactating sows. Acta Agric. Scand. Sect. A Anim. Sci. 2007, 57, 38–45. [Google Scholar] [CrossRef]
- Amdi, C.; Jensen, L.L.; Oksbjerg, N.; Hansen, C.F. Supplementing newborn intrauterine growth restricted piglets with a bolus of porcine colostrum raises rectal temperatures one degree Celsius. J. Anim. Sci. 2017, 95, 2968–2976. [Google Scholar] [CrossRef]
- Moreira, L.P.; Menegat, M.B.; Barros, G.P.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. Effects of colostrum, and protein and energy supplementation on survival and performance of low-birth-weight piglets. Livest. Sci. 2017, 202, 188–193. [Google Scholar] [CrossRef]

| Treatment | RC1 | ||||
|---|---|---|---|---|---|
| N | BWC1 = 37 | BWC2 = 49 | BWC3 = 116 | Overall | |
| Control | 130 | 66.9 ± 34.2 | −439.7 ± 40.1 a | 76.86 ± 21.8 a | −39.2 ± 26.7 |
| Saline | 72 | 189.0 ± 105.0 | 142.3 ± 33.4 b | −135.9 ± 33.4 b | −4.04 ± 29.6 |
| RC2 | |||||
| N | BWC1 = 0 | BWC2 = 39 | BWC3 = 234 | ||
| Control | 154 | - | 295.2 ± 52.0 | 175.9 ± 16.6 | 183.9 ± 19.9 |
| Saline | 119 | - | 254.1 ± 39.9 | 214.6 ± 20.2 | 217.2 ± 17.1 |
| RC3 | |||||
| N | BWC1 = 68 | BWC2 = 174 | BWC3 = 872 | ||
| Control | 491 | 166.0 ± 57.3 | −177.8 ± 28.7 a | 237.4 ± 9.4 a | 205.3 ± 10.05 |
| Saline | 623 | 189.0 ± 55.0 | 246.8 ± 17.2 b | 351.0 ± 9.1 b | 326.9 ± 4.96 |
| Overall | 119.7 ± 3.98 | 99.97 ± 13.0 | 251.1 ± 5.99 | Na | |
| Colostrum Intake < 200 g | |||||
|---|---|---|---|---|---|
| N | BWC1 = 36 | BWC2 = 59 | BWC3 = 115 | Overall | |
| Control | 103 | 6.9 ± 0.37 a | - | 5.5 ± 0.35 | 6.24 ± 0.90 |
| Saline | 107 | 5.3 ± 0.37b | 5.5 ± 0.35 | 5.6 ± 0.36 | 5.82 ± 0.05 |
| Colostrum intake ≥ 200 g | |||||
| N | BWC1 = 0 | BWC2 = 123 | BWC3 = 941 | ||
| Control | 451 | - | 5.8 ± 0.36 a | 4.7 ± 0.34 | 4.87 ± 0.03 |
| Saline | 613 | - | 6.5 ± 0.34 | 4.6 ± 0.33 | 5.02 ± 0.03 |
| Overall | 6.29 ± 0.07 | 6.13 ± 0.05 | 4.87 ± 0.02 | ||
| Parameter | N | BWC1 = 105 | BWC2 = 262 | BWC3 = 1222 | Overall | |
|---|---|---|---|---|---|---|
| Treatment | Control | 753 | 11.7 (8.4–15.0) | 74.4 (68.9–79.3) | 72.5 (69.8–75.0) | 73.3 (71.7–74.8) |
| Saline | 814 | 100 (95.0–100.0) | 78.4 (74.8–81.6) | 90.6 (89.3–91.8) | 88.3 (87.2–89.4) | |
| Sex | F | 750 | 50.0 (43.2–100.0) | 67.4 (62.5–72.0) | 82.7 (80.6–84.6) | 79.8 (78.3–81.2) |
| M | 839 | 100 (96.0–100.0) | 83.6 (79.8–86.8) | 84.2 (82.4–85.9) | 81.6 (80.3–82.9) | |
| Colostrum Intake | <200 g | 335 | 100 (96.0– 100.0) | 84.7 (80.3–88.2) | 78.1 (74.7–81.2) | 71.5 (69.0–74.0) |
| ≥200 g | 1254 | - | 62.6 (61.5–69.6) | 87.7 (86.7–88.6) | 83.1 (82.0–84.1) | |
| Overall | 100 (95.0–100.0) | 76.5 (73.0–79.6) | 83.5 (81.9–84.9) | NA | ||
| Parameters | N | RC1 = 347 | RC2 = 398 | RC3 = 844 | Overall | |
|---|---|---|---|---|---|---|
| Body weight category | BWC1 | 105 | 13.78 (8.96–20.6) | - | - | 52.3 (45.9–58.7) |
| BWC2 | 262 | 39.2 (32.2–46.6) | 62.6 (52.9–71.3) | 80.5 (76.6–83.8) | 72.1 (69.5–74.8) | |
| BWC3 | 1222 | 36.9 (31.3–42.8) | 85.5 (825.3–88.2) | 92.2 (91.0–93.3) | 84.1 (83.0–85.1) | |
| Treatment | Control | 753 | 9.02 (0.01–14.2) | 69.9 (64.6–74.8) | 63.3 (48.9–75.7) | 73.3 (71.7–74.8) |
| Saline | 814 | 100 (95.0–100.0) | 83.8 (79.5–87.3) | 92.7 (87.1–96.0) | 88.3 (87.2–89.4) | |
| Sex | F | 750 | 20.8 (16.5–25.8) | 75.8 (69.7–81.1) | 100 (95.0–100.0) | 79.8 (78.3–81.2) |
| M | 839 | 36.9 (30.9–43.3) | 75.9 (70.1–80.9) | 100 (95.0–100.0) | 81.6 (80.3–82.9) | |
| Colostrum intake | <200 g | 335 | 44.2 (38.3–50.3) | 71.0 (62.2–78.5) | 100 (95.0–100.0) | 71.5 (69.0–74.0) |
| ≥200 g | 1254 | 16.2 (12.3–21.2) | 80.1 (76.2–83.5) | 100 (95.0–100.0) | 83.1 (82.0–84.1) | |
| Overall | 30.6 (27.5–33.8) | 81.3 (79.0–83.6) | 90.4 (89.5–91.3) | NA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucker, B.S.; Petrovski, K.R.; Kirkwood, R.N. Neonatal Piglet Temperature Changes: Effect of Intraperitoneal Warm Saline Injection. Animals 2022, 12, 1312. https://doi.org/10.3390/ani12101312
Tucker BS, Petrovski KR, Kirkwood RN. Neonatal Piglet Temperature Changes: Effect of Intraperitoneal Warm Saline Injection. Animals. 2022; 12(10):1312. https://doi.org/10.3390/ani12101312
Chicago/Turabian StyleTucker, Bryony S., Kiro R. Petrovski, and Roy N. Kirkwood. 2022. "Neonatal Piglet Temperature Changes: Effect of Intraperitoneal Warm Saline Injection" Animals 12, no. 10: 1312. https://doi.org/10.3390/ani12101312
APA StyleTucker, B. S., Petrovski, K. R., & Kirkwood, R. N. (2022). Neonatal Piglet Temperature Changes: Effect of Intraperitoneal Warm Saline Injection. Animals, 12(10), 1312. https://doi.org/10.3390/ani12101312







