Supranutritional Selenium-Yeast Supplementation of Beef Cows during the Last Trimester of Pregnancy Results in Higher Whole-Blood Selenium Concentrations in Their Calves at Weaning, but Not Enough to Improve Nasal Microbial Diversity
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
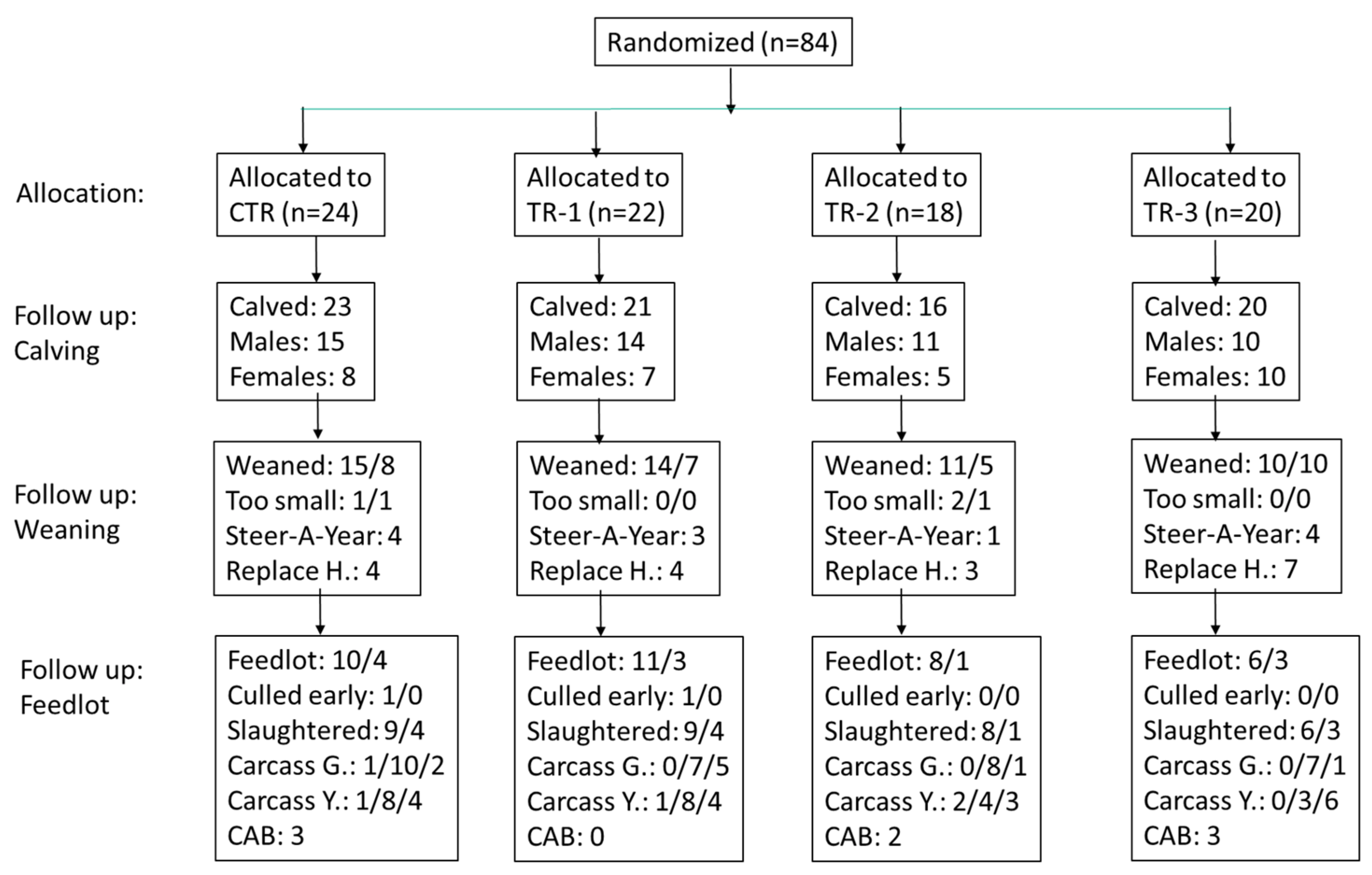
2.1. Animal Ethics Statement and Study Design
2.2. Blood Se Collection and Analyses
2.3. Nasal Microbiota Collection and Analyses
2.4. Statistical Analyses
3. Results
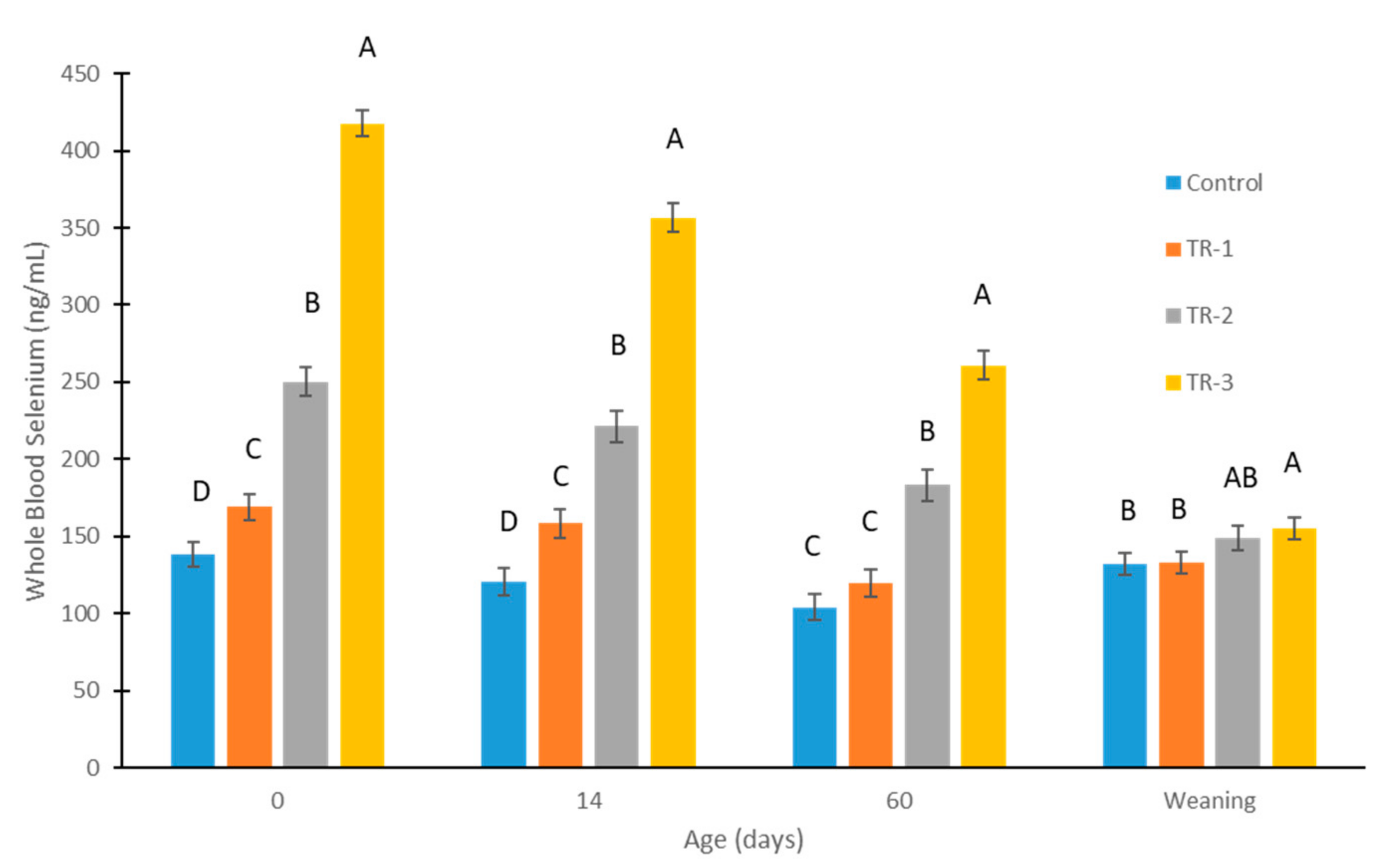
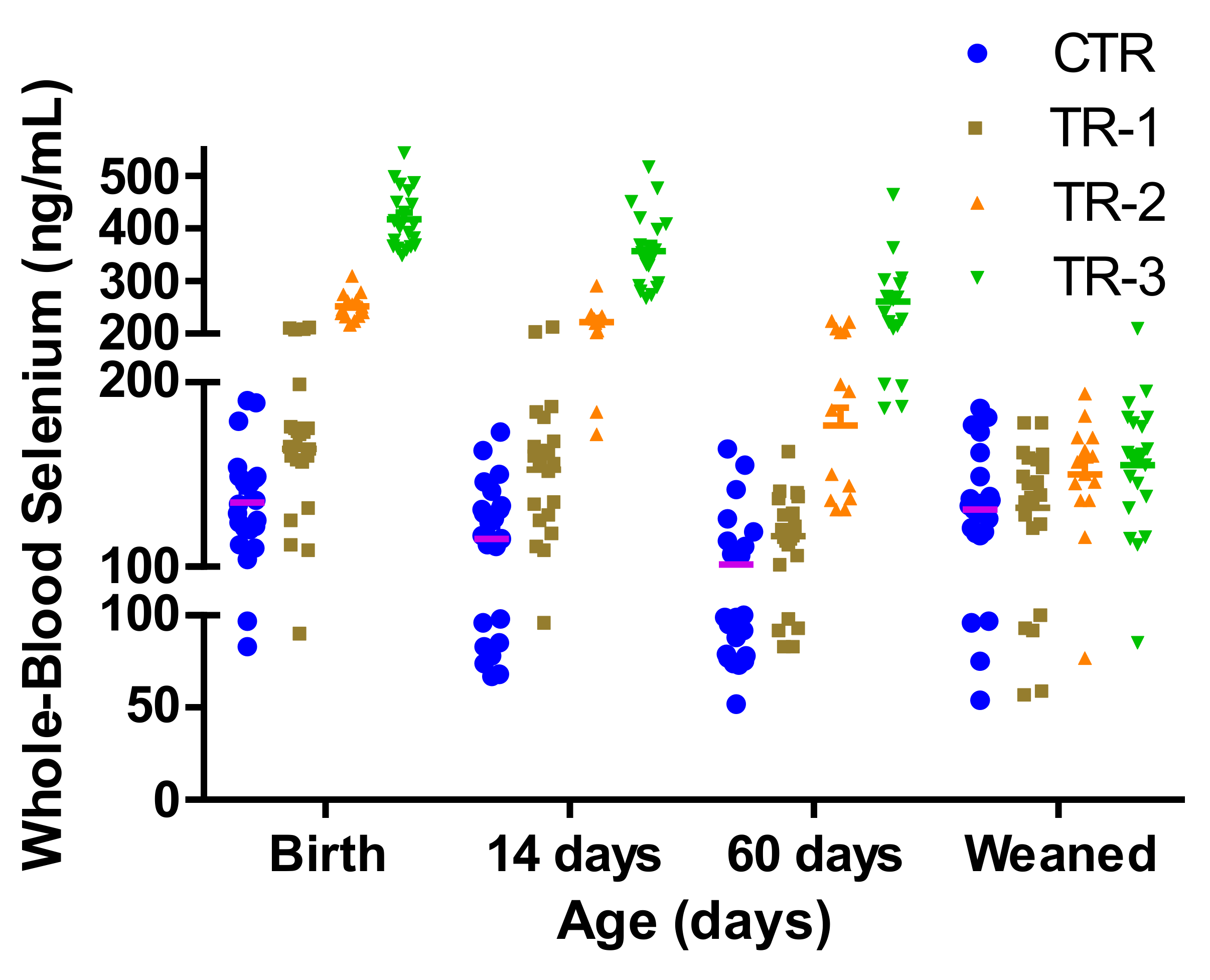
3.1. Effect of Supranutritional Se-Yeast Supplementation of Beef Cows on Whole-Blood Se Status of Their Calves at Weaning
3.2. Effect of Supranutritional Se-Yeast Supplementation of Beef Cows on Growth and Carcass Characteristics of Their Calves
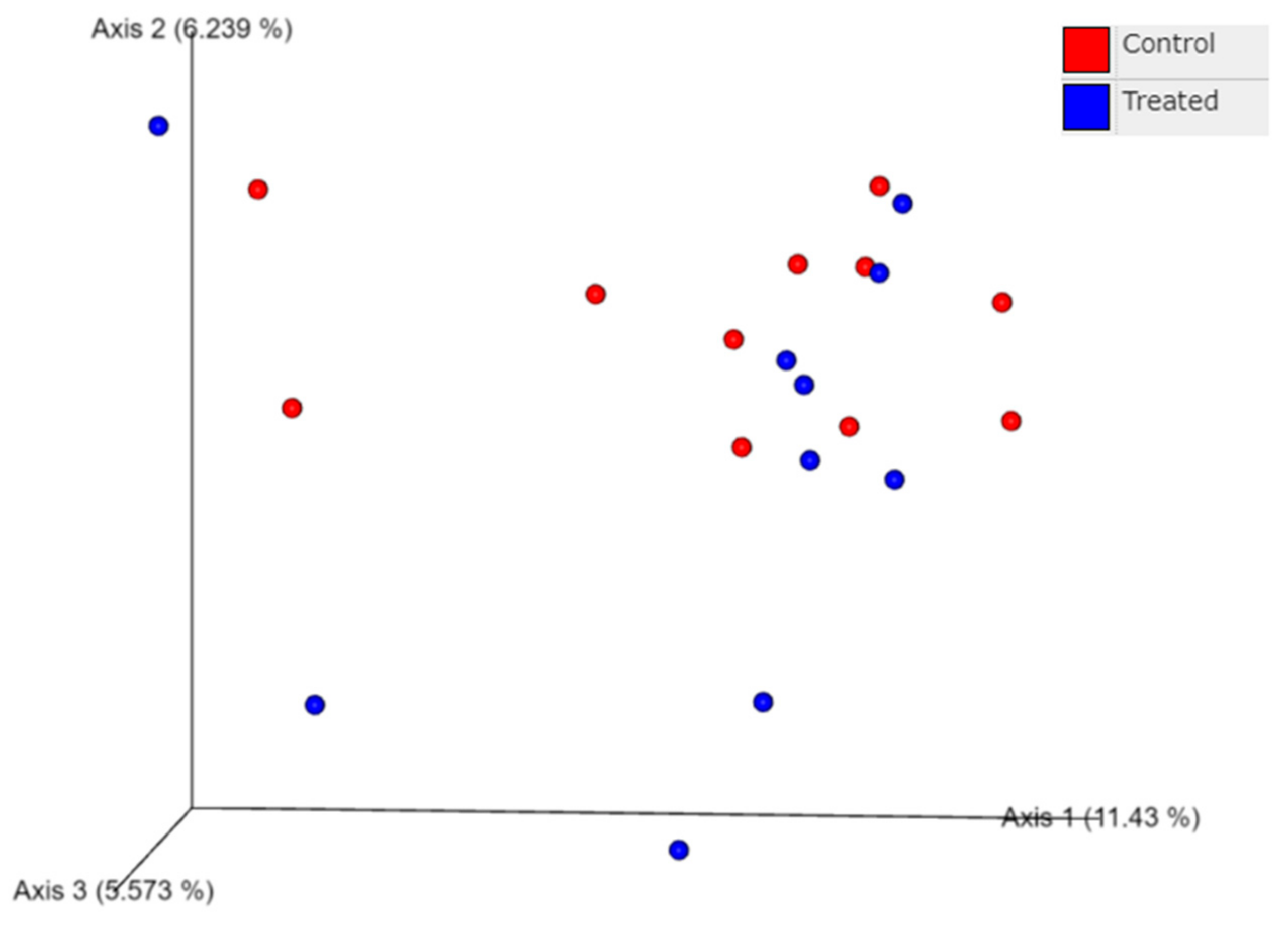
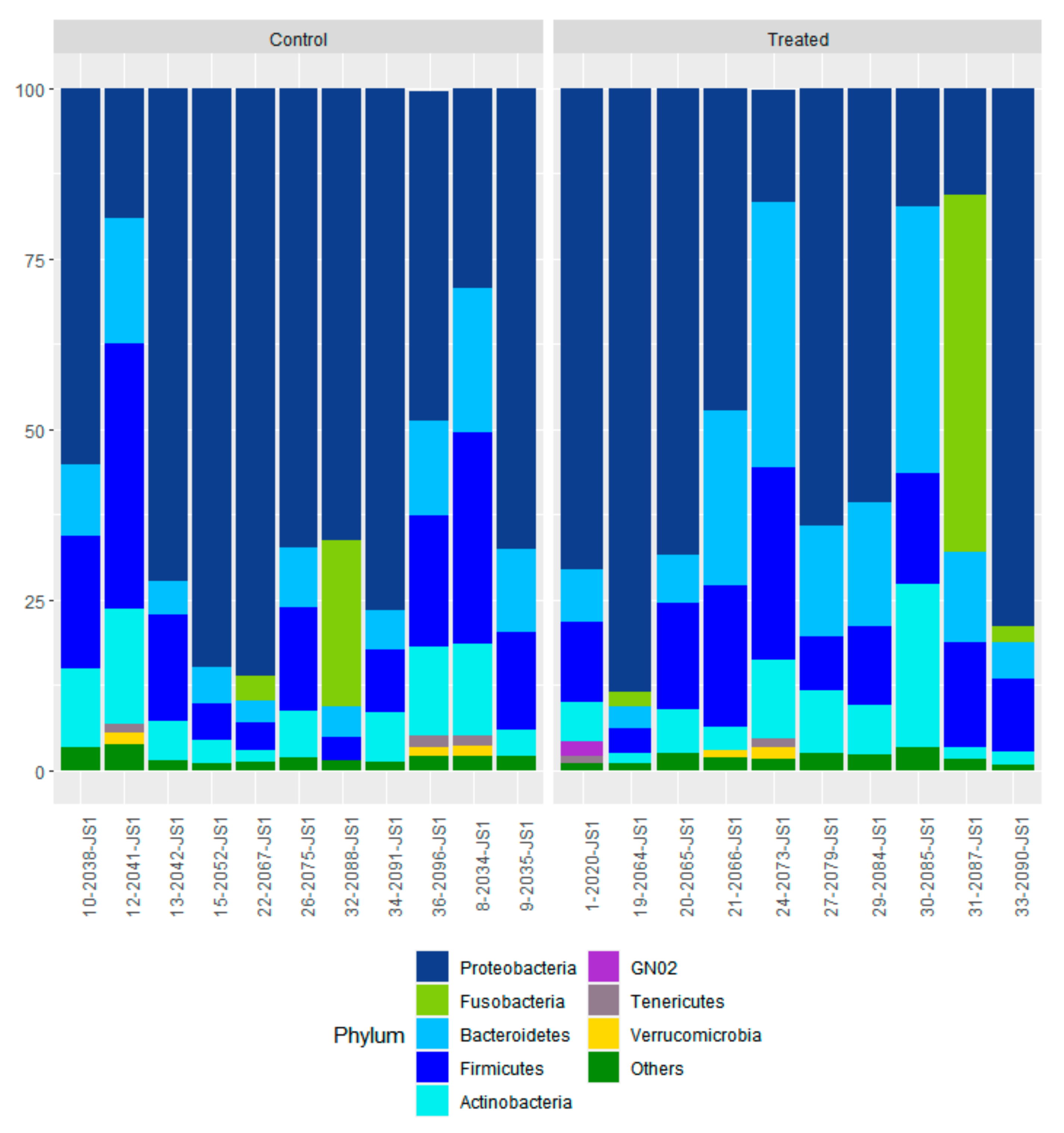
3.3. Effect of Supranutritional Se-Yeast Supplementation of Beef Cows on Nasal Microbiome Genome of Their Calves at Weaning
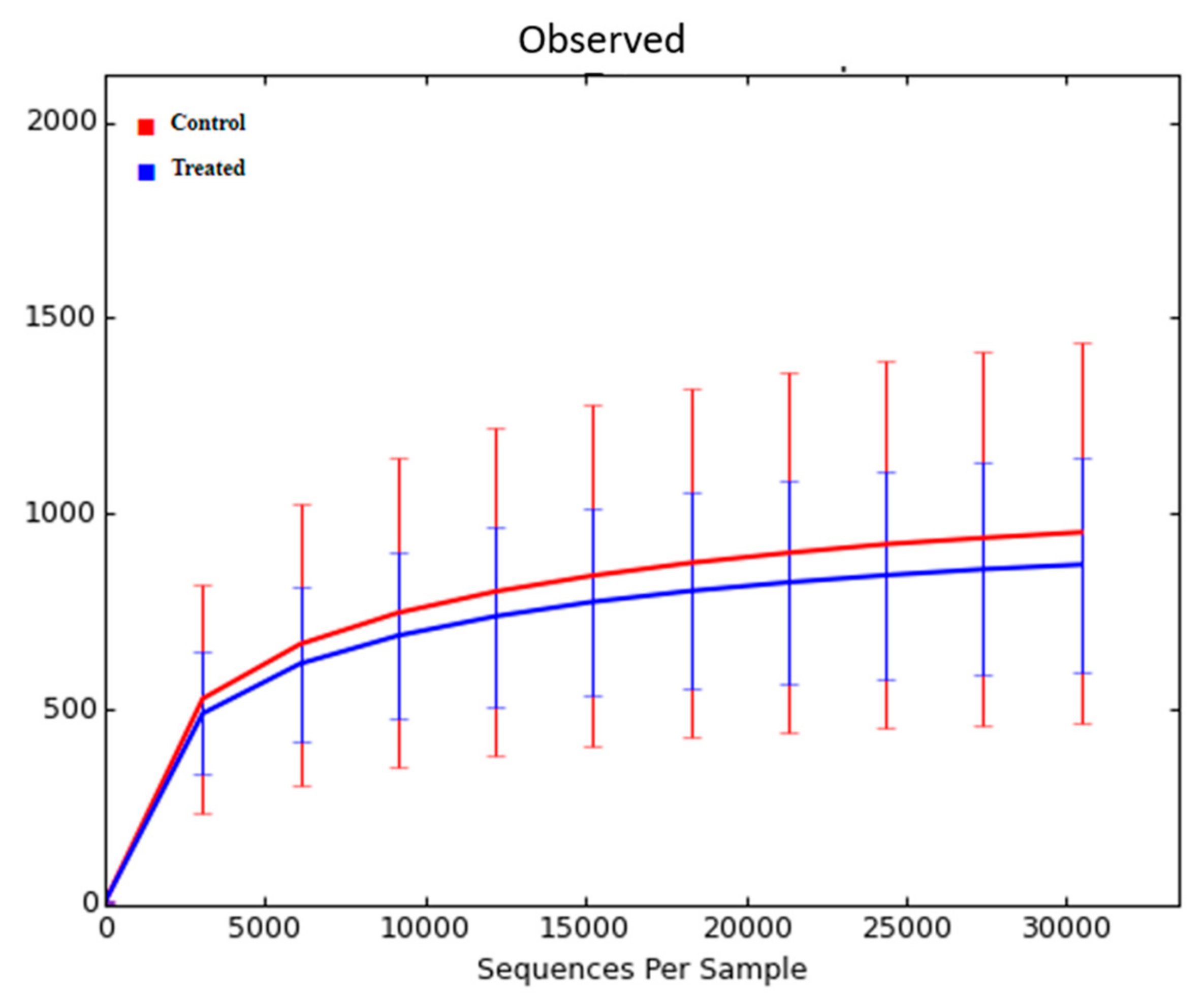
3.4. Effect of Supranutritional Se-Yeast Supplementation of Beef Cows on Nasal Microbiota of Their Calves at Weaning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldfield, J.E. Historical perspectives on selenium. Nutr. Today 2001, 36, 100–102. [Google Scholar] [CrossRef]
- Herdt, T.H.; Hoff, B. The use of blood analysis to evaluate trace mineral status in ruminant livestock. Vet. Clin. N. Am. Food A 2011, 27, 255. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, C.B.; Miller, S.M. Selenium in ruminant nutrition: A review. J. Dairy Sci. 1975, 58, 1561–1577. [Google Scholar] [CrossRef]
- Timsit, E.; Workentine, M.; Schryvers, A.B.; Holman, D.B.; van der Meer, F.; Alexander, T.W. Evolution of the nasopharyngeal microbiota of beef cattle from weaning to 40 days after arrival at a feedlot. Vet. Microbiol. 2016, 187, 75–81. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed, H.; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- Filley, S.J.; Peters, A.; Bouska, C. Effect of selenium fertilizer on forage selenium content. J. Anim. Sci. 2007, 85, 35. [Google Scholar]
- Brummer, F.A.; Gow-Hogge, L.; Mueller, C.; Pirelli, G.; Bobe, G. Short Communication: Mineral assessment of rangeland-managed beef cows in the high desert region of Oregon. Appl. Anim. Sci. 2019, 35, 577–585. [Google Scholar] [CrossRef]
- Brummer, F.A.; Pirelli, G.; Hall, J.A. Selenium Supplementation Strategies for Livestock in Oregon; Oregon State University: Corvallis, OR, USA, 2014; pp. 1–9. [Google Scholar]
- Hall, J.A.; Bobe, G.; Hunter, J.K.; Vorachek, W.R.; Stewart, W.C.; Vanegas, J.A.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J. Effect of feeding selenium-fertilized alfalfa hay on performance of weaned beef calves. PLoS ONE 2013, 8, e58188. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Hugejiletu; Gorman, M.E.; Mosher, W.D.; Pirelli, G.J. Effects of feeding selenium-enriched alfalfa hay on immunity and health of weaned beef calves. Biol. Trace Elem. Res. 2013, 156, 96–110. [Google Scholar] [CrossRef]
- Hall, J.A.; Isaiah, A.; Bobe, G.; Estill, C.T.; Bishop-Stewart, J.K.; Davis, T.Z.; Suchodolski, J.S.; Pirelli, G.J. Feeding selenium-biofortified alfalfa hay during the preconditioning period improves growth, carcass weight, and nasal microbial diversity of beef calves. PLoS ONE 2020, 15, e0242771. [Google Scholar] [CrossRef]
- Hall, J.A.; Isaiah, A.; Estill, C.T.; Pirelli, G.J.; Suchodolski, J.S. Weaned beef calves fed selenium-biofortified alfalfa hay have an enriched nasal microbiota compared with healthy controls. PLoS ONE 2017, 12, e0179215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hall, J.A.; Sendek, R.L.; Chinn, R.M.; Bailey, D.P.; Thonstad, K.N.; Wang, Y.; Forsberg, N.E.; Vorachek, W.R.; Stang, B.V.; Van Saun, R.J.; et al. Higher whole-blood selenium is associated with improved immune responses in footrot-affected sheep. Vet. Res. 2011, 42, 99. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Bobe, G.; Pirelli, G.J.; Mosher, W.D.; Hall, J.A. Organic and inorganic selenium: III. Ewe and progeny performance. J. Anim Sci 2012, 90, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Bobe, G.; Nixon, B.K.; Vorachek, W.R.; Hugejiletu; Nichols, T.; Mosher, W.D.; Pirelli, G.J. Effect of transport on blood selenium and glutathione status in feeder lambs. J. Anim. Sci. 2014, 92, 4115–4122. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Estill, C.T.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of supranutritional maternal and colostral selenium supplementation on passive absorption of immunoglobulin G in selenium-replete dairy calves. J. Dairy Sci. 2014, 97, 4379–4391. [Google Scholar] [CrossRef]
- Hugejiletu, H.; Bobe, G.; Vorachek, W.R.; Gorman, M.E.; Mosher, W.D.; Pirelli, G.J.; Hall, J.A. Selenium supplementation alters gene expression profiles associated with innate immunity in whole-blood neutrophils of sheep. Biol. Trace Elem. Res. 2013, 154, 28–44. [Google Scholar] [CrossRef]
- Holman, D.B.; McAllister, T.A.; Topp, E.; Wright, A.D.G.; Alexander, T.W. The nasopharyngeal microbiota of feedlot cattle that develop bovine respiratory disease. Vet. Microbiol. 2015, 180, 90–95. [Google Scholar] [CrossRef]
- Hall, J.A.; Harwell, A.M.; Van Saun, R.J.; Vorachek, W.R.; Stewart, W.C.; Galbraith, M.L.; Hooper, K.J.; Hunter, J.K.; Mosher, W.D.; Pirelli, G.J. Agronomic biofortification with selenium: Effects on whole blood selenium and humoral immunity in beef cattle. Anim. Feed Sci. Technol. 2011, 164, 184–190. [Google Scholar] [CrossRef]
- Wang, G.J.; Bobe, G.; Filley, S.J.; Pirelli, G.J.; Bohle, M.G.; Davis, T.Z.; Banuelos, G.L.; Hall, J.A. Effects of springtime sodium selenate foliar application and NPKS fertilization on selenium concentrations and selenium species in forages across Oregon. Anim. Feed Sci. Technol. 2021, 276, 114944. [Google Scholar] [CrossRef]
- NRC. Mineral Tolerance of Animals, 2nd ed.; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, 9490. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide. Statistics, version 9.2; SAS Inst Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef]
- Hughes, J.B.; Hellmann, J.J.; Ricketts, T.H.; Bohannan, B.J. Counting the uncountable: Statistical approaches to estimating microbial diversity. Appl. Environ. Microbiol. 2001, 67, 4399–4406. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Di Rienzi, S.C.; Poole, A.C.; Koren, O.; Walters, W.A.; Caporaso, J.G.; Knight, R.; Ley, R.E. Conducting a microbiome study. Cell 2014, 158, 250–262. [Google Scholar] [CrossRef]
- Fukuyama, J. Emphasis on the deep or shallow parts of the tree provides a new characterization of phylogenetic distances. Genome Biol. 2019, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.G.; Bobe, G.; Vorachek, W.R.; Dolan, B.P.; Estill, C.T.; Pirelli, G.J.; Hall, J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn claves. J. Anim. Sci. 2017, 95, 2408–2420. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.S.; Bobe, G.; Klopfenstein, J.J.; Gultekin, Y.; Davis, T.Z.; Ward, A.K.; Hall, J.A. Supranutritional maternal organic selenium supplementation during different trimesters of pregnancy affects the muscle gene transcriptome of newborn beef calves in a time-dependent manner. Genes 2021, 12, 1884. [Google Scholar] [CrossRef]
- Muegge, C.R.; Brennan, K.M.; Schoonmaker, J.P. Supplementation of organic and inorganic selenium to late gestation and early lactation beef cows effect on progeny feedlot performance and carcass characteristics. J. Anim. Sci. 2017, 95, 1356–1362. [Google Scholar] [CrossRef]
- Muegge, C.R.; Brennan, K.M.; Schoonmaker, J.P. Supplementation of organic and inorganic selenium to late gestation and early lactation beef cows effect on cow and preweaning calf performance. J. Anim. Sci. 2016, 94, 3399–3408. [Google Scholar] [CrossRef]
- Grossi, S.; Dell’Anno, M.; Rossi, L.; Compiani, R.; Sgoifo Rossi, C.A. Supplementation of live yeast, mannan oligosaccharide, and organic selenium during the adaptation phase of newly arrived beef cattle: Effects on health status, immune functionality, and growth performance. Antibiotics 2021, 10, 114. [Google Scholar] [CrossRef]

| Body Weight | Control | TR-1 | TR-2 | TR-3 | SEM | p Value |
|---|---|---|---|---|---|---|
| (kg) | (n = 23) | (n = 21) | (n = 14) | (n = 20) | ||
| 0 days (birth) | 39.7 a | 36.8 b | 40.0 a | 38.7 ab | 1.2 | 0.22 |
| 14 days | 56.1 | 53.1 | 52.5 | 55.5 | 2.0 | 0.31 |
| 60 days | 110.2 | 108.6 | 102.9 | 110.8 | 4.0 | 0.38 |
| 224 days (weaning) | 295.7 a | 294.4 ab | 272.1 b | 299.9 a | 9.6 | 0.10 |
| 474 days (carcass) | 411.6 | 404.7 | 383.2 | 391.5 | 13.1 | 0.26 |
| Calves from Control and TR-1 Dams (n = 11) | Calves from TR-2 and TR-3 Dams (n = 10) | p-Value | |
|---|---|---|---|
| Chao1 | 1028 ± 564.4 | 931.1 ± 324.4 | 0.63 |
| Observed ASV | 952.5 ± 510.0 | 870.1 ± 290.6 | 0.65 |
| Shannon Index | 7.4 ± 1.6 | 7.2 ± 0.9 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, J.A.; Isaiah, A.; McNett, E.R.L.; Klopfenstein, J.J.; Davis, T.Z.; Suchodolski, J.S.; Bobe, G. Supranutritional Selenium-Yeast Supplementation of Beef Cows during the Last Trimester of Pregnancy Results in Higher Whole-Blood Selenium Concentrations in Their Calves at Weaning, but Not Enough to Improve Nasal Microbial Diversity. Animals 2022, 12, 1360. https://doi.org/10.3390/ani12111360
Hall JA, Isaiah A, McNett ERL, Klopfenstein JJ, Davis TZ, Suchodolski JS, Bobe G. Supranutritional Selenium-Yeast Supplementation of Beef Cows during the Last Trimester of Pregnancy Results in Higher Whole-Blood Selenium Concentrations in Their Calves at Weaning, but Not Enough to Improve Nasal Microbial Diversity. Animals. 2022; 12(11):1360. https://doi.org/10.3390/ani12111360
Chicago/Turabian StyleHall, Jean A., Anitha Isaiah, Ened R.L. McNett, Joseph J. Klopfenstein, T. Zane Davis, Jan S. Suchodolski, and Gerd Bobe. 2022. "Supranutritional Selenium-Yeast Supplementation of Beef Cows during the Last Trimester of Pregnancy Results in Higher Whole-Blood Selenium Concentrations in Their Calves at Weaning, but Not Enough to Improve Nasal Microbial Diversity" Animals 12, no. 11: 1360. https://doi.org/10.3390/ani12111360
APA StyleHall, J. A., Isaiah, A., McNett, E. R. L., Klopfenstein, J. J., Davis, T. Z., Suchodolski, J. S., & Bobe, G. (2022). Supranutritional Selenium-Yeast Supplementation of Beef Cows during the Last Trimester of Pregnancy Results in Higher Whole-Blood Selenium Concentrations in Their Calves at Weaning, but Not Enough to Improve Nasal Microbial Diversity. Animals, 12(11), 1360. https://doi.org/10.3390/ani12111360








