Cryopreservation Competence of Chicken Oocytes as a Model of Endangered Wild Birds: Effects of Storage Time and Temperature on the Ovarian Follicle Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Chemicals
2.2. Collection of Chicken Ovarian Tissues
2.3. Neutral Red (NR) Staining to Assess Viable Ovarian Follicles in Fresh and Vitrified–Warmed Tissues
2.4. Histological Assessment and Classification of Follicular Structure
2.5. Gene Expression Analysis by Quantitative Reverse Transcription Polymerase Chain Reaction (qPCR)
2.6. Protein Expression Analysis by Western Blotting
2.7. Vitrification and Warming Procedure
2.8. Experimental Design
2.8.1. Study 1: Effect of Different Storage Conditions on Follicle Maintenance in Chicken Ovaries
2.8.2. Study 2: Examine the Freezing Tolerance of Ovarian Follicles Stored for Different Storage Periods
2.9. Statistical Analysis
3. Results
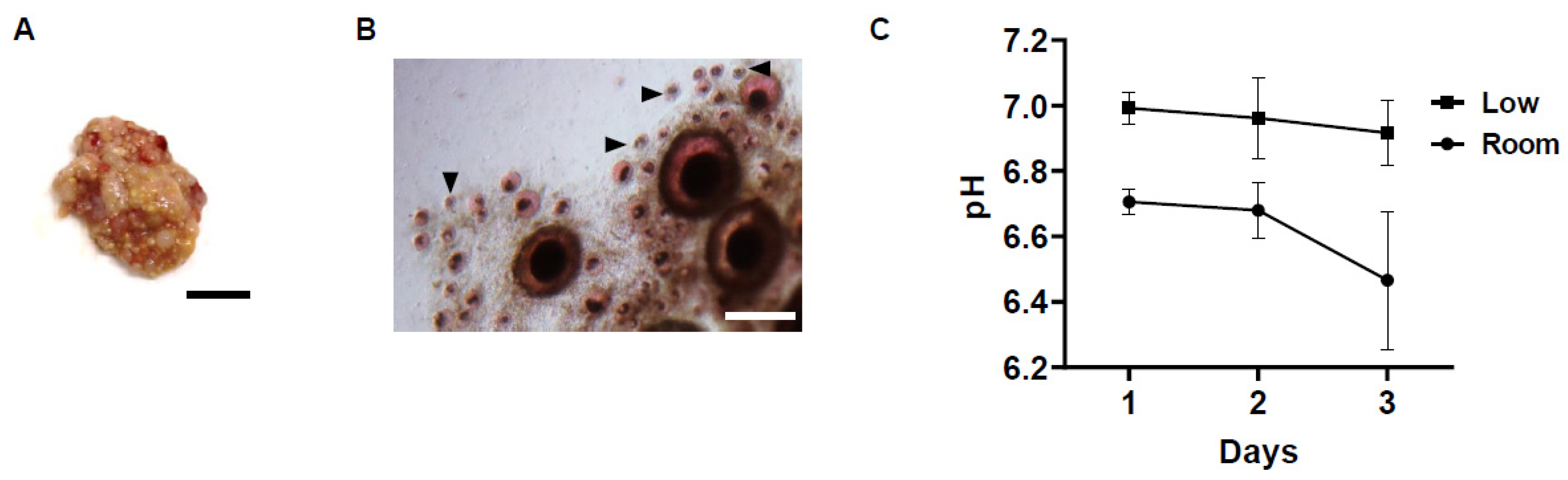
3.1. Changes in pH of Storage Medium and Follicle Viability in Chicken Ovarian Tissues under Different Storage Conditions
3.2. Influence of Different Storage Conditions on Gene and Protein Expressions in Chicken Ovarian Tissues
3.3. Influence of Storage Time on Cryopreservation of Chicken Ovarian Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species (Version2021-3). Available online: https://www.iucnredlist.org (accessed on 15 April 2022).
- Yamashina, Y.; Mano, T. A New Species of Rail from Okinawa Island. J. Yamashina Inst. Ornithol. 1981, 13, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y. Poultry genetic resource conservation using primordial germ cells. J. Reprod. Dev. 2016, 62, 2016–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.E.; Singh, R.P.; Pukazhenthi, B.; Keefer, C.L.; Songsasen, N. Cryopreservation effects on sperm function and fertility in two threatened crane species. Cryobiology 2018, 82, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Wallace, W.H.B.; Baird, D.T. Ovarian cryopreservation for fertility preservation: Indications and outcomes. Reproduction 2008, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nateghi, R.; Alizadeh, A.; Jafari Ahangari, Y.; Fathi, R.; Akhlaghi, A. Ethylene Glycol and Dimethyl Sulfoxide Combination Reduces Cryoinjuries and Apoptotic Gene Expression in Vitrified Laying Hen Ovary. Biopreserv. Biobank. 2017, 15, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Nateghi, R.; Masouleh, A.R.A.M.; Ahangari, Y.J.; Fathi, R.; Akhlaghi, A. Dietary Fish Oil and Vitamin E Reduce Cryoinjuries and Apoptosis in Vitrified Laying Hens’ Ovarian Tissue. Biopreserv. Biobank. 2019, 17, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, Y.; Cheng, K.M.; Silversides, F.G. Production of Donor-Derived Offspring from Cryopreserved Ovarian Tissue in Japanese Quail (Coturnix japonica). Biol. Reprod. 2010, 83, 15–19. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, K.M.; Silversides, F.G. Novel needle-in-straw vitrification can effectively preserve the follicle morphology, viability, and vascularization of ovarian tissue in Japanese quail (Coturnix japonica). Anim. Reprod. Sci. 2012, 134, 197–202. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, K.M.; Silversides, F.G. A model for cryobanking female germplasm in Japanese quail (Coturnix japonica). Poult. Sci. 2013, 92, 2772–2775. [Google Scholar] [CrossRef]
- Liptoi, K.; Buda, K.; Rohn, E.; Drobnyak, A.; Meleg, E.E.; Palinkas-Bodzsar, N.; Vegi, B.; Barna, J. Improvement of the application of gonadal tissue allotransplantation in the in vitro conservation of chicken genetic lines. Anim. Reprod. Sci. 2020, 213, 106280. [Google Scholar] [CrossRef] [PubMed]
- Onuma, M.; Nagamine, T.; Nakaya, Y.; Neagari, Y. Reproductive cycle observation of the Okinawa rail (Gallirallus okinawae) in the wild. J. Vet. Med. Sci. 2011, 73, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Wongsrikeao, P.; Otoi, T.; Karja, N.W.K.; Agung, B.; Nii, M.; Nagai, T. Effects of Ovary Storage Time and Temperature on DNA Fragmentation and Development of Porcine Oocytes. J. Reprod. Dev. 2005, 51, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guignot, F.; Bezard, J.; Palmer, E. Effect of time during transport of excised mare ovaries on oocyte recovery rate and quality after in vitro maturation. Theriogenology 1999, 52, 757–766. [Google Scholar] [CrossRef]
- Evecen, M.; Cirit, Ü.; Demir, K.; Karaman, E.; Hamzaoǧlu, A.I.; Bakirer, G. Developmental competence of domestic cat oocytes from ovaries stored at various durations at 4 °C temperature. Anim. Reprod. Sci. 2009, 116, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Evecen, M.; Cirit, Ü.; Demir, K.; Özdaş, Ö.B.; Taş, M.; Birler, S.; Pabuccuoǧlu, S. Effects of estrous cycle stage and transport temperature of ovaries on in vitro maturation of canine oocytes. Anim. Reprod. Sci. 2010, 117, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.S.; Lu, K.H.; Gordon, I. In vitro fertilization (IVF) and culture (IVC) of bovine oocytes from stored ovaries. Theriogenology 1990, 33, 352. [Google Scholar] [CrossRef]
- Fujihara, M.; Comizzoli, P.; Keefer, C.L.C.L.; Wildt, D.E.D.E.; Songsasen, N. Epidermal growth factor (EGF) sustains in vitro primordial follicle viability by enhancing stromal cell proliferation via MAPK and PI3K pathways in the prepubertal, but not adult, cat ovary. Biol. Reprod. 2014, 90, 86. [Google Scholar] [CrossRef] [PubMed]
- Songsasen, N.; Comizzoli, P.; Nagashima, J.; Fujihara, M.; Wildt, D. The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reprod. Domest. Anim. 2012, 47, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Kuwana, T.; Hashimoto, K.; Nakanishi, A.; Yasuda, Y.; Tajima, A.; Naito, M. Long-term culture of avian embryonic cells in vitro. Int. J. Dev. Biol. 1996, 40, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, M.; Comizzoli, P.; Wildt, D.E.; Songsasen, N. Cat and dog primordial follicles enclosed in ovarian cortex sustain viability after in vitro culture on agarose gel in a protein-free medium. Reprod. Domest. Anim. 2012, 47 (Suppl. 6), 102–108. [Google Scholar] [CrossRef] [Green Version]
- Kito, G.; Aramaki, S.; Tanaka, K.; Soh, T.; Yamauchi, N.; Hattori, M. Temporal and spatial differential expression of chicken germline-specific proteins cDAZL, CDH and CVH during gametogenesis. J. Reprod. Dev. 2010, 56, 341–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aramaki, S.; Kubota, K.; Soh, T.; Yamauchi, N.; Hattori, M.-A. Chicken Dead End Homologue Protein is a Nucleoprotein of Germ Cells Including Primordial Germ Cells. J. Reprod. Dev. 2009, 55, 20154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tellado, M.N.; Alvarez, G.M.; Dalvit, G.C.; Cetica, P.D.; Tellado, M.N.; Alvarez, G.M.; Dalvit, G.C.; Cetica, P.D. The Conditions of Ovary Storage Affect the Quality of Porcine Oocytes. Adv. Reprod. Sci. 2014, 2, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Rall, W.F. Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology 1987, 24, 387–402. [Google Scholar] [CrossRef]
- Amorim, C.A.; Curaba, M.; Van Langendonckt, A.; Dolmans, M.-M.; Donnez, J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod. Biomed. Online 2011, 23, 160–186. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, M.; Kaneko, T.; Inoue-Murayama, M. Vitrification of canine ovarian tissues with polyvinylpyrrolidone preserves the survival and developmental capacity of primordial follicles. Sci. Rep. 2019, 9, 3970. [Google Scholar] [CrossRef] [Green Version]




| Primers | Sequence | Accession No. | |
|---|---|---|---|
| Chicken CASP3 | Forward | AGCAGGGAAACCCAAACTCT | NM_204725.1 |
| Reverse | CTGGTCCACTGTCTGCTTCA | ||
| Chicken CASP9 | Forward | CCCACTCCTGGTGACATCTT | XM_046903261.1 |
| Reverse | AGGTTTCCACGTACCACGAG | ||
| Chicken SOD1 | Forward | GTGGTCCATGCAAAAAGTGA | NM_205064.1 |
| Reverse | AAGCTAAACGAGGTCCAGCA | ||
| Chicken GAPDH | Forward | TGGGAAGCTTACTGGAATGG | NM_204305.1 |
| Reverse | CTTGGCTGGTTTCTCCAGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujihara, M.; Shiraishi, J.-i.; Onuma, M.; Ohta, Y.; Inoue-Murayama, M. Cryopreservation Competence of Chicken Oocytes as a Model of Endangered Wild Birds: Effects of Storage Time and Temperature on the Ovarian Follicle Survival. Animals 2022, 12, 1434. https://doi.org/10.3390/ani12111434
Fujihara M, Shiraishi J-i, Onuma M, Ohta Y, Inoue-Murayama M. Cryopreservation Competence of Chicken Oocytes as a Model of Endangered Wild Birds: Effects of Storage Time and Temperature on the Ovarian Follicle Survival. Animals. 2022; 12(11):1434. https://doi.org/10.3390/ani12111434
Chicago/Turabian StyleFujihara, Mayako, Jun-ichi Shiraishi, Manabu Onuma, Yoshiyuki Ohta, and Miho Inoue-Murayama. 2022. "Cryopreservation Competence of Chicken Oocytes as a Model of Endangered Wild Birds: Effects of Storage Time and Temperature on the Ovarian Follicle Survival" Animals 12, no. 11: 1434. https://doi.org/10.3390/ani12111434






