Effect of Acute Exposure to the Ionic Liquid 1-Methyl-3-octylimidazolium Chloride on the Embryonic Development and Larval Thyroid System of Zebrafish
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish and Experimental Design
2.3. Total RNA Extraction and Quantization RT-PCR
2.4. Thyroid Hormone Determination
2.5. The Statistical Analysis
3. Results
3.1. Effect of [C8mim]Cl Exposure on the Embryonic Development of Zebrafish
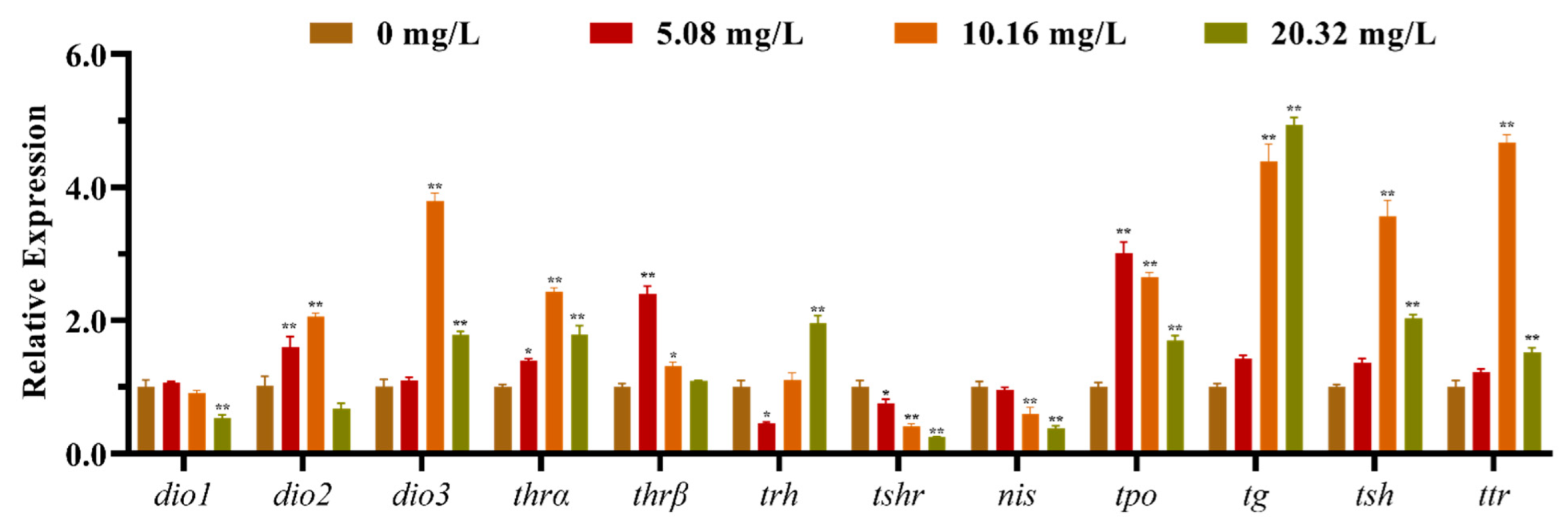
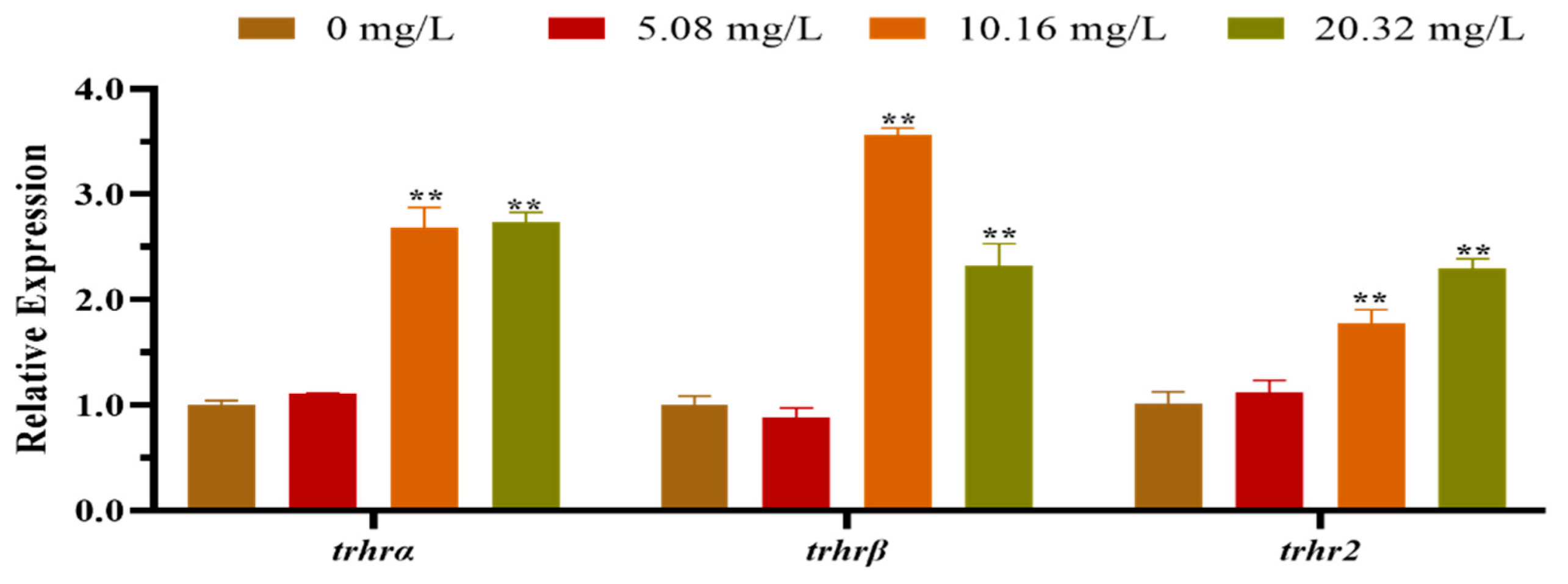
3.2. Effect of [C8mim]Cl on the Expression of HPT Axis-Related Genes
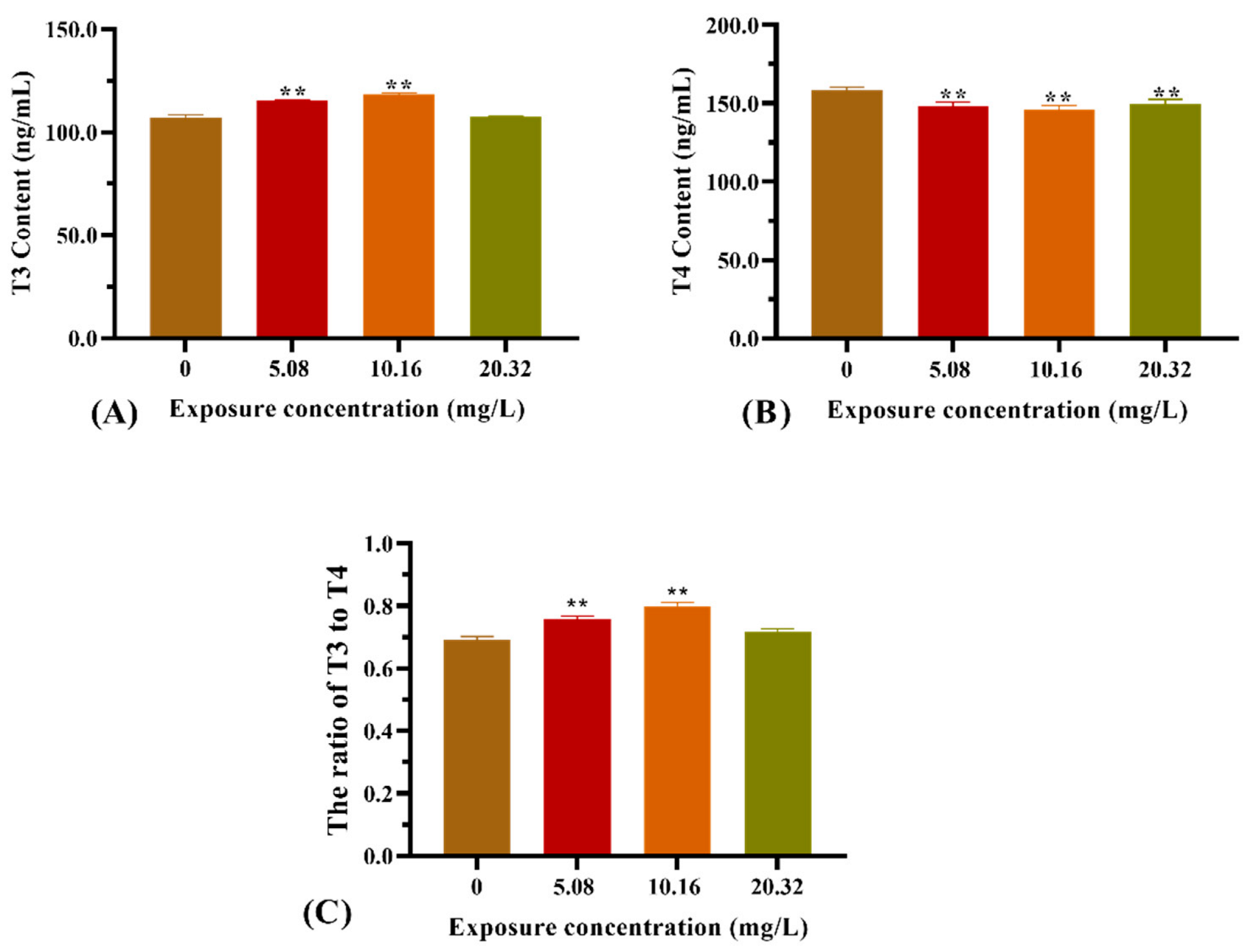
3.3. Thyroid Hormone Content in Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Canongia Lopes, J.N.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic LiquidsÐNew Solutions for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- McNeice, P.; Marr, P.C.; Marr, A.C. Basic Ionic Liquids for Catalysis: The Road to Greater Stability. Catal. Sci. Technol. 2021, 11, 726–741. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, F.Q.; Ge, L.Y.; Hu, Y.J.; Xia, Z.N. Recent Applications of Hydrophilic Interaction Liquid Chromatography in Pharmaceutical Analysis. J. Sep. Sci. 2017, 40, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Veerasingam, M.; Murugesan, B.; Mahalingam, S. Ionic Liquid Mediated Morphologically Improved Lanthanum Oxide Nanoparticles by Andrographis Paniculata Leaves Extract and Its Biomedical Applications. J. Rare Earths 2020, 38, 281–291. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins Currents Status and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Probert, P.M.; Leitch, A.C.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Abdelghany, T.M.; Lakey, A.F.; Cooke, M.P.; Talbot, H.; Wills, C.; et al. Identification of a Xenobiotic as a Potential Environmental Trigger in Primary Biliary Cholangitis. J. Hepatol. 2018, 69, 1123–1135. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, S.M.; Li, X.Y.; Zhang, B.J.; Wang, J.J. Acute Effects of 1-Octyl-3-Methylimidazolium Bromide Ionic Liquid on the Antioxidant Enzyme System of Mouse Liver. Ecotoxicol. Environ. Saf. 2008, 71, 903–908. [Google Scholar] [CrossRef]
- Li, X.Y.; Zeng, S.H.; Dong, X.Y.; Ma, J.G.; Wang, J.J. Acute Toxicity and Responses of Antioxidant Systems to 1-Methyl-3- Octylimidazolium Bromide at Different Developmental Stages of Goldfish. Ecotoxicology 2012, 21, 253–259. [Google Scholar] [CrossRef]
- Ma, J.; Chen, X.; Xin, G.; Li, X. Chronic Exposure to the Ionic Liquid [C8mim]Br Induces Inflammation in Silver Carp Spleen: Involvement of Oxidative Stress-Mediated P38MAPK/NF-ΚB Signalling and MicroRNAs. Fish. Shellfish Immunol. 2019, 84, 627–638. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Cui, M.; Li, W.; Li, X. Negative Impact of the Imidazolium-Based Ionic Liquid [C8mim]Br on Silver Carp (Hypophthalmichthys molitrix): Long-Term and Low-Level Exposure. Chemosphere 2018, 213, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Hijo, A.A.C.T.; Barros, H.D.F.Q.; Maximo, G.J.; Cazarin, C.B.B.; da Costa, L.B.E.; Pereira, J.F.B.; Junior, M.R.M.; Meirelles, A.J.A. Subacute Toxicity Assessment of Biobased Ionic Liquids in Rats. Food Res. Int. 2020, 134, 109125. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, R.; Ferrán, B.; Alonso, J.; Martí-Pàmies, I.; Aguiló, S.; Calvayrac, O.; Rodríguez, C.; Martínez-González, J. NR4A Receptors Up-Regulate the Antiproteinase Alpha-2 Macroglobulin (A2M) and Modulate MMP-2 and MMP-9 in Vascular Smooth Muscle Cells. Thromb. Haemost. 2015, 113, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Ruthsatz, K.; Dausmann, K.H.; Drees, C.; Becker, L.I.; Hartmann, L.; Reese, J.; Sabatino, N.M.; Peck, M.A.; Glos, J. Altered Thyroid Hormone Levels Affect Body Condition at Metamorphosis in Larvae of Xenopus Laevis. J. Appl. Toxicol. 2018, 38, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- De Escobar, G.M.; Obregón, M.J.; Del Rey, F.E. Role of Thyroid Hormone during Early Brain Development. Eur. J. Endocrinol. Suppl. 2004, 151, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Chopra, I.J.; Solomon, D.H.; Chopra, U.; Wu, S.Y.; Fisher, D.A.; Nakamura, Y. Pathways of Metabolism of Thyroid Hormones. Recent Prog. Horm. Res. 1978, 34, 521–567. [Google Scholar] [CrossRef]
- Wang, W.; Ma, Q.; Ding, X.; Xu, Y.; He, M.; Xu, J.; Liu, J.; Ji, C.; Zhang, J. Developmental Toxicity of Bromoacetamide via the Thyroid Hormone Receptors-Mediated Disruption of Thyroid Hormone Homeostasis in Zebrafish Embryos. Ecotoxicol. Environ. Saf. 2022, 233, 113334. [Google Scholar] [CrossRef]
- Buchholz, D.R.; Paul, B.D.; Fu, L.; Shi, Y.B. Molecular and Developmental Analyses of Thyroid Hormone Receptor Function in Xenopus Laevis, the African Clawed Frog. Gen. Comp. Endocrinol. 2006, 145, 1–19. [Google Scholar] [CrossRef]
- Kim, H.; Ji, K. Ecotoxicology and Environmental Safety Effects of Tetramethyl Bisphenol F on Thyroid and Growth Hormone-Related Endocrine Systems in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2022, 237, 113516. [Google Scholar] [CrossRef]
- Davidsen, N.; Ramhøj, L.; Lykkebo, C.A.; Kugathas, I.; Poulsen, R.; Rosenmai, A.K.; Evrard, B.; Darde, T.A.; Axelstad, M.; Bahl, M.I.; et al. PFOS-Induced Thyroid Hormone System Disrupted Rats Display Organ-Specific Changes in Their Transcriptomes. Environ. Pollut. 2022, 305, 119340. [Google Scholar] [CrossRef]
- Liu, Z.; Shangguan, Y.; Zhu, P.; Sultan, Y.; Feng, Y.; Li, X.; Ma, J. Developmental Toxicity of Glyphosate on Embryo-Larval Zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 236, 113493. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Shangguan, Y.; Zhu, Y.; Sultan, Y.; Feng, Y.; Zhang, B.; Liu, Y.; Ma, J.; Li, X. Negative Impacts of Microcystin-LR and Glyphosate on Zebrafish Intestine: Linked with Gut Microbiota and MicroRNAs? Environ. Pollut. 2021, 286, 117685. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Ding, W.; Ma, J.; Zhang, B.; Li, X. MicroRNA-16 Participates in the Cell Cycle Alteration of HepG2 Cells Induced by MC-LR. Ecotoxicol. Environ. Saf. 2020, 192, 110295. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, Y.A.; El-Hashash, M.A.; Al-Ali, K. Synthesis of Bioactive Quinazolin-4(3H)-One Derivatives via Microwave Activation Tailored by Phase-Transfer Catalysis. Acta Pharm. 2020, 70, 161–178. [Google Scholar] [CrossRef] [Green Version]
- Neves, Y.F.; Eloi, A.C.L.; de Freitas, H.M.M.; Soares, E.G.O.; Rivillo, D.; Demétrio da Silva, V.; Schrekker, H.S.; Badel, J.L. Imidazolium Salts as Alternative Compounds to Control Diseases Caused by Plant Pathogenic Bacteria. J. Appl. Microbiol. 2020, 128, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, Y.; Gao, Y.; Liu, Z.; Ding, J.; Zhang, C.; Liu, W.; Zhang, H.; Zhuang, S. Thyroid Dysfunction of Zebrafish (Danio rerio) after Early-Life Exposure and Discontinued Exposure to Tetrabromobiphenyl (BB-80) and OH-BB-80. Environ. Sci. Technol. 2022, 56, 2519–2528. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Yan, W.; Li, J.; Yu, L.; Wang, J.; Li, G.; Chen, N.; Steinman, A.D. Microcystin-RR Exposure Results in Growth Impairment by Disrupting Thyroid Endocrine in Zebrafish Larvae. Aquat. Toxicol. 2015, 164, 16–22. [Google Scholar] [CrossRef]
- Yang, M.; Hu, J.; Li, S.; Ma, Y.; Gui, W.; Zhu, G. Thyroid Endocrine Disruption of Acetochlor on Zebrafish (Danio rerio) Larvae. J. Appl. Toxicol. 2016, 36, 844–852. [Google Scholar] [CrossRef]
- Yu, L.; Chen, M.; Liu, Y.; Gui, W.; Zhu, G. Thyroid Endocrine Disruption in Zebrafish Larvae Following Exposure to Hexaconazole and Tebuconazole. Aquat. Toxicol. 2013, 138–139, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Blanton, M.L.; Specker, J.L. The Hypothalamic-Pituitary-Thyroid (HPT) Axis in Fish and Its Role in Fish Development and Reproduction. Crit. Rev. Toxicol. 2007, 37, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, X.; Deng, J.; Hecker, M.; Al-Khedhairy, A.; Giesy, J.P.; Zhou, B. Effects of Prochloraz or Propylthiouracil on the Cross-Talk between the HPG, HPA, and HPT Axes in Zebrafish. Environ. Sci. Technol. 2011, 45, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liang, K.; Liu, J.; Yang, L.; Guo, Y.; Liu, C.; Zhou, B. Exposure of Zebrafish Embryos/Larvae to TDCPP Alters Concentrations of Thyroid Hormones and Transcriptions of Genes Involved in the Hypothalamic-Pituitary-Thyroid Axis. Aquat. Toxicol. 2013, 126, 207–213. [Google Scholar] [CrossRef]
- Kim, H.; Ji, K. Exposure to Humidifier Disinfectants Induces Developmental Effects and Disrupts Thyroid Endocrine Systems in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2019, 184, 109663. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, I.; Rodríguez-Ortiz, R.; Villalobos, P.; Martínez-Torres, A.; Solís-Saínz, J.C.; Orozco, A. Knock-down of Specific Thyroid Hormone Receptor Isoforms Impairs Body Plan Development in Zebrafish. Front. Endocrinol. 2019, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Pascual, A.; Aranda, A. Thyroid Hormone Receptors, Cell Growth and Differentiation. Biochim. Biophys. Acta—Gen. Subj. 2013, 1830, 3908–3916. [Google Scholar] [CrossRef]
- Yan, W.; Zhou, Y.; Yang, J.; Li, S.; Hu, D.; Wang, J.; Chen, J.; Li, G. Waterborne Exposure to Microcystin-LR Alters Thyroid Hormone Levels and Gene Transcription in the Hypothalamic-Pituitary-Thyroid Axis in Zebrafish Larvae. Chemosphere 2012, 87, 1301–1307. [Google Scholar] [CrossRef]
- Yu, L.; Deng, J.; Shi, X.; Liu, C.; Yu, K.; Zhou, B. Exposure to DE-71 Alters Thyroid Hormone Levels and Gene Transcription in the Hypothalamic-Pituitary-Thyroid Axis of Zebrafish Larvae. Aquat. Toxicol. 2010, 97, 226–233. [Google Scholar] [CrossRef]
- Chang, J.; Wang, H.; Xu, P.; Guo, B.; Li, J.; Wang, Y.; Li, W. Oral and Dermal Diflubenzuron Exposure Causes a Hypothalamic–Pituitary–Thyroid (HPT) Axis Disturbance in the Mongolian Racerunner (Eremias argus). Environ. Pollut. 2018, 232, 338–346. [Google Scholar] [CrossRef]
- Manchado, M.; Infante, C.; Asensio, E.; Planas, J.V.; Cañavate, J.P. Thyroid Hormones Down-Regulate Thyrotropin β Subunit and Thyroglobulin during Metamorphosis in the Flatfish Senegalese Sole (Solea senegalensis Kaup). Gen. Comp. Endocrinol. 2008, 155, 447–455. [Google Scholar] [CrossRef]
- Waugh, D.T. Fluoride Exposure Induces Inhibition of Sodium/Iodide Symporter (NIS) Contributing to Impaired Iodine Absorption and Iodine Deficiency: Molecular Mechanisms of Inhibition and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohán, O.; la Vieja, A.; Paroder, V.; Riedel, C.; Artani, M.; Reed, M.; Ginter, C.S.; Carrasco, N. The Sodium/Iodide Symporter (NIS): Characterization, Regulation, and Medical Significance. Endocr. Rev. 2003, 24, 48–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Yang, Y.; Chen, Y.; Tang, W.; Wang, F.; Diao, X. Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF. Int. J. Environ. Res. Public Health 2015, 12, 13069–13084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, J.T.; Dunn, A.D. Update on Intrathyroidal Iodine Metabolism. Thyroid 2001, 11, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Murk, A.T.J.; Rijntjes, E.; Blaauboer, B.J.; Clewell, R.; Crofton, K.M.; Dingemans, M.M.L.; David Furlow, J.; Kavlock, R.; Köhrle, J.; Opitz, R.; et al. Mechanism-Based Testing Strategy Using in Vitro Approaches for Identification of Thyroid Hormone Disrupting Chemicals. Toxicol. Vitr. 2013, 27, 1320–1346. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, R.; Li, D.; Hu, Q.; Wang, Y. Subacute Microcystin-LR Exposure Alters the Metabolism of Thyroid Hormones in Juvenile Zebrafish (Danio rerio). Toxins 2015, 7, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yang, L.; Han, J.; Zou, Y.; Wang, Y.; Feng, C.; Zhou, B. Early-Life Exposure to Tris (1,3-Dichloro-2-Propyl) Phosphate Caused Multigenerational Neurodevelopmental Toxicity in Zebrafish via Altering Maternal Thyroid Hormones Transfer and Epigenetic Modifications. Environ. Pollut. 2021, 285, 117471. [Google Scholar] [CrossRef]
- Yao, F.; Li, Y.; Ru, H.; Wu, L.; Xiao, Z.; Ni, Z.; Chen, D.; Zhong, L. Thyroid Disruption and Developmental Toxicity Caused by Triphenyltin (TPT) in Zebrafish Embryos/Larvae. Toxicol. Appl. Pharmacol. 2020, 394, 114957. [Google Scholar] [CrossRef]
- Xu, C.; Niu, L.; Liu, J.; Sun, X.; Zhang, C.; Ye, J.; Liu, W. Maternal Exposure to Fipronil Results in Sulfone Metabolite Enrichment and Transgenerational Toxicity in Zebrafish Offspring: Indication for an Overlooked Risk in Maternal Transfer? Environ. Pollut. 2019, 246, 876–884. [Google Scholar] [CrossRef]
- Wu, L.; Ru, H.; Ni, Z.; Zhang, X.; Xie, H.; Yao, F.; Zhang, H.; Li, Y.; Zhong, L. Comparative Thyroid Disruption by o,p’-DDT and p,p’-DDE in Zebrafish Embryos/Larvae. Aquat. Toxicol. 2019, 216, 105280. [Google Scholar] [CrossRef]
- Kawakami, Y.; Shin, D.H.; Kitano, T.; Adachi, S.; Yamauchi, K.; Ohta, H. Transactivation Activity of Thyroid Hormone Receptors in Fish (Conger myriaster) in Response to Thyroid Hormones. Comp. Biochem. Physiol.—B Biochem. Mol. Biol. 2006, 144, 503–509. [Google Scholar] [CrossRef]
- Power, D.M.; Elias, N.P.; Richardson, S.J.; Mendes, J.; Soares, C.M.; Santos, C.R.A. Evolution of the Thyroid Hormone-Binding Protein, Transthyretin. Gen. Comp. Endocrinol. 2000, 119, 241–255. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.K.; Chan, K.M. Disruption of the Hypothalamic-Pituitary-Thyroid Axis in Zebrafish Embryo-Larvae Following Waterborne Exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ling, S.; Guan, K.; Luo, X.; Chen, L.; Han, J.; Zhang, W.; Mai, B.; Zhou, B. Bioconcentration, Biotransformation, and Thyroid Endocrine Disruption of Decabromodiphenyl Ethane (Dbdpe), A Novel Brominated Flame Retardant, in Zebrafish Larvae. Environ. Sci. Technol. 2019, 53, 8437–8446. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the Local Control of Thyroid Hormone Action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Geyten, S.; Byamungu, N.; Reyns, G.E.; Kühn, E.R.; Darras, V.M. Iodothyronine Deiodinases and the Control of Plasma and Tissue Thyroid Hormone Levels in Hyperthyroid Tilapia (Oreochromis niloticus). J. Endocrinol. 2005, 184, 467–479. [Google Scholar] [CrossRef] [Green Version]

| Gene | Sense Primers (5′-3′) | Accession No. |
|---|---|---|
| β-actin | FR 5′-TTCACCACCACAGCCGAAAGA-3′ RP 5′-TACCGCAAGATTCCATACCCA-3′ | NM_131031.2 |
| dio1 | FR 5′-AGCGTGCACAAAAACTTGGAG-3′ RP 5′-TCCGATGCCTCCCTGATAGATA -3′ | NM_001007283.2 |
| dio2 | FR 5′-GGCGGATGGTGGAGGAATT-3′ RP 5′-CACTGAGGAGGCAAGGAGAA-3′ | NM_212789.4 |
| dio3 | FR 5′-AAGATGTTCACGCTGGAGTC-3′ RP 5′-GCTGCCGAAGTTGAGGATC-3′ | NM_001177935.3 |
| nis | FR 5′-TCTCTATGGCTTGCTGTTGGA-3′ RP 5′-GATGCTGTGGTGTTGAGTGTT-3′ | XM_021478270.1 |
| tg | FR 5′-CGTCCACCAATCGGAGACCTCA-3′ RP 5′-AACACCAGAACCGGCGCATTC-3′ | NM_001329865.1 |
| thrα | FR 5′-TATGGTGACGGAGGCTCATCGG-3′ RP 5′-ACTCGTGTGATGGCAGGCGTA-3′ | NM_131396.1 |
| thrβ | FR 5′- GCATCGCTGTTGGCATGGCTA-3′ RP 5′- ACTCTTCCTGTGTGGGCTCTGG-3′ | XM_009306720.3 |
| tpo | FR 5′-CTGCGGCGTGAAGGAGATA-3′ RP 5′-CCAATACAACGGCTTCCAACT-3′ | XM_021467270.1 |
| trh | FR 5′-GATACACCTGAGGAGTCCACTT-3′ RP 5′-CTGATCGTTCTCGCTGTTCTG-3′ | NM_001012365.2 |
| trhr2 | FR 5′-GCATCCGCCAGATTCAGGTA-3′ RP 5′-TACAACCACCACAGCCAACA-3′ | NM_001044346.1 |
| trhrα | FR 5′-CGGAGACAGGTGACGAAGAT-3′ RP 5′-AGAGACGGCAGAACAGAAGG-3′ | XM_682154.7 |
| trhrβ | FR 5′-CACTCTTGCCACTGTCCTCTA-3′ RP 5′-TGTAACCTGTCTTCTGGATGCT-3′ | NM_001114688.1 |
| tsh | FR 5′- GCTGTCAACACCACCATCTG-3′ RP 5′-GTGAGGATCTGCATGTGAAGG-3′ | NM_181494.2 |
| tshr | FR 5′-ACGAGACAAGGCTAACAAGAGT-3′ RP 5′-TCAGGCTGTGGAAGGAATGC-3′ | NM_001145763.2 |
| ttr | FR 5′- CTCACTGTCCTCTGACGGTAA-3′ RP 5′- CACTTTCCCACTGGCAATCTT-3′ | NM_001005598.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Chen, Y.; Sultan, Y.; Ma, J.; Feng, Y.; Li, X. Effect of Acute Exposure to the Ionic Liquid 1-Methyl-3-octylimidazolium Chloride on the Embryonic Development and Larval Thyroid System of Zebrafish. Animals 2022, 12, 1353. https://doi.org/10.3390/ani12111353
Ding W, Chen Y, Sultan Y, Ma J, Feng Y, Li X. Effect of Acute Exposure to the Ionic Liquid 1-Methyl-3-octylimidazolium Chloride on the Embryonic Development and Larval Thyroid System of Zebrafish. Animals. 2022; 12(11):1353. https://doi.org/10.3390/ani12111353
Chicago/Turabian StyleDing, Weikai, Yangli Chen, Yousef Sultan, Junguo Ma, Yiyi Feng, and Xiaoyu Li. 2022. "Effect of Acute Exposure to the Ionic Liquid 1-Methyl-3-octylimidazolium Chloride on the Embryonic Development and Larval Thyroid System of Zebrafish" Animals 12, no. 11: 1353. https://doi.org/10.3390/ani12111353






