Partial Substitution of Corn Grain in the Diet with Beet Pulp Reveals Increased Ruminal Acetate Proportion and Circulating Insulin Levels in Korean Cattle Steers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Diets
2.2. Blood Collection and Analyses
2.3. Rumen Fluid Collection and Analysis
2.4. Analysis of the Chemical Composition of the Feeds
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
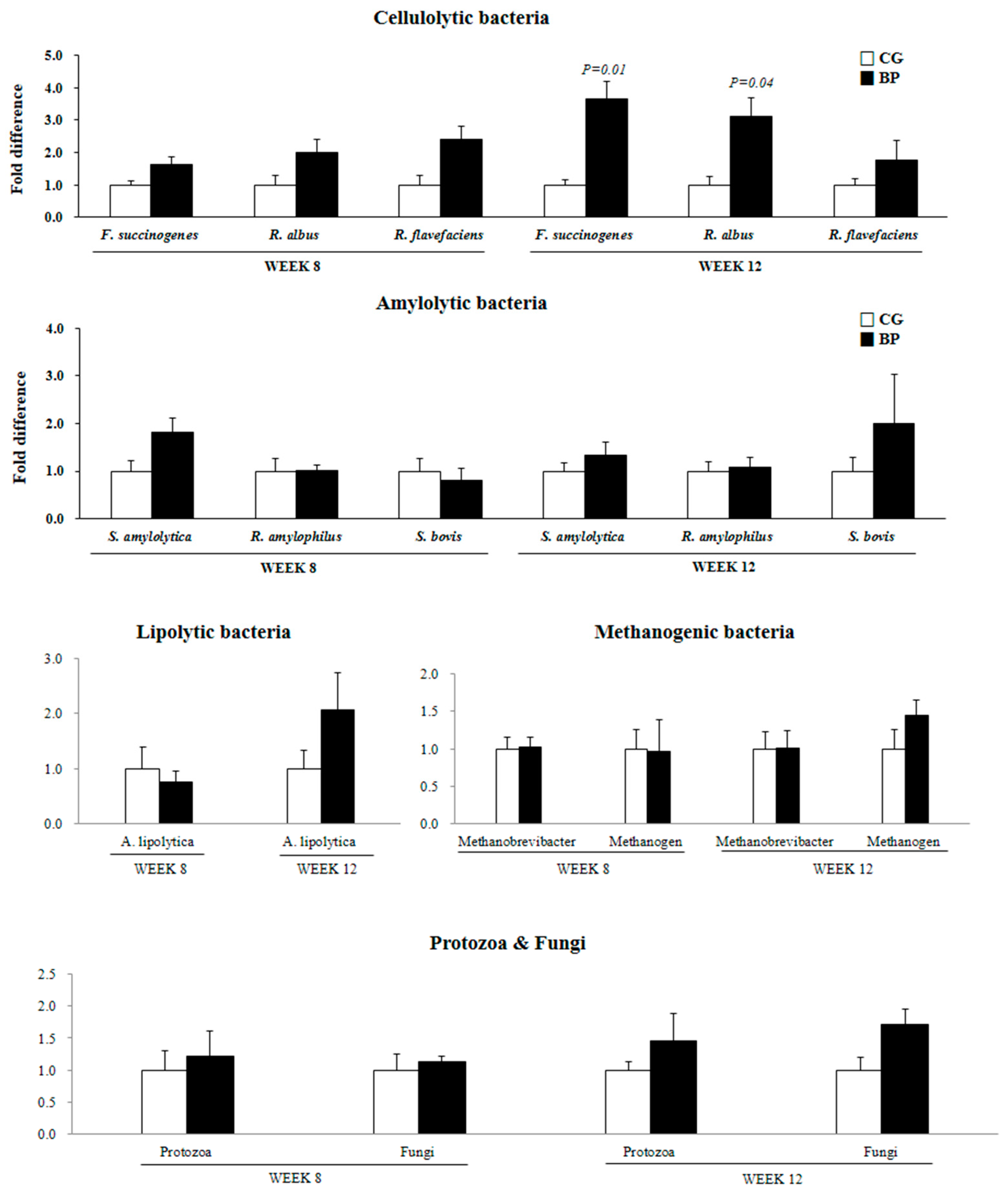
3.2. Ruminal Fermentation Characteristics and Microbial Population
3.3. Blood Metabolites and Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.J.; Beak, S.H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A Review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.V.; Nguyen, O.C.; Malau-Aduli, A.E.O. Main regulatory factors of marbling level in beef cattle. Vet. Anim. Sci. 2021, 14, 100219. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.L.; Cundiff, L.V.; Koch, R.M. Effect of marbling degree on beef palatability in Bos taurus and Bos indicus cattle. J. Anim. Sci. 1994, 72, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Crouse, J.D. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 1984, 114, 792–800. [Google Scholar] [CrossRef]
- Nafikov, R.A.; Beitz, D.C. Carbohydrate and lipid metabolism in farm animals. Proc. J. Nutr. 2007, 137, 702–705. [Google Scholar] [CrossRef] [Green Version]
- Schoonmaker, J.P.; Cecava, M.J.; Faulkner, D.B.; Fluharty, F.L.; Zerby, H.N.; Loerch, S.C. Effect of source of energy and rate of growth on performance, carcass characteristics, ruminal fermentation, and serum glucose and insulin of early-weaned steers. J. Anim. Sci. 2003, 81, 843–855. [Google Scholar] [CrossRef]
- Nayananjalie, W.A.D.; Wiles, T.R.; Gerrard, D.E.; McCann, M.A.; Hanigan, M.D. Acetate and glucose incorporation into subcutaneous, intramuscular, and visceral fat of finishing steers. J. Anim. Sci. 2015, 93, 2451–2459. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Lee, H.J.; Kim, D.W.; Park, J.G.; Han, I.K. Effects of carbon precursors and hormones on the lipogenesis and lipolysis of hanwoo cattle adipose tissues. Asian-Australas. J. Anim. Sci. 2000, 13, 300–306. [Google Scholar] [CrossRef]
- Song, M.K.; Sohn, H.J.; Hong, S.K.; Kim, H.C. Utilization of substrate for the in vitro lipid synthesis in the adipose tissue of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2001, 14, 1560–1563. [Google Scholar] [CrossRef]
- Huntington, G.B. Starch utilization by ruminants: From basics to the bunk. J. Anim. Sci. 1997, 75, 852–867. [Google Scholar] [CrossRef]
- Maiga, H.A.; Marx, G.D.; Crary, V.W.; Linn, J.G. Dairy Update: Alternative Feeds for Dairy Cattle in Northwest Minesota; University of Minnesota Extension Service: Minneapolis, MN, USA, 1997; pp. 5–6. [Google Scholar]
- Abo-Zeid, H.M.; El-Zaiat, H.M.; Morsy, A.S.; Attia, M.F.A.; Abaza, M.A.; Sallam, S.M.A. Effects of replacing dietary maize grains with increasing levels of sugar beet pulp on rumen fermentation constituents and performance of growing buffalo calves. Anim. Feed Sci. Technol. 2017, 234, 128–138. [Google Scholar] [CrossRef]
- Marounek, M.; Bartos, S.; Brezina, P. Factors influencing the production of volatile fatty acids from hemicellulose, pectin and starch by mixed culture of rumen microorganisms. Z. Tierphysiol. Tierernaehr. Futtermittelkd. 1985, 53, 50–58. [Google Scholar] [CrossRef]
- Voelker, J.A.; Allen, M.S. Pelleted beet pulp substituted for high-moisture corn: 2. Effects on digestion and ruminal digestion kinetics in lactating dairy cows. J. Dairy Sci. 2003, 86, 3553–3561. [Google Scholar] [CrossRef]
- Gao, X.; Oba, M. Characteristics of dairy cows with a greater or lower risk of subacute ruminal acidosis: Volatile fatty acid absorption, rumen digestion, and expression of genes in rumen epithelial cells. J. Dairy Sci. 2016, 99, 8733–8745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, A.N.; Lubbadah, W.F. Feeding high levels of beet pulp in high concentrate dairy rations. J. Dairy Sci. 1971, 54, 95–99. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Kang, H.J.; Piao, M.Y.; Park, S.J.; Na, S.W.; Kim, H.J.; Baik, M. Effects of ambient temperature and rumen-protected fat supplementation on growth performance, rumen fermentation and blood parameters during cold season in Korean cattle steers. Asian-Australas. J. Anim. Sci. 2019, 32, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.S.; Chai, Z.; Song, L.J.; Liu, J.X.; Wu, Y.M. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef] [Green Version]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, J.; Park, S.J.; Jung, D.; Na, S.W.; Kim, H.J.; Baik, M. Effects of cold temperature and fat supplementation on growth performance and rumen and blood parameters in early fattening stage of Korean cattle steers. Anim. Feed Sci. Technol. 2020, 269, 114624. [Google Scholar] [CrossRef]
- Denman, S.E.; McSweeney, C.S. Development of a Real-Time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; McSweeney, C.S. Quantitative (Real-Time) PCR. In Methods in Gut Microbial Ecology for Ruminants; Denman, S.E., McSweeny, C.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 105–115. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- Mertens, D.R.; Allen, M.; Carmany, J.; Clegg, J.; Davidowicz, A.; Drouches, M.; Frank, K.; Gambin, D.; Garkie, M.; Gildemeister, B.; et al. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, S.; Sari, M.; Erafanimajd, N.; Kiani, A.; Ponnampalam, E.N. Supplementation of sugar beet pulp and roasted canola seed in a concentrate diet altered carcass traits, muscle (Longissimus dorsi) composition and meat sensory properties of Arabian fattening lambs. Small Rumin. Res. 2017, 153, 95–102. [Google Scholar] [CrossRef]
- Cuvelier, C.; Cabaraux, J.F.; Dufrasne, I.; Clinquart, A.; Hocquette, J.F.; Istasse, L.; Hornick, J.L. Performance, slaughter characteristics and meat quality of young bulls from Belgian Blue, Limousin and Aberdeen Angus breeds fattened with a sugar-beet pulp or a cereal-based diet. Anim. Sci. 2006, 82, 125–132. [Google Scholar] [CrossRef]
- Yang, W.Z.; Beauchemin, K.A. Physically effective fiber: Method of determination and effects on chewing, ruminal acidosis, and digestion by dairy cows. J. Dairy Sci. 2006, 89, 2618–2633. [Google Scholar] [CrossRef] [Green Version]
- Vojvodić, A.; Komes, D.; Vovk, I.; Belščak-Cvitanović, A.; Bušić, A. Compositional evaluation of selected agro-industrial wastes as valuable sources for the recovery of complex carbohydrates. Food Res. Int. 2016, 89, 565–573. [Google Scholar] [CrossRef]
- Voelker, J.A.; Allen, M.S. Pelleted beet pulp substituted for high-moisture corn: 3. Effects on ruminal fermentation, pH, and microbial protein efficiency in lactating dairy cows. J. Dairy Sci. 2003, 86, 3562–3570. [Google Scholar] [CrossRef]
- Feng, P.; Hunt, C.W.; Pritchard, G.T.; Parish, S.M. Effect of barley variety and dietary barley content on digestive function in beef steers fed grass hay-based diets. J. Anim. Sci. 1995, 73, 3476–3484. [Google Scholar] [CrossRef]
- Alamouti, A.A.; Alikhani, M.; Ghorbani, G.R.; Teimouri-Yansari, A.; Bagheri, M. Response of early lactation Holstein Cows to partial replacement of neutral detergent soluble fibre for starch in diets varying in forage particle size. Livest. Sci. 2014, 160, 60–68. [Google Scholar] [CrossRef]
- Mojtahedi, M.; Danesh Mesgaran, M. Effects of the Inclusion of dried molassed sugar beet pulp in a low-forage diet on the digestive process and blood biochemical parameters of Holstein steers. Livest. Sci. 2011, 141, 95–103. [Google Scholar] [CrossRef]
- McGavin, M.J.; Forsberg, C.W.; Crosby, B.; Bell, A.W.; Dignard, D.; Thomas, D.Y. Structure of the Cel-3 Gene from Fibrobacter Succinogenes S85 and characteristics of the encoded gene product, Endoglucanase 3. J. Bacteriol. 1989, 171, 5587–5595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, S.; Kobayashi, Y. Fibrolytic rumen bacteria: Their ecology and functions. Asian-Australas. J. Anim. 2009, 22, 131–138. [Google Scholar] [CrossRef]
- Gibson, T.; Stimmler, L.; Jarrett, R.J.; Rutland, P.; Shiu, M. Diurnal variation in the effects of insulin on blood glucose, plasma non-esterified fatty acids and growth hormone. Diabetologia 1975, 11, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Takizawa, S.; Ogawa, M. Plasma insulin, metabolite concentrations, and carcass characteristics of Japanese Black, Japanese Brown, and Holstein steers. J. Anim. Sci. 1997, 75, 3287–3293. [Google Scholar] [CrossRef] [Green Version]
- Wegner, J.; Huff, P.; Xie, C.P.; Schneider, F.; Teuscher, F.; Mir, P.S.; Mir, Z.; Kazala, E.C.; Weselake, R.J.; Ender, K. Relationship of plasma leptin concentration to intramuscular fat content in beef from crossbred Wagyu cattle. Can. J. Anim. Sci. 2001, 81, 451–457. [Google Scholar] [CrossRef] [Green Version]
| Dietary Treatment | Individual Feed | |||||
|---|---|---|---|---|---|---|
| Item | Corn Grain | Beet Pulp | Concentrate | Corn Grain | Beet Pulp | Oat Straw |
| Ingredients, g/kg dry matter (DM) | - | - | - | - | ||
| Concentrate 1 | 600 | 600 | ||||
| Oat straw | 250 | 250 | ||||
| Supplemented corn grain | 150 | 0 | - | - | - | - |
| Supplemented beet pulp | 0 | 135 | - | - | - | - |
| Protected fat | 0 | 15 | ||||
| Total | 1000 | 1000 | ||||
| Chemical composition, g/kg | ||||||
| DM | 890 | 900 | 878 | 856 | 894 | 911 |
| Crude protein (CP) | 127 | 122 | 160 | 84.0 | 90.9 | 53.9 |
| Ether extract (EE) | 34 | 34 | 41.4 | 35.6 | 8.00 | 19.4 |
| Ash | 54 | 58 | 72.2 | 11.2 | 45.0 | 35.1 |
| Neutral detergent fiber (NDF) | 310 | 350 | 238 | 102 | 425 | 640 |
| Acid detergent fiber | 149 | 162 | 86.4 | 27.9 | 231 | 403 |
| Non-fiber carbohydrates (NFC) 2 | 475 | 436 | 488 | 767 | 431 | 252 |
| Starch | 320 | 204 | 333 | 720 | 9.8 | 14 |
| Calcium | 91 | 100 | 13.9 | 0.20 | 8.50 | 2.10 |
| Phosphorus | 40 | 40 | 5.80 | 2.30 | 0.70 | 1.50 |
| Total digestible nutrient (TDN) 3, % | 71.8 | 69.4 | 72.7 | 85.0 | 69.1 | 55.9 |
| Metabolizable energy (ME) 4, MJ/kg | 10.6 | 10.5 | ||||
| Items | Treatment 1 | |||
|---|---|---|---|---|
| CG | BP | SEM | p-Value | |
| Initial body weight, kg | 484 | 485 | 5.37 | 0.98 |
| Final body weight, kg | 558 | 564 | 5.70 | 0.90 |
| Average daily gain, kg | 0.89 | 0.93 | 0.040 | 0.55 |
| Total dry matter (DM) intake 2, kg/d | 7.80 | 7.47 | 0.207 | 0.45 |
| Concentrate DM intake, kg/d | 4.75 | 4.58 | 0.153 | 0.61 |
| Supplement DM intake, kg/d | 1.23 | 1.07 | 0.05 | 0.17 |
| Forage DM intake, kg/d | 1.82 | 1.82 | 0.001 | 0.33 |
| Total feed intake 2, g/kg of body weight | 15.01 | 14.39 | 0.39 | 0.28 |
| Concentrate DM intake, g/kg of body weight | 9.14 | 8.82 | 0.290 | 0.61 |
| Supplement DM intake, g/kg of body weight | 2.37 | 2.07 | 0.107 | 0.17 |
| Forage DM intake, g/kg of body weight | 3.50 | 3.50 | 0.002 | 0.67 |
| Feed efficiency (gain/feed) | 0.115 | 0.123 | 0.004 | 0.44 |
| Items | Treatment 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| CG | BP | Mean | SEM | Treatment | Time | Treatment × Time | |
| pH | |||||||
| Week 0 | 6.54 | 6.50 | 6.52 y | 0.06 | 0.53 | 0.001 | 0.78 |
| Week 4 | 6.74 | 6.89 | 6.81 x | 0.11 | |||
| Week 8 | 6.43 | 6.57 | 6.49 y | 0.09 | |||
| Week 12 | 6.91 | 6.99 | 6.94 x | 0.09 | |||
| Ammonia nitrogen, mg/dL | |||||||
| Week 0 | 23.4 | 25.8 | 24.6 | 2.40 | 0.89 | 0.86 | 0.25 |
| Week 4 | 32.3 | 20.6 | 26.5 | 1.70 | |||
| Week 8 | 23.2 | 21.4 | 22.3 | 2.29 | |||
| Week 12 | 19.2 | 26.9 | 23.1 | 5.70 | |||
| Total volatile fatty acid, mM | |||||||
| Week 0 | 112 | 111 | 111 | 6.60 | 0.83 | 0.28 | 0.64 |
| Week 4 | 123 | 125 | 124 | 5.40 | |||
| Week 8 | 127 | 122 | 124 | 5.70 | |||
| Week 12 | 109 | 123 | 115 | 9.20 | |||
| Acetate, mol/100 mol | |||||||
| Week 0 | 59.2 | 58.8 | 59.0 z | 0.53 | 0.11 | 0.001 | 0.006 |
| Week 4 | 60.0 b | 63.4 a | 61.7 y | 0.62 | |||
| Week 8 | 62.8 | 64.2 | 63.5 x | 0.51 | |||
| Week 12 | 62.9 | 64.1 | 63.5 x | 0.51 | |||
| Propionate, mol/100 mol | |||||||
| Week 0 | 17.5 | 17.9 | 17.7 y | 0.55 | 0.06 | 0.001 | 0.07 |
| Week 4 | 17.9 | 16.2 | 17.1 y | 0.57 | |||
| Week 8 | 22.2 a | 19.7 b | 20.9 x | 0.55 | |||
| Week 12 | 21.8 a | 19.7 b | 20.8 x | 0.45 | |||
| Butyrate, mol/100 mol | |||||||
| Week 0 | 16.3 | 15.6 | 15.9 x | 0.01 | 0.13 | 0.001 | 0.07 |
| Week 4 | 16.2 a | 13.4 b | 14.8 y | 0.01 | |||
| Week 8 | 12.0 | 13.4 | 12.7 y | 0.01 | |||
| Week 12 | 12.3 | 13.4 | 12.9 y | 0.01 | |||
| Acetate to propionate ratio | |||||||
| Week 0 | 3.40 | 3.32 | 3.36 y | 0.09 | 0.02 | 0.001 | 0.005 |
| Week 4 | 3.37 a | 3.98 a | 3.67 x | 0.15 | |||
| Week 8 | 2.85 b | 3.28 a | 3.06 y | 0.09 | |||
| Week 12 | 2.89 b | 3.26 a | 3.08 y | 0.08 | |||
| Item | Treatment 1 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| CG | BP | Mean | SEM | Treatment | Time | Treatment × Time | |
| Glucose, mg/dL | |||||||
| Week 0 | 80.8 | 79.2 | 80.0 | 2.36 | 0.99 | 0.09 | 0.76 |
| Week 4 | 73.5 | 74.8 | 74.1 | 1.18 | |||
| Week 8 | 77.2 | 76.2 | 76.7 | 2.42 | |||
| Week 12 | 74.7 | 75.0 | 74.8 | 1.19 | |||
| Triglyceride, mg/dL | |||||||
| Week 0 | 23.3 | 20.7 | 22.0 y | 1.29 | 0.11 | 0.04 | 0.88 |
| Week 4 | 24.3 | 19.7 | 22.0 y | 1.71 | |||
| Week 8 | 23.0 | 20.3 | 21.7 y | 1.70 | |||
| Week 12 | 18.8 | 16.0 | 17.4 x | 1.00 | |||
| Total cholesterol, mg/dL | |||||||
| Week 0 | 130 | 123 | 127 y | 6.2 | 0.44 | 0.001 | 0.02 |
| Week 4 | 158 | 145 | 151 y | 10.9 | |||
| Week 8 | 175 | 209 | 192 x | 11.6 | |||
| Week 12 | 170 b | 202 a | 156 x | 9.6 | |||
| Nonesterified fatty acids, mg/dL | |||||||
| Week 0 | 134 | 137 | 136 x | 9.1 | 0.10 | 0.004 | 0.15 |
| Week 4 | 105 | 90.5 | 98.3 y | 5.9 | |||
| Week 8 | 130 | 108 | 120 x | 14.5 | |||
| Week 12 | 173 a | 119 b | 148 x | 11.1 | |||
| Mean | 135 a | 114 b | |||||
| Leptin, ng/mL | |||||||
| Week 0 | 9.79 | 9.01 | 9.40 x | 0.450 | 0.49 | 0.0008 | 0.94 |
| Week 4 | 7.20 | 7.19 | 7.17 y, z | 0.673 | |||
| Week 8 | 6.23 | 5.55 | 5.89 y | 0.458 | |||
| Week 12 | 8.65 | 8.64 | 8.65 x, z | 0.500 | |||
| Insulin, ng/mL | |||||||
| Week 0 | 1.08 | 1.27 | 1.18 x | 0.142 | 0.03 | 0.001 | 0.27 |
| Week 4 | 1.16 | 1.54 | 1.35 x | 0.136 | |||
| Week 8 | 0.55 | 0.84 | 0.70 y | 0.082 | |||
| Week 12 | 0.79 b | 1.52 a | 1.15 x | 0.152 | |||
| Mean | 0.90 b | 1.29 a | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, I.; Na, S.W.; Kang, H.J.; Park, S.J.; Jung, D.J.S.; Beak, S.H.; Lee, J.; Kim, D.-H.; Kim, H.J.; Malekkhahi, M.; et al. Partial Substitution of Corn Grain in the Diet with Beet Pulp Reveals Increased Ruminal Acetate Proportion and Circulating Insulin Levels in Korean Cattle Steers. Animals 2022, 12, 1419. https://doi.org/10.3390/ani12111419
Jeong I, Na SW, Kang HJ, Park SJ, Jung DJS, Beak SH, Lee J, Kim D-H, Kim HJ, Malekkhahi M, et al. Partial Substitution of Corn Grain in the Diet with Beet Pulp Reveals Increased Ruminal Acetate Proportion and Circulating Insulin Levels in Korean Cattle Steers. Animals. 2022; 12(11):1419. https://doi.org/10.3390/ani12111419
Chicago/Turabian StyleJeong, Inhyuk, Sang Weon Na, Hyeok Joong Kang, Seung Ju Park, Da Jin Sol Jung, Seok Hyeon Beak, Jaesung Lee, Do-Hyun Kim, Hyun Jin Kim, Mohammad Malekkhahi, and et al. 2022. "Partial Substitution of Corn Grain in the Diet with Beet Pulp Reveals Increased Ruminal Acetate Proportion and Circulating Insulin Levels in Korean Cattle Steers" Animals 12, no. 11: 1419. https://doi.org/10.3390/ani12111419
APA StyleJeong, I., Na, S. W., Kang, H. J., Park, S. J., Jung, D. J. S., Beak, S. H., Lee, J., Kim, D. -H., Kim, H. J., Malekkhahi, M., Ranaweera, K. K. T. N., & Baik, M. (2022). Partial Substitution of Corn Grain in the Diet with Beet Pulp Reveals Increased Ruminal Acetate Proportion and Circulating Insulin Levels in Korean Cattle Steers. Animals, 12(11), 1419. https://doi.org/10.3390/ani12111419






