Detection of Low MAP Shedder Prevalence in Large Free-Stall Dairy Herds by Repeated Testing of Environmental Samples and Pooled Milk Samples
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Herds
- Count of positive test results in each study herd;
- Count of individual fecal samples taken in each study herd;
- Imperfect sensitivity of individual fecal culture or real-time PCR: 0.74 [45] (we assumed that fecal PCR is at least as sensitive as fecal culture);
- Specificity of individual fecal culture or real-time PCR: 1.00.
2.2. Environmental Samples
- milking area (waiting pen);
- main alleyway;
- lactating cow floor (fresh cow pen).
2.3. Milk Samples and Milk Pools
2.4. Laboratory Methods
2.5. Statistical Analysis and Model
2.6. Comparison of Laboratory Methods
3. Results
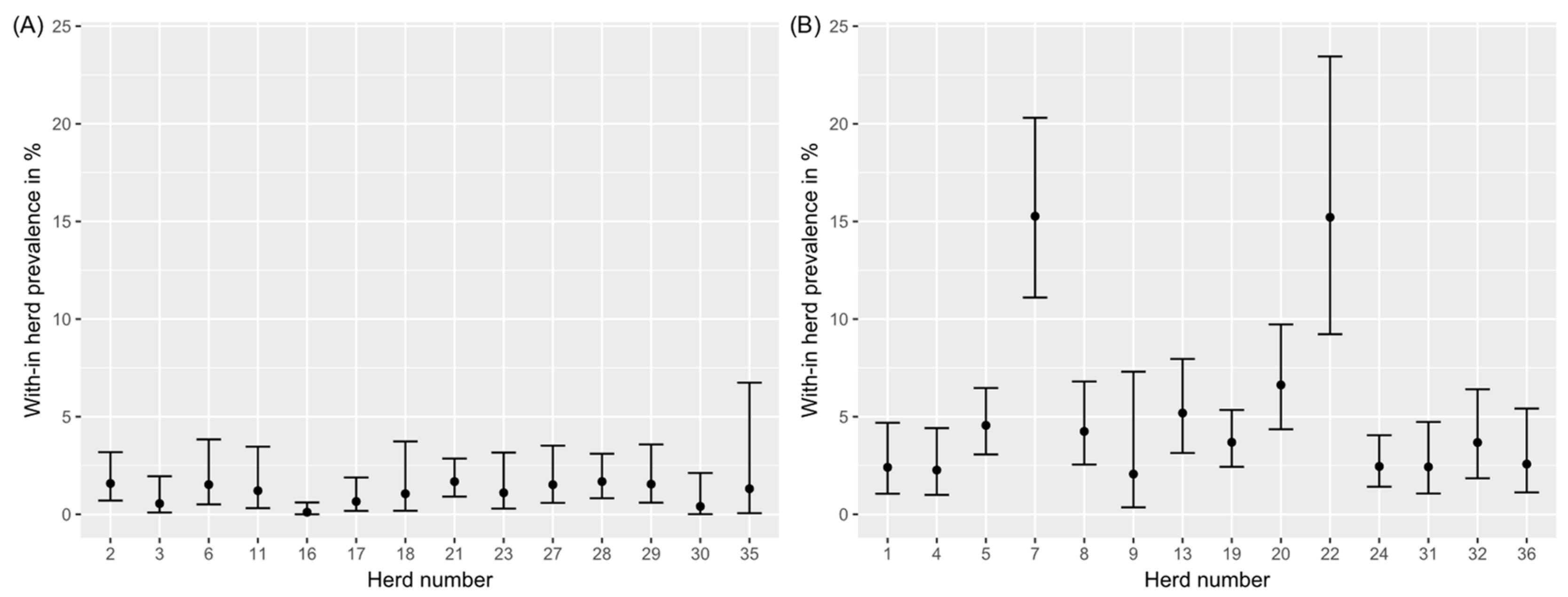
3.1. Study Herds
3.2. Environmental Samples
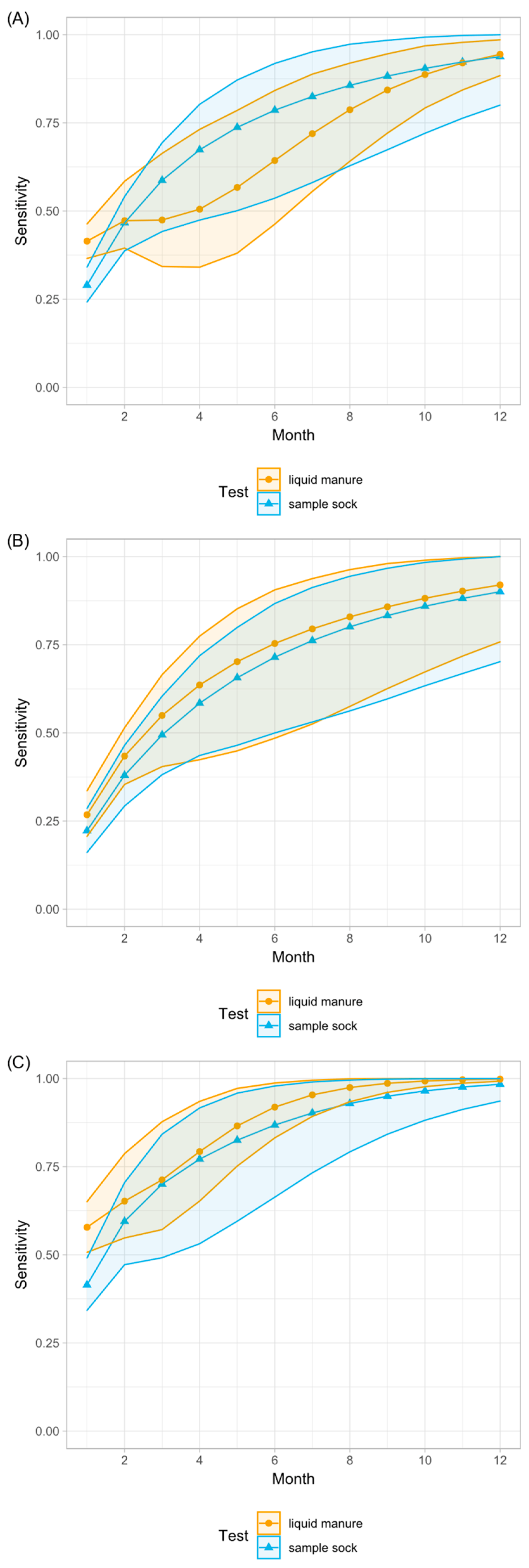
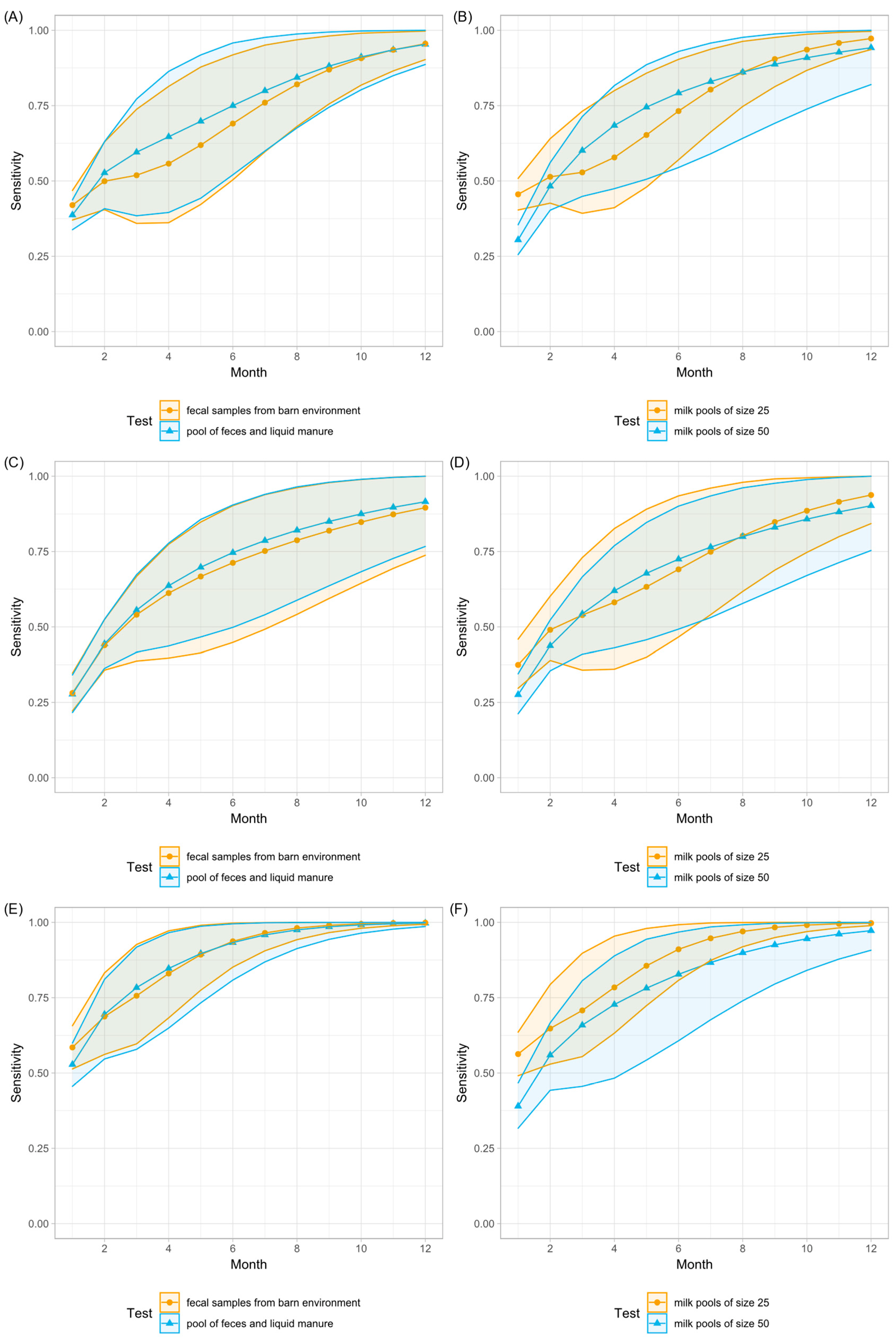
3.3. Milk Samples and Milk Pools
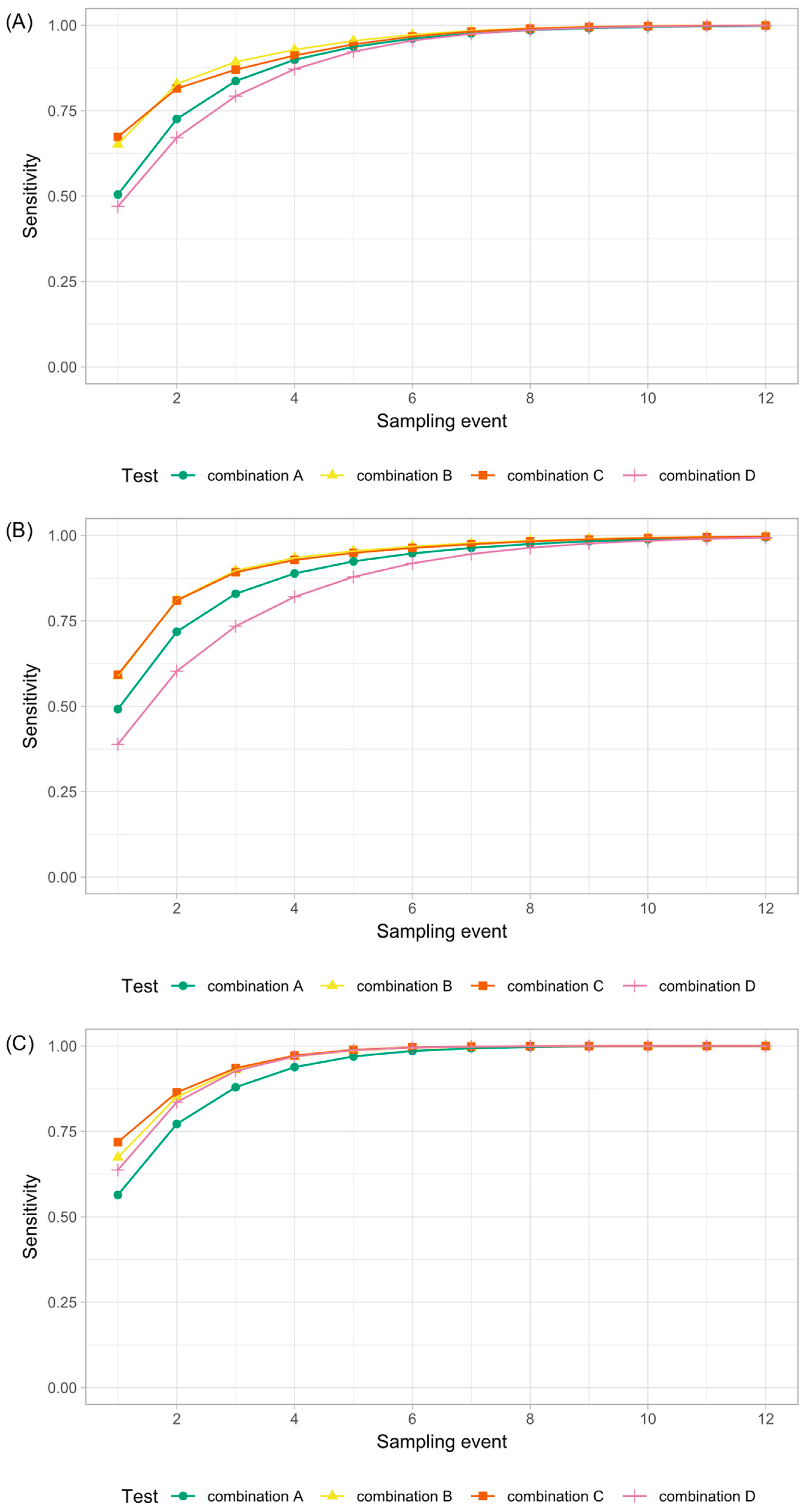
3.4. Statistical Analysis and Model
3.5. Comparison of Laboratory Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number of Sampling Events with a MAP-Positive Result at Herd Level | |||||||
|---|---|---|---|---|---|---|---|
| Herd Number | Estimated True within-Herd Prevalence of MAP-Shedders | Sample Sock | Liquid Manure | Fecal Samples from the Barn Environment | Pool of Feces a and Liquid Manure | Milk Pools of Size 25 | Milk Pools of Size 50 |
| 1 | 2.41 | 0 | 0 | 1 | 1 | 2 | 2 |
| 2 | 1.58 | 2 | 1 | 2 | 1 | 2 | 1 d |
| 3 | 0.55 | 0 | 1 | 0 | 0 | 0 | 0 |
| 4 | 2.27 | 6 | 8 | 11 | 8 | 7 | 5 |
| 5 | 4.56 | 6 | 10 | 9 | 7 | 12 | 6 |
| 6 | 1.52 | 1 | 2 b | 4 | 5 | 3 | 2 d |
| 7 | 15.27 | 12 | 12 | 12 | 12 | 12 | 11 |
| 8 | 4.25 | 2 | 12 | 8 | 6 | 8 | 3 |
| 9 | 2.06 | 1 | 5 | 3 | 2 | 9 | 4 |
| 10 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | 1.21 | 0 | 0 | 0 | 0 | 2 | 1 |
| 12 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 5.19 | 0 | 0 | 4 | 2 | 0 | 0 |
| 14 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 d |
| 15 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0.11 | 0 | 2 | 0 | 1 | 4 | 2 |
| 17 | 0.66 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 1.06 | 0 | 1 | 1 | 1 | 2 | 0 d |
| 19 | 3.69 | 4 | 5 c | 7 | 8 | 6 | 1 |
| 20 | 6.63 | 8 | 8 | 7 | 7 | 8 | 8 |
| 21 | 1.68 | 0 | 1 | 1 | 0 | 3 | 2 |
| 22 | 15.21 | 7 | 11 | 11 | 9 | 9 | 4 |
| 23 | 1.10 | 3 | 2 | 4 | 4 | 8 | 5 |
| 24 | 2.45 | 7 | 9 | 9 | 7 | 10 | 6 d |
| 25 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 d |
| 26 | 0.00 | 0 | 0 | 0 | 0 | 3 | 1 |
| 27 | 1.52 | 2 | 2 | 6 | 3 | 5 | 2 |
| 28 | 1.68 | 1 | 2 | 1 | 1 | 9 | 4 |
| 29 | 1.55 | 8 | 9 | 9 | 10 | 12 | 12 |
| 30 | 0.40 | 0 | 1 | 2 | 1 | 1 | 1 |
| 31 | 2.43 | 4 | 6 | 8 | 7 | 2 | 1 |
| 32 | 3.68 | 4 | 5 | 8 | 5 | 8 | 4 |
| 33 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| 34 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 |
| 35 | 1.31 | 7 | 12 | 9 | 10 | 9 | 4 |
| 36 | 2.57 | 0 | 4 | 0 | 3 | 0 | 0 |
Appendix B
References
- Whitlock, R.H.; Buergelt, C. Preclinical and clinical manifestations of paratuberculosis (including pathology). Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 345–356. [Google Scholar] [CrossRef]
- Tiwari, A.; VanLeeuwen, J.A.; McKenna, S.L.; Keefe, G.P.; Barkema, H.W. Johne’s disease in Canada: Part I: Clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can. Vet. J. 2006, 47, 874–882. [Google Scholar] [PubMed]
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; et al. Control of paratuberculosis: Who, why and how. A review of 48 countries. BMC Vet. Res. 2019, 15, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waddell, L.A.; Rajić, A.; Stärk, K.D.; Mc, E.S. The zoonotic potential of Mycobacterium avium ssp. paratuberculosis: A systematic review and meta-analyses of the evidence. Epidemiol. Infect. 2015, 143, 3135–3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynne, J.W.; Bull, T.J.; Seemann, T.; Bulach, D.M.; Wagner, J.; Kirkwood, C.D.; Michalski, W.P. Exploring the zoonotic potential of Mycobacterium avium subspecies paratuberculosis through comparative genomics. PLoS ONE 2011, 6, e22171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sechi, L.A.; Dow, C.T. Mycobacterium avium ss. paratuberculosis Zoonosis—The Hundred Year War—Beyond Crohn’s Disease. Front. Immunol. 2015, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami, M.; Shafiei, M.; Ghasemian, A.; Valizadeh, S.; Al-Marzoqi, A.H.; Shokouhi Mostafavi, S.K.; Nojoomi, F.; Mirforughi, S.A. Mycobacterium avium paratuberculosis and Mycobacterium avium complex and related subspecies as causative agents of zoonotic and occupational diseases. J. Cell Physiol. 2019, 234, 12415–12421. [Google Scholar] [CrossRef]
- Donat, K.; Soschinka, A.; Erhardt, G.; Brandt, H.R. Paratuberculosis: Decrease in milk production of German Holstein dairy cows shedding Mycobacterium avium ssp. paratuberculosis depends on within-herd prevalence. Animal 2014, 8, 852–858. [Google Scholar] [CrossRef] [Green Version]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Kudahl, A.B.; Nielsen, S.S. Effect of paratuberculosis on slaughter weight and slaughter value of dairy cows. J. Dairy Sci. 2009, 92, 4340–4346. [Google Scholar] [CrossRef] [Green Version]
- Rangel, S.J.; Paré, J.; Doré, E.; Arango, J.C.; Côté, G.; Buczinski, S.; Labrecque, O.; Fairbrother, J.H.; Roy, J.P.; Wellemans, V.; et al. A systematic review of risk factors associated with the introduction of Mycobacterium avium spp. paratuberculosis (MAP) into dairy herds. Can. Vet. J. 2015, 56, 169–177. [Google Scholar]
- Stabel, J.R.; Kimura, K.; Robbe-Austerman, S. Augmentation of secreted and intracellular gamma interferon following johnin purified protein derivative sensitization of cows naturally infected with Mycobacterium avium subsp. paratuberculosis. J. Vet. Diagn. Investig. 2007, 19, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Jungersen, G.; Mikkelsen, H.; Grell, S.N. Use of the johnin PPD interferon-gamma assay in control of bovine paratuberculosis. Vet. Immunol. Immunopathol. 2012, 148, 48–54. [Google Scholar] [CrossRef]
- Corneli, S.; Di Paolo, A.; Vitale, N.; Torricelli, M.; Petrucci, L.; Sebastiani, C.; Ciullo, M.; Curcio, L.; Biagetti, M.; Papa, P.; et al. Early Detection of Mycobacterium avium subsp. paratuberculosis Infected Cattle: Use of Experimental Johnins and Innovative Interferon-Gamma Test Interpretative Criteria. Front. Vet. Sci. 2021, 8, 638890. [Google Scholar] [CrossRef]
- Collins, M.T.; Wells, S.J.; Petrini, K.R.; Collins, J.E.; Schultz, R.D.; Whitlock, R.H. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin. Diagn. Lab. Immunol. 2005, 12, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Kalis, C.H.; Barkema, H.W.; Hesselink, J.W.; van Maanen, C.; Collins, M.T. Evaluation of two absorbed enzyme-linked immunosorbent assays and a complement fixation test as replacements for fecal culture in the detection of cows shedding Mycobacterium avium subspecies paratuberculosis. J. Vet. Diagn. Investig. 2002, 14, 219–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, T.E.; Gardner, I.A.; Collins, M.T.; Whitlock, R.H. Effects of prevalence and testing by enzyme-linked immunosorbent assay and fecal culture on the risk of introduction of Mycobacterium avium subsp. paratuberculosis-infected cows into dairy herds. J. Vet. Diagn. Investig. 2004, 16, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, S.J.; Wagner, B.A. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J. Am. Vet. Med. Assoc. 2000, 216, 1450–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkeby, C.; Græsbøll, K.; Nielsen, S.S.; Toft, N.; Halasa, T. Epidemiological and economic consequences of purchasing livestock infected with Mycobacterium avium subsp. paratuberculosis. BMC Vet. Res. 2017, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- More, S.J.; Sergeant, E.S.; Strain, S.; Cashman, W.; Kenny, K.; Graham, D. The effect of alternative testing strategies and bio-exclusion practices on Johne’s disease risk in test-negative herds. J. Dairy Sci. 2013, 96, 1581–1590. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.J.; Benedictus, G. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Rev. Sci. Tech. 2001, 20, 151–179. [Google Scholar] [CrossRef] [PubMed]
- Kalis, C.H.; Collins, M.T.; Barkema, H.W.; Hesselink, J.W. Certification of herds as free of Mycobacterium paratuberculosis infection: Actual pooled faecal results versus certification model predictions. Prev. Vet. Med. 2004, 65, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.J.; Whitlock, R.H.; Wagner, B.A.; Collins, J.; Garry, F.; Hirst, H.; Lawrence, J.; Saville, W.J.; Naugle, A.L.L. Sensitivity of test strategies used in the Voluntary Johne’s Disease Herd Status Program for detection of Mycobacterium paratuberculosis infection in dairy cattle herds. J. Am. Vet. Med. Assoc. 2002, 220, 1053–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donat, K.; Kube, J.; Dressel, J.; Einax, E.; Pfeffer, M.; Failing, K. Detection of Mycobacterium avium subspecies paratuberculosis in environmental samples by faecal culture and real-time PCR in relation to apparent within-herd prevalence as determined by individual faecal culture. Epidemiol. Infect. 2015, 143, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Tavornpanich, S.; Wells, S.J.; Fossler, C.P.; Roussel, A.J.; Gardner, I.A. Evaluation of an alternative method of herd classification for infection with paratuberculosis in cattle herds in the United States. Am. J. Vet. Res. 2012, 73, 248–256. [Google Scholar] [CrossRef]
- Pouillot, R.; Dufour, B.; Durand, B. A deterministic and stochastic simulation model for intra-herd paratuberculosis transmission. Vet. Res. 2004, 35, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Sergeant, E.S.G.; McAloon, C.G.; Tratalos, J.A.; Citer, L.R.; Graham, D.A.; More, S.J. Evaluation of national surveillance methods for detection of Irish dairy herds infected with Mycobacterium avium ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 2525–2538. [Google Scholar] [CrossRef] [Green Version]
- van Weering, H.; van Schaik, G.; van der Meulen, A.; Waal, M.; Franken, P.; van Maanen, K. Diagnostic performance of the Pourquier ELISA for detection of antibodies against Mycobacterium avium subspecies paratuberculosis in individual milk and bulk milk samples of dairy herds. Vet. Microbiol. 2007, 125, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Lavers, C.J.; Barkema, H.W.; Dohoo, I.R.; McKenna, S.L.; Keefe, G.P. Evaluation of milk ELISA for detection of Mycobacterium avium subspecies paratuberculosis in dairy herds and association with within-herd prevalence. J. Dairy Sci. 2014, 97, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.J.; Rood, K.; Biswas, P.; Byrem, T.M. Herd-level prevalence of Johne’s disease in Utah and adjacent areas of the intermountain west as detected by a bulk-tank milk surveillance project. J. Dairy Sci. 2010, 93, 5792–5797. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S.; Toft, N. Bulk tank milk ELISA for detection of antibodies to Mycobacterium avium subsp. paratuberculosis: Correlation between repeated tests and within-herd antibody-prevalence. Prev. Vet. Med. 2014, 113, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Tavornpanich, S.; Muñoz-Zanzi, C.A.; Wells, S.J.; Raizman, E.A.; Carpenter, T.E.; Johnson, W.O.; Gardner, I.A. Simulation model for evaluation of testing strategies for detection of paratuberculosis in Midwestern US dairy herds. Prev. Vet. Med. 2008, 83, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Lavers, C.J.; McKenna, S.L.; Dohoo, I.R.; Barkema, H.W.; Keefe, G.P. Evaluation of environmental fecal culture for Mycobacterium avium subspecies paratuberculosis detection in dairy herds and association with apparent within-herd prevalence. Can. Vet. J. 2013, 54, 1053–1060. [Google Scholar]
- Donat, K.; Hahn, N.; Eisenberg, T.; Schlez, K.; Köhler, H.; Wolter, W.; Rohde, M.; Pützschel, R.; Rösler, U.; Failing, K. Within-herd prevalence thresholds for the detection of Mycobacterium avium subspecies paratuberculosis-positive dairy herds using boot swabs and liquid manure samples. Epidemiol. Infect. 2016, 144, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, T.; Wolter, W.; Lenz, M.; Schlez, K.; Zschöck, M. Boot swabs to collect environmental samples from common locations in dairy herds for Mycobacterium avium ssp. paratuberculosis (MAP) detection. J. Dairy Res. 2013, 80, 485–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.L.; Schukken, Y.H.; Pradhan, A.K.; Smith, J.M.; Whitlock, R.H.; Van Kessel, J.S.; Wolfgang, D.R.; Grohn, Y.T. Environmental contamination with Mycobacterium avium subsp. paratuberculosis in endemically infected dairy herds. Prev. Vet. Med. 2011, 102, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Donat, K.; Schau, U.; Soschinka, A. Identification of Mycobacterium avium ssp. paratuberculosis infected dairy herds by environmental sampling. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Pillars, R.B.; Grooms, D.L.; Kaneene, J.B. Longitudinal study of the distribution of Mycobacterium avium subsp. paratuberculosis in the environment of dairy herds in the Michigan Johne’s disease control demonstration herd project. Can. Vet. J. 2009, 50, 1039–1046. [Google Scholar]
- Lombard, J.E.; Wagner, B.A.; Smith, R.L.; McCluskey, B.J.; Harris, B.N.; Payeur, J.B.; Garry, F.B.; Salman, M.D. Evaluation of environmental sampling and culture to determine Mycobacterium avium subspecies paratuberculosis distribution and herd infection status on US dairy operations. J. Dairy Sci. 2006, 89, 4163–4171. [Google Scholar] [CrossRef] [Green Version]
- Khol, J.; Vill, M.; Dünser, M.; Geisbauer, E.; Tichy, A.; Baumgartner, W. Environmental faecal sampling, a new approach in diagnosis and surveillance of paratuberculosis in Austrian cattle herds. Wien. Tierärztl. Mschr. 2009, 96, 279–285. [Google Scholar]
- Zoche-Golob, V.; Donat, K.; Barkema, H.W.; De Buck, J.; Kastelic, J.; Wolf, R. Predicting sensitivity of repeated environmental sampling for Mycobacterium avium subsp. paratuberculosis in dairy herds using a Bayesian latent class model. Vet. J. 2021, 275, 105728. [Google Scholar] [CrossRef] [PubMed]
- Khol, J.L.; Eisenberg, S.; Noll, I.; Zschöck, M.; Eisenberg, T.; Donat, K. Two-stage control of paratuberculosis: Herd-status surveillance as the basis for operational measures to reduce the prevalence.—Experiences from Lower Saxony, Hesse, Thuringia and Tyrol. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2019, 47, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Donat, K.; Einax, E.; Klassen, A. Evaluation of the Thuringian Bovine Johne’s Disease Control Program-A Case Study. Animals 2022, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Sergeant, E. epiR: Tools for the Analysis of Epidemiological Data; R package version 2.0.26; 2021. Available online: https://cran.r-project.org/web/packages/epiR/index.html (accessed on 27 April 2022).
- Sockett, D.C.; Carr, D.J.; Collins, M.T. Evaluation of conventional and radiometric fecal culture and a commercial DNA probe for diagnosis of Mycobacterium paratuberculosis infections in cattle. Can. J. Vet. Res. 1992, 56, 148–153. [Google Scholar]
- Wolf, R.; Barkema, H.W.; De Buck, J.; Orsel, K. Sampling location, herd size, and season influence Mycobacterium avium ssp. paratuberculosis environmental culture results. J. Dairy Sci. 2015, 98, 275–287. [Google Scholar] [CrossRef]
- Wolf, R.; Donat, K.; Khol, J.L.; Barkema, H.W.; Kastelic, J.; Wagner, P. Detection of Mycobacterium avium subspecies paratuberculosis infected cattle herds using environmental samples: A review. Berl. Münchener Tierärztliche Wochenschr. 2017, 130, 4–12. [Google Scholar] [CrossRef]
- Raizman, E.A.; Wells, S.J.; Godden, S.M.; Bey, R.F.; Oakes, M.J.; Bentley, D.C.; Olsen, K.E. The distribution of Mycobacterium avium ssp. paratuberculosis in the environment surrounding Minnesota dairy farms. J. Dairy Sci. 2004, 87, 2959–2966. [Google Scholar] [CrossRef]
- Hahn, N.; Failing, K.; Eisenberg, T.; Schlez, K.; Zschöck, P.M.; Donat, K.; Einax, E.; Köhler, H. Evaluation of different diagnostic methods for the detection of Mycobacterium avium subsp. paratuberculosis in boot swabs and liquid manure samples. BMC Vet. Res. 2017, 13, 259. [Google Scholar] [CrossRef] [Green Version]
- Englund, S.; Ballagi-Pordány, A.; Bölske, G.; Johansson, K.E. Single PCR and nested PCR with a mimic molecule for detection of Mycobacterium avium subsp. paratuberculosis. Diagn. Microbiol. Infect. Dis. 1999, 33, 163–171. [Google Scholar] [CrossRef]
- Græsbøll, K.; Andresen, L.O.; Halasa, T.; Toft, N. Opportunities and challenges when pooling milk samples using ELISA. Prev. Vet. Med. 2017, 139, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Köhler, H.; Ziller, M.; Gierke, F.; Donat, K. Within-pool prevalence limits for the identification of paratuberculosis infected herds using antibody detection in pooled milk samples. Berl. Munch. Tierarztl. Wochenschr 2017, 130, 34–41. [Google Scholar] [CrossRef]
- Wang, C.; Hanson, T.E. Estimation of sensitivity and specificity of multiple repeated binary tests without a gold standard. Stat. Med. 2019, 38, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, X.; Nelson, K.P. Bayesian hierarchical latent class models for estimating diagnostic accuracy. Stat. Methods Med. Res. 2020, 29, 1112–1128. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing, Vienna, Austria, 20–22 March 2003; pp. 1–10. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Denwood, M.J. runjags: An R package providing interface utilities, model templates, parallel computing methods and additional distributions for MCMC models in JAGS. J. Stat. Softw. 2016, 71, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Chamchoy, T.; Williams, D.R.; Adaska, J.M.; Anderson, R.J.; Aly, S.S. Environmental sampling to assess the bioburden of Mycobacterium avium subspecies paratuberculosis in drylot pens on California dairies. PeerJ 2019, 7, e8081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aly, S.S.; Anderson, R.J.; Whitlock, R.H.; Fyock, T.L.; McAdams, S.; Adaska, J.M.; Jiang, J.; Gardner, I.A. Reliability of environmental sampling to quantify Mycobacterium avium subspecies paratuberculosis on California free-stall dairies. J. Dairy Sci. 2009, 92, 3634–3642. [Google Scholar] [CrossRef] [Green Version]
- Köchler, J.; Gschaider, S.; Spergser, J.; Tichy, A.; Mader, C.; Vill, M.; Ortner, P.; Kössler, J.; Khol, J.L. Reproducibility of negative boot swab samples for paratuberculosis in cattle herds in Tyrol (Austria). Berl. Munch. Tierarztl. Wochenschr 2017, 130, 29–33. [Google Scholar] [CrossRef]
- Groenendaal, H.; Nielen, M.; Jalvingh, A.W.; Horst, S.H.; Galligan, D.T.; Hesselink, J.W. A simulation of Johne’s disease control. Prev. Vet. Med. 2002, 54, 225–245. [Google Scholar] [CrossRef]
- Kudahl, A.B.; Nielsen, S.S.; Ostergaard, S. Strategies for time of culling in control of paratuberculosis in dairy herds. J. Dairy Sci. 2011, 94, 3824–3834. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S.; Toft, N. Effect of management practices on paratuberculosis prevalence in Danish dairy herds. J. Dairy Sci. 2011, 94, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.S.; Toft, N. Ante mortem diagnosis of paratuberculosis: A review of accuracies of ELISA, interferon-gamma assay and faecal culture techniques. Vet. Microbiol. 2008, 129, 217–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, M.; Gardner, I.A. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev. Vet. Med. 2000, 45, 3–22. [Google Scholar] [CrossRef]
- Scott, H.M.; Sorensen, O.; Wu, J.T.; Chow, E.Y.; Manninen, K.; VanLeeuwen, J.A. Seroprevalence of Mycobacterium avium subspecies paratuberculosis, Neospora caninum, Bovine leukemia virus, and Bovine viral diarrhea virus infection among dairy cattle and herds in Alberta and agroecological risk factors associated with seropositivity. Can. Vet. J. 2006, 47, 981–991. [Google Scholar] [PubMed]
- Sorensen, O.; Rawluk, S.; Wu, J.; Manninen, K.; Ollis, G. Mycobacterium paratuberculosis in dairy herds in Alberta. Can. Vet. J. 2003, 44, 221–226. [Google Scholar]
- Corbett, C.S.; Naqvi, S.A.; Bauman, C.A.; De Buck, J.; Orsel, K.; Uehlinger, F.; Kelton, D.F.; Barkema, H.W. Prevalence of Mycobacterium avium ssp. paratuberculosis infections in Canadian dairy herds. J. Dairy Sci. 2018, 101, 11218–11228. [Google Scholar] [CrossRef] [Green Version]
- Field, N.L.; Mee, J.F.; McAloon, C.G. Characteristics (sensitivity and specificity) of herd-level diagnostic tests for Mycobacterium avium subspecies paratuberculosis in cattle—A systematic review. Vet. J. 2022, 279, 105786. [Google Scholar] [CrossRef]
- Gardner, I.A.; Stryhn, H.; Lind, P.; Collins, M.T. Conditional dependence between tests affects the diagnosis and surveillance of animal diseases. Prev. Vet. Med. 2000, 45, 107–122. [Google Scholar] [CrossRef]
- Kruschke, J.K. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan, 2nd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Collins, M.T. Diagnosis of paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 581–591. [Google Scholar] [CrossRef]
- Schoneberg, C.; Kreienbrock, L.; Campe, A. An Iterative, Frequentist Approach for Latent Class Analysis to Evaluate Conditionally Dependent Diagnostic Tests. Front. Vet. Sci. 2021, 8, 588176. [Google Scholar] [CrossRef]
- Helman, S.K.; Mummah, R.O.; Gostic, K.M.; Buhnerkempe, M.G.; Prager, K.C.; Lloyd-Smith, J.O. Estimating prevalence and test accuracy in disease ecology: How Bayesian latent class analysis can boost or bias imperfect test results. Ecol. Evol. 2020, 10, 7221–7232. [Google Scholar] [CrossRef]
- Martin, P.A.; Cameron, A.R.; Greiner, M. Demonstrating freedom from disease using multiple complex data sources 1: A new methodology based on scenario trees. Prev. Vet. Med. 2007, 79, 71–97. [Google Scholar] [CrossRef] [PubMed]

| Sample Type | Laboratory Method | Mean | Median | 95% CI |
|---|---|---|---|---|
| Sample sock | PCR + culture b | 0.290 | 0.290 | 0.242–0.341 |
| Liquid manure | PCR + culture b | 0.415 | 0.415 | 0.366–0.463 |
| Pool of fecal samples from the barn Environment a and liquid manure | PCR + culture b | 0.387 | 0.387 | 0.338–0.437 |
| Fecal samples from the barn environment a | PCR + culture b | 0.420 | 0.420 | 0.370–0.468 |
| Milk pools of size 25 | ELISA | 0.456 | 0.456 | 0.404–0.508 |
| Milk pools of size 50 | ELISA | 0.305 | 0.305 | 0.256–0.355 |
| Sample Type | Laboratory Method | Mean | Median | 95% CI |
|---|---|---|---|---|
| Sample sock | PCR + culture b | 0.993 | 0.995 | 0.981–1.00 |
| Liquid manure | PCR + culture b | 0.991 | 0.993 | 0.976–1.00 |
| Pool of fecal samples from the barn Environment a and liquid manure | PCR + culture b | 0.994 | 0.995 | 0.982–1.00 |
| Fecal samples from the barn environment a | PCR + culture b | 0.992 | 0.994 | 0.978–1.00 |
| Milk pools of size 25 | ELISA | 0.977 | 0.979 | 0.953–0.997 |
| Milk pools of size 50 | ELISA | 0.991 | 0.992 | 0.978–1.00 |
| Only Culture | Only PCR | PCR + Culture b | ||||
|---|---|---|---|---|---|---|
| Sample Type | Median | 95% CI | Median | 95% CI | Median | 95% CI |
| Sample sock | 0.203 | 0.163–0.246 | 0.237 | 0.191–0.284 | 0.290 | 0.242–0.341 |
| Liquid manure | 0.205 | 0.163–0.247 | 0.376 | 0.328–0.428 | 0.415 | 0.366–0.463 |
| Pool of fecal samples from the barn environment a and liquid manure | 0.248 | 0.202–0.295 | 0.335 | 0.286–0.384 | 0.387 | 0.338–0.437 |
| Fecal samples from the barn environment a | 0.343 | 0.292–0.398 | 0.348 | 0.299–0.397 | 0.420 | 0.370–0.468 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichert, A.; Kasbohm, E.; Einax, E.; Wehrend, A.; Donat, K. Detection of Low MAP Shedder Prevalence in Large Free-Stall Dairy Herds by Repeated Testing of Environmental Samples and Pooled Milk Samples. Animals 2022, 12, 1343. https://doi.org/10.3390/ani12111343
Wichert A, Kasbohm E, Einax E, Wehrend A, Donat K. Detection of Low MAP Shedder Prevalence in Large Free-Stall Dairy Herds by Repeated Testing of Environmental Samples and Pooled Milk Samples. Animals. 2022; 12(11):1343. https://doi.org/10.3390/ani12111343
Chicago/Turabian StyleWichert, Annika, Elisa Kasbohm, Esra Einax, Axel Wehrend, and Karsten Donat. 2022. "Detection of Low MAP Shedder Prevalence in Large Free-Stall Dairy Herds by Repeated Testing of Environmental Samples and Pooled Milk Samples" Animals 12, no. 11: 1343. https://doi.org/10.3390/ani12111343






