Comparison between Image-Guided Transbronchial Cryobiopsies and Thoracoscopic Lung Biopsies in Canine Cadaver: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
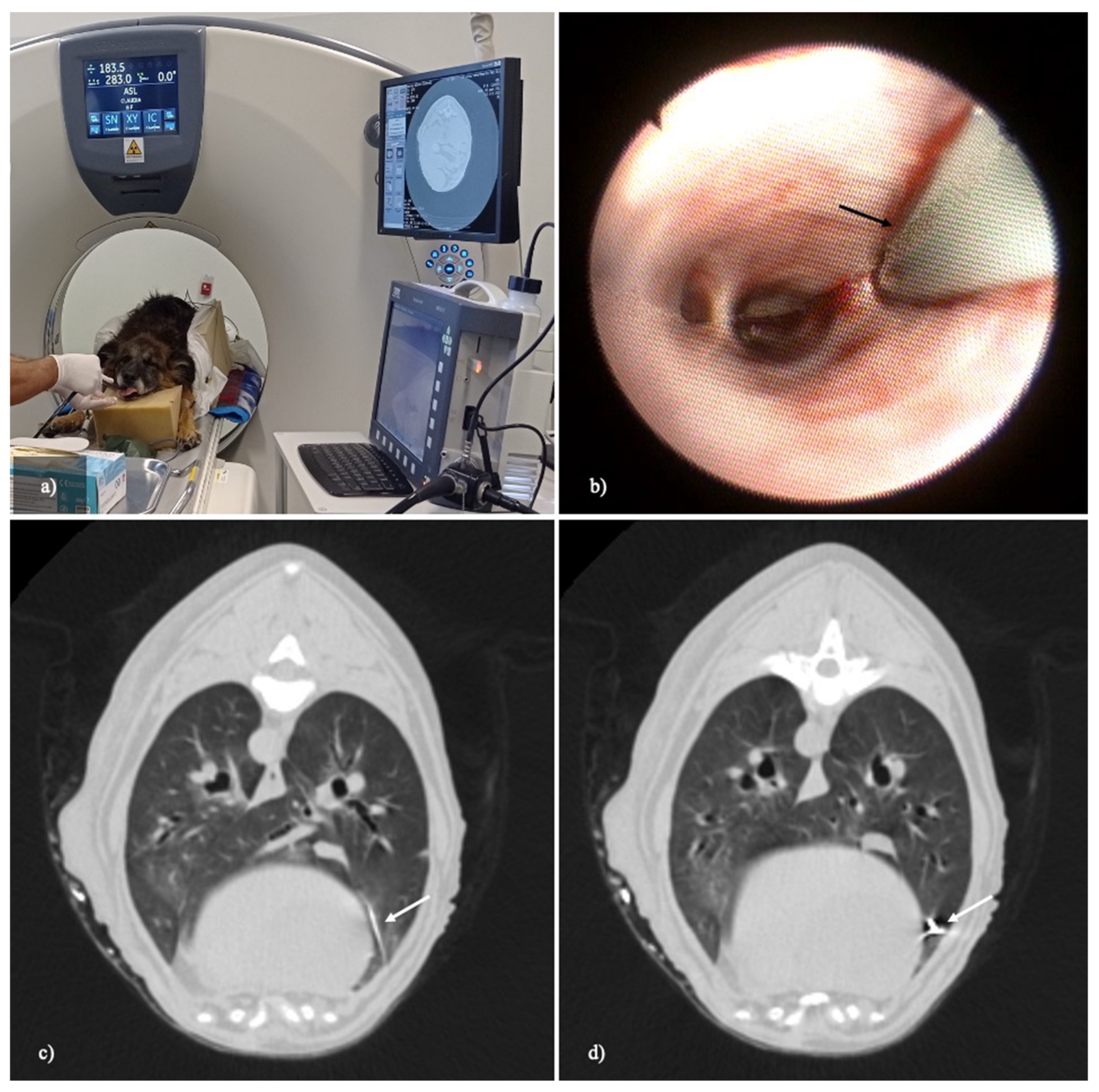
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Boedec, K.; Roady, P.J.; O’Brien, R.T. A Case of Atypical Diffuse Feline Fibrotic Lung Disease. J. Feline Med. Surg. 2014, 16, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C. Interstitial Lung Diseases in Dogs and Cats Part I: The Idiopathic Interstitial Pneumonias. Vet. J. 2019, 243, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä-Laurila, H.P.; Rajamäki, M.M. Idiopathic Pulmonary Fibrosis in West Highland White Terriers. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.R.; Griffey, S.M.; Walsh, P. Use of Keyhole Lung Biopsy for Diagnosis of Interstitial Lung Diseases in Dogs and Cats: 13 Cases (1998–2001). J. Am. Vet. Med. Assoc. 2002, 221, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.A. Pulmonary Parenchymal Disease. In Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat; Ettinger, S., Feldman, E., Eds.; Saunders, Elsevier: St. Louis, MO, USA, 2010; Volume 2. [Google Scholar]
- Laurila, H.P.; Rajamäki, M.M. Update on Canine Idiopathic Pulmonary Fibrosis in West Highland White Terriers. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Thierry, F.; Handel, I.; Hammond, G.; King, L.G.; Corcoran, B.M.; Schwarz, T. Further Characterization of Computed Tomographic and Clinical Features for Staging and Prognosis of Idiopathic Pulmonary Fibrosis in West Highland White Terriers. Vet. Radiol. Ultrasound 2017, 58, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heikkilä, H.P.; Lappalainen, A.K.; Day, M.J.; Clercx, C.; Rajamäki, M.M. Clinical, Bronchoscopic, Histopathologic, Diagnostic Imaging, and Arterial Oxygenation Findings in West Highland White Terriers with Idiopathic Pulmonary Fibrosis. J. Vet. Intern. Med. 2011, 25, 433–439. [Google Scholar] [CrossRef]
- Lobetti, R.; Milner, R.; Lane, E. Chronic Idiopathic Pulmonary Fibrosis in Five Dogs. J. Am. Anim. Hosp. Assoc. 2001, 37, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Koster, L.; Kirberger, R. A Syndrome of Severe Idiopathic Pulmonary Parenchymal Disease with Pulmonary Hypertension in Pekingese. Vet. Med. Res. Rep. 2016, 7, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Roels, E.; Fastrès, A.; Merveille, A.-C.; Bolen, G.; Teske, E.; Clercx, C.; Mc Entee, K. The Prevalence of Pulmonary Hypertension Assessed Using the Pulmonary Vein-to-right Pulmonary Artery Ratio and Its Association with Survival in West Highland White Terriers with Canine Idiopathic Pulmonary Fibrosis. BMC Vet. Res. 2021, 17, 171. [Google Scholar] [CrossRef]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM Consensus Statement Guidelines for the Diagnosis, Classification, Treatment, and Monitoring of Pulmonary Hypertension in Dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Sutherland-Smith, J.; Hankin, E.J.; Cunningham, S.M.; Sato, A.F.; Barton, B.A. Comparison of a Computed Tomographic Pulmonary Trunk to Aorta Diameter Ratio with Echocardiographic Indices of Pulmonary Hypertension in Dogs. Vet. Radiol. Ultrasound 2018, 59, 18–26. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Johnson, V.S.; Corcoran, B.M.; Wotton, P.R.; Schwarz, T.; Sullivan, M. Thoracic High-Resolution Computed Tomographic Findings in Dogs with Canine Idiopathic Pulmonary Fibrosis. J. Small Anim. Pract. 2005, 46, 381–388. [Google Scholar] [CrossRef]
- Roels, E.; Couvreur, T.; Farnir, F.; Clercx, C.; Verschakelen, J.; Bolen, G. Comparison between Sedation and General Anesthesia for High Resolution Computed Tomographic Characterization of Canine Idiopathic Pulmonary Fibrosis in West Highland White Terriers. Vet. Radiol. Ultrasound 2017, 58, 284–294. [Google Scholar] [CrossRef] [Green Version]
- Holopainen, S.; Rautala, E.; Lilja-Maula, L.; Lohi, H.; Rajamäki, M.M.; Lappalainen, A.K. Thoracic High Resolution CT Using the Modified VetMousetrapTM Device Is a Feasible Method for Diagnosing Canine Idiopathic Pulmonary Fibrosis in Awake West Highland White Terriers. Vet. Radiol. Ultrasound 2019, 60, 525–532. [Google Scholar] [CrossRef]
- Stavri, A.; Masseau, I.; Reinero, C.R. Reversibility of Clinical and Computed Tomographic Lesions Mimicking Pulmonary Fibrosis in a Young Cat. BMC Vet. Res. 2021, 17, 380. [Google Scholar] [CrossRef]
- Al-Hyani, O.H. A Comparative Study for Lung Biopsy in Dogs. Iraqi J. Vet. Sci. 2011, 25, 35–40. [Google Scholar] [CrossRef]
- Bauer, T.G. Lung Biopsy. Vet. Clin. N. Am. Small Anim. Pract. 2000, 30, 1207–1225. [Google Scholar] [CrossRef]
- Marvel, S.; Monnet, E. Ex Vivo Evaluation of Canine Lung Biopsy Techniques: Ex Vivo Evaluation of Canine Lung Biopsy Techniques. Vet. Surg. 2013, 42, 473–477. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Culp, W.T.N.; Pascoe, P.J.; Arzi, N.V. Use of the Ligasure Vessel-Sealing Device for Thoracoscopic Peripheral Lung Biopsy in Healthy Dogs: Ligasure Thoracoscopic Lung Biopsy in Dogs. Vet. Surg. 2012, 41, 523–528. [Google Scholar] [CrossRef]
- Babiak, A.; Hetzel, J.; Krishna, G.; Fritz, P.; Moeller, P.; Balli, T.; Hetzel, M. Transbronchial Cryobiopsy: A New Tool for Lung Biopsies. Respiration 2009, 78, 203–208. [Google Scholar] [CrossRef]
- Hetzel, J.; Wells, A.U.; Costabel, U.; Colby, T.V.; Walsh, S.L.F.; Verschakelen, J.; Cavazza, A.; Tomassetti, S.; Ravaglia, C.; Böckeler, M.; et al. Transbronchial Cryobiopsy Increases Diagnostic Confidence in Interstitial Lung Disease: A Prospective Multicentre Trial. Eur. Respir. J. 2020, 56, 1901520. [Google Scholar] [CrossRef]
- Cavazza, A.; Colby, T.V.; Dubini, A.; Tomassetti, S.; Ravaglia, C.; Poletti, V.; Mengoli, M.C.; Tagliavini, E.; Rossi, G. Transbronchial Cryobiopsy in the Diagnosis of Diffuse Lung Disease. Surg. Pathol. Clin. 2020, 13, 197–208. [Google Scholar] [CrossRef]
- Dhooria, S.; Mehta, R.M.; Srinivasan, A.; Madan, K.; Sehgal, I.S.; Pattabhiraman, V.; Yadav, P.; Sivaramakrishnan, M.; Mohan, A.; Bal, A.; et al. The Safety and Efficacy of Different Methods for Obtaining Transbronchial Lung Cryobiopsy in Diffuse Lung Diseases. Clin. Respir. J. 2018, 12, 1711–1720. [Google Scholar] [CrossRef]
- Steinfort, D.P.; D’Agostino, R.D.; Vrjlic, I.; Einsiedel, P.; Prasad, J.D.; Jennings, B.R.; Heinze, S.; Irving, L.B. CT-Fluoroscopic Guidance for Performance of Targeted Transbronchial Cryobiopsy: A Preliminary Report. Respiration 2018, 96, 472–479. [Google Scholar] [CrossRef]
- Meyer, C.A.; White, C.S.; Wu, J.; Futterer, S.F.; Templeton, P.A. Real-Time CT Fluoroscopy: Usefulness in Thoracic Drainage. Am. J. Roentgenol. 1998, 171, 1097–1101. [Google Scholar] [CrossRef]
- Vignoli, M.; Rossi, F.; Chierici, C.; Terragni, R.; De Lorenzi, D.; Stanga, M.; Olivero, D. Needle Tract Implantation after Fine Needle Aspiration Biopsy (FNAB) of Transitional Cell Carcinoma of the Urinary Bladder and Adenocarcinoma of the Lung. Schweizer Archiv für Tierheilkunde 2007, 149, 314–318. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wisner, E.R. Advances in Respiratory Imaging. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 879–900. [Google Scholar] [CrossRef]
- Iftikhar, I.H.; Alghothani, L.; Sardi, A.; Berkowitz, D.; Musani, A.I. Transbronchial Lung Cryobiopsy and Video-Assisted Thoracoscopic Lung Biopsy in the Diagnosis of Diffuse Parenchymal Lung Disease: A Meta-Analysis of Diagnostic Test Accuracy. Ann. Am. Thorac. Soc. 2017, 14, 1197–1211. [Google Scholar] [CrossRef]
- Poletti, V.; Ravaglia, C.; Tomassetti, S. Transbronchial Cryobiopsy in Diffuse Parenchymal Lung Diseases. Curr. Opin. Pulm. Med. 2016, 22, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, C.; Wells, A.U.; Tomassetti, S.; Gurioli, C.; Gurioli, C.; Dubini, A.; Cavazza, A.; Colby, T.V.; Piciucchi, S.; Puglisi, S.; et al. Diagnostic Yield and Risk/Benefit Analysis of Trans-Bronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Large Cohort of 699 Patients. BMC Pulm. Med. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.R.; Hur, J.; Lee, S.M.; Lee, H.-J.; Hong, Y.J.; Nam, J.E.; Kim, H.S.; Kim, Y.J.; Choi, B.W.; Kim, T.H.; et al. CT Fluoroscopy-Guided Lung Biopsy versus Conventional CT-Guided Lung Biopsy: A Prospective Controlled Study to Assess Radiation Doses and Diagnostic Performance. Eur. Radiol. 2011, 21, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Froelich, J.J.; Ishaque, N.; Regn, J.; Saar, B.; Walthers, E.M.; Klose, K.J. Guidance of Percutaneous Pulmonary Biopsies with Real-Time CT Fluoroscopy. Eur. J. Radiol. 2002, 42, 74–79. [Google Scholar] [CrossRef]
- Daly, B.; Templeton, P.A. Real-Time CT Fluoroscopy: Evolution of an Interventional Tool. Radiology 1999, 211, 309–315. [Google Scholar] [CrossRef]
- Paulson, E.K.; Sheafor, D.H.; Enterline, D.S.; McAdams, H.P.; Yoshizumi, T.T. CT Fluoroscopy-Guided Interventional Procedures: Techniques and Radiation Dose to Radiologists. Radiology 2001, 220, 161–167. [Google Scholar] [CrossRef]
- Stoeckelhuber, B.M.; Leibecke, T.; Schulz, E.; Melchert, U.H.; Bergmann-Koester, C.U.; Helmberger, T.; Gellissen, J. Radiation Dose to the Radiologist’s Hand During Continuous CT Fluoroscopy-Guided Interventions. Cardiovasc. Intervent. Radiol. 2005, 28, 589–594. [Google Scholar] [CrossRef]
- Carlson, S.K.; Bender, C.E.; Classic, K.L.; Zink, F.E.; Quam, J.P.; Ward, E.M.; Oberg, A.L. Benefits and Safety of CT Fluoroscopy in Interventional Radiologic Procedures. Radiology 2001, 219, 515–520. [Google Scholar] [CrossRef]
- Walsh, P.J.; Remedios, A.M.; Ferguson, J.F.; Walker, D.D.; Cantwell, S.; Duke, T. Thoracoscopic Versus Open Partial Pericardectomy in Dogs: Comparison of Postoperative Pain and Morbidity. Vet. Surg. 1999, 28, 472–479. [Google Scholar] [CrossRef]
- Hetzel, J.; Maldonado, F.; Ravaglia, C.; Wells, A.U.; Colby, T.V.; Tomassetti, S.; Ryu, J.H.; Fruchter, O.; Piciucchi, S.; Dubini, A.; et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 2018, 95, 188–200. [Google Scholar] [CrossRef]
- Ravaglia, C.; Wells, A.U.; Tomassetti, S.; Dubini, A.; Cavazza, A.; Piciucchi, S.; Sverzellati, N.; Gurioli, C.; Gurioli, C.; Costabel, U.; et al. Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Disease: Comparison between Biopsy from 1 Segment and Biopsy from 2 Segments—Diagnostic Yield and Complications. Respiration 2017, 93, 285–292. [Google Scholar] [CrossRef]
- Ravaglia, C.; Bonifazi, M.; Wells, A.U.; Tomassetti, S.; Gurioli, C.; Piciucchi, S.; Dubini, A.; Tantalocco, P.; Sanna, S.; Negri, E.; et al. Safety and Diagnostic Yield of Transbronchial Lung Cryobiopsy in Diffuse Parenchymal Lung Diseases: A Comparative Study versus Video-Assisted Thoracoscopic Lung Biopsy and a Systematic Review of the Literature. Respiration 2016, 91, 215–227. [Google Scholar] [CrossRef]
- Hussein, S.A.M.; Elhadidy, A.A.; Amin, H.M.; Tantawy, A.A.; Negm, M.S. Transbronchial Cryobiopsy as a New Tool for Lung Biopsies in Diagnosis of Diffuse Parenchymal Lung Diseases. Egypt. J. Chest Dis. Tuberc. 2020, 69, 649–658. [Google Scholar]
- Casoni, G.L.; Tomassetti, S.; Cavazza, A.; Colby, T.V.; Dubini, A.; Ryu, J.H.; Carretta, E.; Tantalocco, P.; Piciucchi, S.; Ravaglia, C.; et al. Transbronchial Lung Cryobiopsy in the Diagnosis of Fibrotic Interstitial Lung Diseases. PLoS ONE 2014, 9, e86716. [Google Scholar] [CrossRef] [Green Version]
- Mehrad, M.; Colby, T.V.; Rossi, G.; Cavazza, A. Transbronchial Cryobiopsy in the Diagnosis of Fibrotic Interstitial Lung Disease. Arch. Pathol. Lab. Med. 2020, 144, 1501–1508. [Google Scholar] [CrossRef] [Green Version]
- Johannson, K.A.; Marcoux, V.S.; Ronksley, P.E.; Ryerson, C.J. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease: A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2016, 13, 1828–1838. [Google Scholar] [CrossRef]
- Troy, L.K.; Grainge, C.; Corte, T.J.; Williamson, J.P.; Vallely, M.P.; Cooper, W.A.; Mahar, A.; Myers, J.L.; Lai, S.; Mulyadi, E.; et al. Diagnostic Accuracy of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease Diagnosis (COLDICE): A Prospective, Comparative Study. Lancet Respir. Med. 2020, 8, 171–181. [Google Scholar] [CrossRef]
- Franke, K.-J.; Linzenbold, W.; Nuessle, D.; Enderle, M.; Boesmueller, H.; Nilius, G.; Hetzel, J. A New Tool for Transbronchial Cryobiopsies in the Lung: An Experimental Feasibility Ex Vivo Study. Respiration 2016, 91, 228–234. [Google Scholar] [CrossRef]




| CASE | TBLC LL × ll (mm) | VATS LL × ll (mm) | TBLC Morphometric Area (µm2) | VATS Morphometric Area (µm2) | TBLC Diagnostic/ Non-Diagnostic | VATS Diagnostic/ Non-Diagnostic |
|---|---|---|---|---|---|---|
| CASE 1 8-year-old, female, 25 kg, German Shepherd dog | 5 × 4 | 11 × 7 | 9,396,718.853 | 408,094,846.000 | yes | yes |
| 6 × 4 | 10 × 6 | 10,324,954.857 | 188,784,016.243 | yes | yes | |
| 6 × 4.5 | 6 × 3 | 12,140,963.542 | 14,741,529.047 | yes | yes | |
| 7 × 4 | 6 × 4 | 14,106,518.510 | 13,670,544.785 | yes | yes | |
| 6 × 4 | 7 × 5 | 9,986,131.659 | 26,115,367.832 | yes | yes | |
| 6 × 5 | 10 × 4 | 10,704,181.184 | 24,115,367.852 | yes | yes | |
| 7 × 4 | 12 × 9 | 7,428,219.660 | 29,827,114.240 | yes | not (Pleura, fat 100%) | |
| 5 × 4 | 10 × 7 | 8,475,004.000 | 38,789,118.787 | yes | not (pleura) | |
| 5 × 4 | 10 × 6 | 9,095,582.381 | 21,318,396.660 | yes | Not (pleura, fat 90%) | |
| 8 × 5 | 7 × 6 | 15,025,663.235 | 16,778,496.575 | yes | yes | |
| CASE 2 4-year-old, male, 20 kg, Breton dog | 6 × 4 | 6 × 5 | 1,983,314.112 | 15,245,819.671 | yes | yes |
| 4 × 3 | 11 × 5 | 8,943,111.418 | 22,510,133.198 | yes | yes | |
| 6 × 5 | 6 × 5 | 14,874,303.049 | 9,141,015.205 | yes | Not (pleura 100%) | |
| 7.5 × 4.5 | 6 × 5 | 22,810,981.388 | 19,253,547.092 | yes | Not (pleura, fat 100%) | |
| 10 × 6 | 9 × 7 | 36,447,651.581 | 24,082,825.732 | Not (bronchus 80%) | Not (pleura 90%) | |
| CASE 3 10-year-old, male, 22 kg mixed breed | 6 ×4 | 4 × 3 | 13,795,552.710 | 7,606,530.042 | / | / |
| 7 × 4 | 4 × 4 | 14,982,551.040 | 9,761,885.312 | / | / | |
| 8 × 4 | 11 × 5 | 7,421,963.532 | 22,196,350.990 | / | / | |
| 6 × 5 | 15 × 10 | 5,016,010.512 | 38,943,399.500 | / | / | |
| 7 × 4 | 6 × 2 | 176,640,219.500 | 7,413,050.998 | / | / | |
| 7 × 4 | 6 × 4 | 14,789,211.350 | 11,842,863.000 | / | / |
| Lung Biopsy | Mean Area (mm2) Macroscopic (LL × ll) | Mean Area (mm2) Morphometric | Non-Diagnostic |
|---|---|---|---|
| VATS | 48 (12–108) | 46.20 | 40% (6/15) |
| TBLC | 28 (12–60) | 20.20 | 6.6% (1/15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falerno, I.; Tamburro, R.; Collivignarelli, F.; Della Salda, L.; Navas, L.; Terragni, R.; Crisi, P.E.; Paolini, A.; Simeoni, F.; Vignoli, M. Comparison between Image-Guided Transbronchial Cryobiopsies and Thoracoscopic Lung Biopsies in Canine Cadaver: A Pilot Study. Animals 2022, 12, 1388. https://doi.org/10.3390/ani12111388
Falerno I, Tamburro R, Collivignarelli F, Della Salda L, Navas L, Terragni R, Crisi PE, Paolini A, Simeoni F, Vignoli M. Comparison between Image-Guided Transbronchial Cryobiopsies and Thoracoscopic Lung Biopsies in Canine Cadaver: A Pilot Study. Animals. 2022; 12(11):1388. https://doi.org/10.3390/ani12111388
Chicago/Turabian StyleFalerno, Ilaria, Roberto Tamburro, Francesco Collivignarelli, Leonardo Della Salda, Luigi Navas, Rossella Terragni, Paolo Emidio Crisi, Andrea Paolini, Francesco Simeoni, and Massimo Vignoli. 2022. "Comparison between Image-Guided Transbronchial Cryobiopsies and Thoracoscopic Lung Biopsies in Canine Cadaver: A Pilot Study" Animals 12, no. 11: 1388. https://doi.org/10.3390/ani12111388
APA StyleFalerno, I., Tamburro, R., Collivignarelli, F., Della Salda, L., Navas, L., Terragni, R., Crisi, P. E., Paolini, A., Simeoni, F., & Vignoli, M. (2022). Comparison between Image-Guided Transbronchial Cryobiopsies and Thoracoscopic Lung Biopsies in Canine Cadaver: A Pilot Study. Animals, 12(11), 1388. https://doi.org/10.3390/ani12111388








