Insects as Feed for Companion and Exotic Pets: A Current Trend
Abstract
:Simple Summary
Abstract
1. Introduction
2. Reasons for Using Insects in Pet Food
2.1. Trends in Dog and Cat Feed
2.2. Nutritional Benefits
2.3. Environmental Benefits
- Insects require less water and land use. For example, 1 g of edible chicken protein requires 2 to 3 times more land and 50% more water compared to mealworms. One gram of beef protein requires 8 to 14 times more land and about 5 times more water compared to mealworms. To produce house fly meal, 98% less land is required than to produce a 50:50 mixture of fish meal and soybean meal [68].
- Greenhouse gas emissions are lower. Broiler chickens emit 32% to 167% more CO2 equivalent emissions, pigs emit 51% to 287% more CO2 equivalents [69], while beef cattle emit 6 to 13 times more CO2 equivalents compared to mealworms [68]. In addition, insect production generates lower ammonia emission, as their excreta are very dry, producing a very slow uric acid-urea-ammonia conversion [55]. One study determined that methane production was almost undetectable in mealworms and crickets [16].
- Insects are capable of transforming waste into high-quality feed. Mealworms and black soldier flies can feed on fruit and vegetable by-products, household waste, slaughter plant waste, milling waste and others [71]. According to Food for the Future [72], 1 kg of black soldier fly larvae can consume 25 tons of organic household waste in 1 week and convert it into 5 tons of larvae, which in turn generate 1700 kg of larval meal and 15 tons of organic fertilizer. Interestingly, mealworms and superworms are able to feed on certain plastics such as polypropylene or polystyrene, through depolymerization and biodegradation of plastics by its microbiota [73].
3. Current Use of Insects as Food Ingredients for Dogs, Cats and Exotic Pets
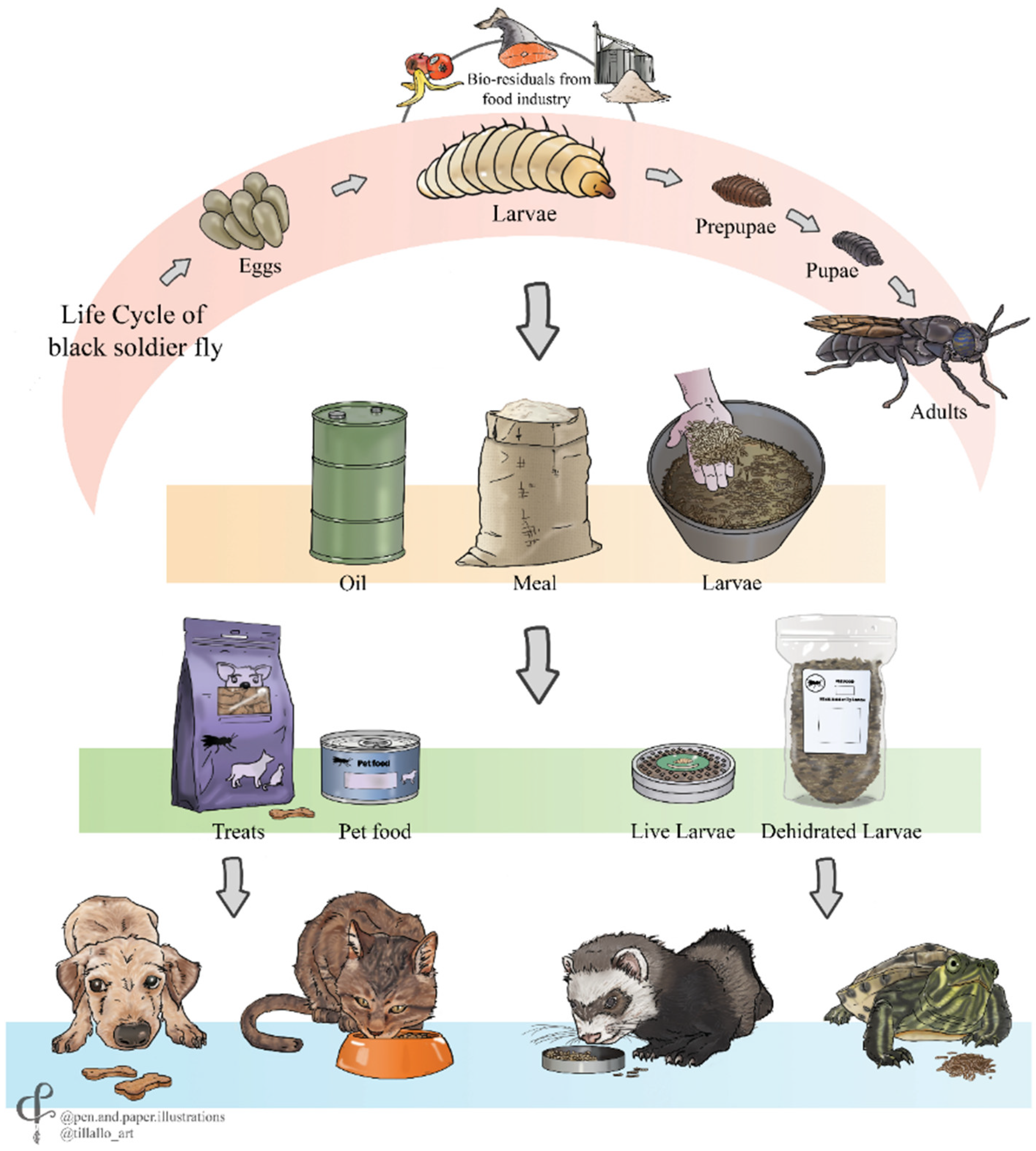
3.1. Rearing and Processing of Insects for Pet Food Ingredients
3.2. Benefits of Using Insects in Dog and Cat Feed
3.3. Outstanding Issues in Edible Insects in Dog and Cat Feed
3.4. Use of Insects as Feed in the Exotic Pet Industry
3.5. Pet Owner Perception of the Use of Insects in Dog and Cat Feed
3.6. Legislation for the Use of Insects in Animal Feed
3.6.1. European Union (EU)
3.6.2. United States
3.6.3. Canada
3.6.4. Australia
3.6.5. China
3.6.6. North Korea
3.6.7. South Korea
3.6.8. Mexico
3.6.9. Chile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Growth from Knowledge. Global Pet Ownership Survey. 2016. Available online: https://cdn2.hubspot.net/hubfs/2405078/cms-pdfs/fileadmin/user_upload/country_one_pager/nl/documents/global-gfk-survey_pet-ownership_2016.pdf (accessed on 1 May 2021).
- Alexander, P.; Berri, A.; Moran, D.; Reay, D.; Rounsevell, M.D.A. The global environmental paw print of pet food. Glob. Environ. Chang. 2020, 65, 102153. [Google Scholar] [CrossRef]
- Euromonitor. Pet Food World Market Share (USD Million); Euromonitor International: London, UK, 2020. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006.
- AAFCO. Methods for Substantiating Nutritional Adequacy of Dog and Cat Food. Available online: https://www.aafco.org/Portals/0/SiteContent/Regulatory/Committees/Pet-Food/Reports/Pet_Food_Report_2013_Midyear-Proposed_Revisions_to_AAFCO_Nutrient_Profiles.pdf (accessed on 21 July 2021).
- Acuff, H.L.; Dainton, A.N.; Dhakal, J.; Kiprotich, S.; Aldrich, G. Sustainability and pet food. Vet. Clin. North Am. Small Anim. Pract. 2021, 51, 563–581. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, K.; Leury, B.J. Review: Insect meal: A future source of protein feed for pigs? Animal 2019, 13, 3022–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, B.; Munekata, P.E.S.; Zhu, Z.; Barba, F.J.; Toldrá, F.; Putnik, P.; Bursać Kovačević, D.; Lorenzo, J.M. Challenges and opportunities regarding the use of alternative protein sources. Aquaculture and insects. Adv. Food Nutr. Res. 2019, 89, 259–295. [Google Scholar] [PubMed]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Meeker, D.L.; Meisinger, J.L. Companion animals symposium: Rendered ingredients significantly influence sustainability, quality, and safety of pet food. J. Anim. Sci. 2015, 93, 835–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donadelli, R.A.; Jones, C.K.; Beyer, R.S. The amino acid composition and protein quality of various egg, poultry meal by-products, and vegetable proteins used in the production of dog and cat diets. Poult. Sci. 2019, 98, 1371–1378. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Dobermann, D.; Swift, J.A.; Field, L.M. Opportunities and hurdles of edible insects for food and feed. Nutr. Bull. 2017, 42, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Ao, X.; Yoo, J.S.; Wu, Z.L.; Kim, I.H. Can dried mealworm (Tenebrio molitor) larvae replace fish meal in weaned pigs? Livest. Sci. 2020, 239, 104103. [Google Scholar] [CrossRef]
- Halloran, A.; Hanboonsong, Y.; Roos, N.; Bruun, S. Life cycle assessment of cricket farming in north-eastern Thailand. J. Clean. Prod. 2017, 156, 83–94. [Google Scholar] [CrossRef]
- Bosch, G.; Vervoort, J.J.M.; Hendriks, W.H. In Vitro digestibility and fermentability of selected insects for dog foods. Anim. Feed Sci. Technol. 2016, 221, 174–184. [Google Scholar] [CrossRef]
- Jarett, J.K.; Carlson, A.; Rossoni Serao, M.; Strickland, J.; Serfilippi, L.; Ganz, H.H. Diets with and without edible cricket support a similar level of diversity in the gut microbiome of dogs. PeerJ 2019, 7, e7661. [Google Scholar] [CrossRef]
- Lei, X.J.; Kim, T.H.; Park, J.H.; Kim, I.H. Evaluation of supplementation of defatted black soldier fly (Hermetia illucens) larvae meal in beagle dogs. Ann. Anim. Sci. 2019, 19, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Viana, L.M.; Mothé, C.G.; Mothé, M.G. Natural food for domestic animals: A national and international technological review. Res. Vet. Sci. 2020, 130, 11–18. [Google Scholar] [CrossRef]
- Euromonitor. Premiumisation: How Value-Seeking Trends in the US Will Shape the Future of Pet Food. 2019. Available online: https://www.euromonitor.com/premiumisation-how-value-seeking-trends-in-the-us-will-shape-the-future-of-petfood/report (accessed on 4 May 2021).
- Buff, P.R.; Carter, R.A.; Bauer, J.E.; Kersey, J.H. Natural pet food: A review of natural diets and their impact on canine and feline physiology. J. Anim. Sci. 2014, 92, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Cave, N.; Abood, S.; Shoveller, A.; Adolphe, J.; Verbrugghe, A. An observational study of pet feeding practices and how these have changed between 2008 and 2018. Vet. Rec. 2020, 186, 643. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.A.; Bauer, J.E.; Kersey, J.H.; Buff, P.R. Awareness and evaluation of natural pet food products in the United States. J. Am. Vet. Med. Assoc. 2014, 245, 1241–1248. [Google Scholar] [CrossRef]
- Brozić, D.; Mikulec, Ž.; Samardžija, M.; Đuričić, D.; Valpotić, H. Raw meat-based diet (BARF) in dogs and cats nutrition. Ветеринарски Журнал Републике Српске 2020, 19, 314–321. [Google Scholar] [CrossRef]
- van Bree, F.P.J.; Bokken, G.C.A.M.; Mineur, R.; Franssen, F.; Opsteegh, M.; van der Giessen, J.W.B.; Lipman, L.J.A.; Overgaauw, P.A.M. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec. 2018, 182, 50. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef]
- Kölle, P.; Schmidt, M. BARF (Biologisch Artgerechte Rohfütterung) als ernährungsform bei hunden. Tierärztliche Prax. Ausgabe K Kleintiere/Heimtiere 2015, 43, 409–419. [Google Scholar] [CrossRef]
- Böhm, T.; Klinger, C.; Gedon, N.; Udraite, L.; Hiltenkamp, K.; Mueller, R. Effekt eines insektenprotein-basierten futters auf die symptomatik von futtermittelallergischen hunden. Tierärztliche Prax. Ausgabe K Kleintiere/Heimtiere 2018, 46, 297–302. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Companion animals symposium: Future aspects and perceptions of companion animal nutrition and sustainability. J. Anim. Sci. 2015, 93, 823. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzo, B.; Olivotto, I.; Gasco, L.; Loponte, R.; Bovera, F. Evaluation of an insect meal of the black soldier fly (Hermetia illucens) as soybean substitute: Intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Vet. Sci. 2018, 117, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Lima, R.C.; Maia, M.R.G.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Cunha, L.M. Impact of defatting freeze-dried edible crickets (Acheta domesticus and Gryllodes sigillatus) on the nutritive value, overall liking and sensory profile of cereal bars. LWT 2019, 113, 108335. [Google Scholar] [CrossRef]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C.; et al. First insights on black soldier fly (Hermetia illucens L.) larvae meal dietary administration in siberian sturgeon (Acipenser baerii brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Kröger, S.; Heide, C.; Zentek, J. Evaluation of an extruded diet for adult dogs containing larvae meal from the black soldier fly (Hermetia illucens). Anim. Feed Sci. Technol. 2020, 270, 114699. [Google Scholar] [CrossRef]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A potential source of protein and other nutrients for feed and food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Batal, A.; Dale, N. Feedstuffs Ingredient Analysis Table: 2016 Edition. Available online: https://feedstuffs.farmcentric.com/mdfm/Feeess50/author/427/2015/11/Feedstuffs_RIBG_Ingredient_Analysis_Table_2016.pdf (accessed on 15 March 2021).
- Ramos-Elorduy, J.; Moreno, J.M.P.; Prado, E.E.; Perez, M.A.; Otero, J.L.; de Guevara, O.L. Nutritional value of edible insects from the state of Oaxaca, Mexico. J. Food Compos. Anal. 1997, 10, 142–157. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; van Loon, J.J.A.; van Loon, L.J.C. Consideration of insects as a source of dietary protein for human consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Bosch, G.; Zhang, S.; Oonincx, D.G.A.B.; Hendriks, W.H. Protein quality of insects as potential ingredients for dog and cat foods. J. Nutr. Sci. 2014, 3, e29. [Google Scholar] [CrossRef] [Green Version]
- Luna, D.; Carrasco, C.; Álvarez, D.; González, C.; Egaña, J.I.; Figueroa, J. Exploring anhedonia in kennelled dogs: Could coping styles affect hedonic preferences for sweet and umami flavours? Animals 2020, 10, 2087. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Swanson, K.S. Effect of using insects as feed on animals: Pet dogs and cats. J. Insects Food Feed 2021, 7, 795–805. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos Aguilar, J.G. An overview of lipids from insects. Biocatal. Agric. Biotechnol. 2021, 33, 101967. [Google Scholar] [CrossRef]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect derived lauric acid as promising alternative strategy to antibiotics in the antimicrobial resistance scenario. Front. Microbiol. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Lekshmi Sheela, D.; Nazeem, P.A.; Narayanankutty, A.; Manalil, J.J.; Raghavamenon, A.C. In Silico and wet lab studies reveal the cholesterol lowering efficacy of lauric acid, a medium chain fat of coconut oil. Plant Foods Hum. Nutr. 2016, 71, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Araque, R.; Egas-Montenegro, E. Edible insects: A food alternative for the sustainable development of the planet. Int. J. Gastron. Food Sci. 2021, 23, 100304. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Halloran, A.; Hansen, H.H.; Jensen, L.S.; Bruun, S. Comparing environmental impacts from insects for feed and food as an alternative to animal production. In Edible Insects in Sustainable Food Systems; Halloran, A., Flore, R., Vantomme, P., Roos, N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–180. ISBN 978-3-319-74011-9. [Google Scholar]
- Kazimierska, K.; Biel, W.; Witkowicz, R. Mineral composition of cereal and cereal-free dry dog foods versus nutritional guidelines. Molecules 2020, 25, 5173. [Google Scholar] [CrossRef]
- Bolat, B.; Ugur, A.E.; Oztop, M.H.; Alpas, H. Effects of high hydrostatic pressure assisted degreasing on the technological properties of insect powders obtained from Acheta domesticus & Tenebrio molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, X.; Jiao, M.; Anoopkumar-Dukie, S.; Zeng, Y.; Mei, H. Housefly (Musca domestica) larvae powder, preventing oxidative stress injury via regulation of UCP4 and CyclinD1 and modulation of JNK and P38 signaling in APP/PS1 mice. Food Funct. 2019, 10, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Dunkel, F.V. Edible Insects. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 341–355. ISBN 9780128027769. [Google Scholar]
- Weru, J.; Chege, P.; Kinyuru, J. Nutritional potential of edible insects: A systematic review of published data. Int. J. Trop. Insect Sci. 2021, 41, 2015–2037. [Google Scholar] [CrossRef]
- Hernández-Álvarez, A.-J.; Mondor, M.; Piña-Domínguez, I.-A.; Sánchez-Velázquez, O.-A.; Melgar Lalanne, G. Drying technologies for edible insects and their derived ingredients. Dry. Technol. 2021, 39, 1991–2009. [Google Scholar] [CrossRef]
- Pratondo, A.; Bramantoro, A. Classification of Zophobas morio and Tenebrio molitor using transfer learning. PeerJ Comput. Sci. 2022, 8, e884. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. The superworm, Zophobas morio (Coleoptera:Tenebrionidae): A ‘sleeping giant’ in nutrient sources. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Józefiak, A.; Kozłowski, K.; Jankowski, J.; Józefiak, D. Tenebrio molitor and Zophobas morio full-fat meals in broiler chicken diets: Effects on nutrients digestibility, digestive enzyme activities, and cecal microbiome. Animals 2019, 9, 1128. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Chung, H.; Shin, Y.P.; Kim, M.-A.; Natarajan, S.; Veerappan, K.; Kim, S.H.; Park, J.; Hwang, J.S. Uncovering antimicrobial peptide from Zophobas atratus using transcriptome analysis. Int. J. Pept. Res. Ther. 2021, 27, 1827–1835. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans–A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, T.; Abbasi, S.A. Reducing the global environmental impact of livestock production: The minilivestock option. J. Clean. Prod. 2016, 112, 1754–1766. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; González, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Food for the Future (F4F). Available online: https://f4f.cl/ (accessed on 25 May 2021).
- Yang, S.-S.; Ding, M.-Q.; He, L.; Zhang, C.-H.; Li, Q.-X.; Xing, D.-F.; Cao, G.-L.; Zhao, L.; Ding, J.; Ren, N.-Q.; et al. Biodegradation of polypropylene by yellow mealworms (Tenebrio molitor) and superworms (Zophobas atratus) via gut-microbe-dependent depolymerization. Sci. Total Environ. 2021, 756, 144087. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.; Salinas-Castro, A. Edible insects processing: Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [Green Version]
- Dzepe, D.; Nana, P.; Kuietche, H.M.; Kimpara, J.M.; Magatsing, O.; Tchuinkam, T.; Djouaka, R. Feeding strategies for small-scale rearing black soldier fly larvae (Hermetia illucens) as organic waste recycler. SN Appl. Sci. 2021, 3, 252. [Google Scholar] [CrossRef]
- Xiao, H.-W.; Bai, J.-W.; Sun, D.-W.; Gao, Z.-J. The application of superheated steam impingement blanching (SSIB) in agricultural products processing—A review. J. Food Eng. 2014, 132, 39–47. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio molitor L.) as an alternative protein source for monogastric animal: A review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Purschke, B.; Brüggen, H.; Scheibelberger, R.; Jäger, H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2018, 244, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Caparros Megido, R.; Desmedt, S.; Blecker, C.; Béra, F.; Haubruge, É.; Alabi, T.; Francis, F. Microbiological load of edible insects found in Belgium. Insects 2017, 8, 12. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial dynamics during industrial rearing, processing, and storage of tropical house crickets (Gryllodes sigillatus) for human consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Zhou, J.; Yuan, M.-M.; Dong, H.; Cheng, G.-Q.; Wang, Y.-J.; Xia, J.-Y.; Zhang, L. Dietary supplementation with housefly (Musca domestica) maggot meal in growing beagles: Hematology, serum biochemistry, immune responses and oxidative damage. Ann. Anim. Sci. 2020, 20, 1351–1364. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, A.; Mazurkiewicz, J.; Świątkiewicz, S.; Siwek, M.; Bednarczyk, M.; Szumacher-Strabel, M.; Cieślak, A.; Benzertiha, A.; et al. Effects of replacing soybean oil with selected insect fats on broilers. Anim. Feed Sci. Technol. 2018, 240, 170–183. [Google Scholar] [CrossRef]
- Plantinga, E.A.; Bosch, G.; Hendriks, W.H. Estimation of the dietary nutrient profile of free-roaming feral cats: Possible implications for nutrition of domestic cats. Br. J. Nutr. 2011, 106, S35–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolley, L.-A.; Murphy, B.P.; Geyle, H.M.; Legge, S.M.; Palmer, R.A.; Dickman, C.R.; Doherty, T.S.; Edwards, G.P.; Riley, J.; Turpin, J.M.; et al. Introduced cats eating a continental fauna: Invertebrate consumption by feral cats (Felis catus) in Australia. Wildl. Res. 2020, 47, 610. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Pawełczyk, P.; Różyńska, J.; Golusik, J.; Mikołajczak, Z.; Józefiak, D. Do insects smell attractive to dogs? A comparison of dog reactions to insects and commercial feed aromas–a preliminary study. Ann. Anim. Sci. 2018, 18, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Paßlack, N.; Zentek, J. Akzeptanz, verträglichkeit und scheinbare nährstoffverdaulichkeit von alleinfuttermitteln auf basis von Hermetia-illucens-larvenmehl bei katzen. Tierärztliche Prax. Ausgabe K Kleintiere/Heimtiere 2018, 46, 213–221. [Google Scholar] [CrossRef]
- Feng, T.; Hu, Z.; Tong, Y.; Yao, L.; Zhuang, H.; Zhu, X.; Song, S.; Lu, J. Preparation and evaluation of mushroom (Lentinus edodes) and mealworm (Tenebrio molitor) as dog food attractant. Heliyon 2020, 6, e05302. [Google Scholar] [CrossRef]
- Hu, Y.; He, F.; Mangian, H.; Lambrakis, L.; Saad, F.M.; de Godoy, M.R.C. Insect meals as novel protein sources in wet pet foods for adult cats. J. Anim. Sci. 2020, 98, 315. [Google Scholar] [CrossRef]
- Kilburn, L.R.; Carlson, A.T.; Lewis, E.; Serao, M.C.R. Cricket (Gryllodes sigillatus) meal fed to healthy adult dogs does not affect general health and minimally impacts apparent total tract digestibility. J. Anim. Sci. 2020, 98, skaa083. [Google Scholar] [CrossRef] [Green Version]
- Freel, T.A.; McComb, A.; Koutsos, E.A. Digestibility and safety of dry black soldier fly larvae meal and black soldier fly larvae oil in dogs. J. Anim. Sci. 2021, 99, skab047. [Google Scholar] [CrossRef] [PubMed]
- Penazzi, L.; Schiavone, A.; Russo, N.; Nery, J.; Valle, E.; Madrid, J.; Martinez, S.; Hernandez, F.; Pagani, E.; Ala, U.; et al. In Vivo and in vitro digestibility of an extruded complete dog food containing black soldier fly (Hermetia illucens) larvae meal as protein source. Front. Vet. Sci. 2021, 8, 653411. [Google Scholar] [CrossRef] [PubMed]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible insects in a food safety and nutritional perspective: A critical review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food safety issues related to uses of insects for feeds and foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murefu, T.R.; Macheka, L.; Musundire, R.; Manditsera, F.A. Safety of wild harvested and reared edible insects: A review. Food Control. 2019, 101, 209–224. [Google Scholar] [CrossRef]
- Imathiu, S. Benefits and food safety concerns associated with consumption of edible insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Edible Insects. Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Mézes, M. Food safety aspect of insects: A review. Acta Aliment. 2018, 47, 513–522. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E.; Lorini, C.; Oliva, N.; Bonaccorsi, G. Insects as food: A review on risks assessments of Tenebrionidae and Gryllidae in relation to a first machines and plants development. Food Control. 2020, 108, 106877. [Google Scholar] [CrossRef]
- Fröhling, A.; Bußler, S.; Durek, J.; Schlüter, O.K. Thermal impact on the culturable microbial diversity along the processing chain of flour from crickets (Acheta domesticus). Front. Microbiol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- van Broekhoven, S.; Gutierrez, J.M.; De Rijk, T.C.; De Nijs, W.C.M.; Van Loon, J.J.A. Degradation and excretion of the Fusarium toxin deoxynivalenol by an edible insect, the yellow mealworm (Tenebrio molitor L.). World Mycotoxin J. 2017, 10, 163–169. [Google Scholar] [CrossRef]
- Camenzuli, L.; Van Dam, R.; de Rijk, T.; Andriessen, R.; Van Schelt, J.; Van der Fels-Klerx, H. Tolerance and excretion of the mycotoxins aflatoxin B1, zearalenone, deoxynivalenol, and ochratoxin A by Alphitobius diaperinus and Hermetia illucens from contaminated substrates. Toxins 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Smet, J.; Wynants, E.; Cos, P.; Van Campenhout, L. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and impact on exploitation potential. Appl. Environ. Microbiol. 2018, 84, e02722-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2015, 35, 261–271. [Google Scholar] [CrossRef]
- De Smet, J.; Vandeweyer, D.; Van Moll, L.; Lachi, D.; Van Campenhout, L. Dynamics of Salmonella inoculated during rearing of black soldier fly larvae (Hermetia illucens). Food Res. Int. 2021, 149, 110692. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Liess, M.; Liebmann, L.; Vormeier, P.; Weisner, O.; Altenburger, R.; Borchardt, D.; Brack, W.; Chatzinotas, A.; Escher, B.; Foit, K.; et al. Pesticides are the dominant stressors for vulnerable insects in lowland streams. Water Res. 2021, 201, 117262. [Google Scholar] [CrossRef]
- Poma, G.; Fujii, Y.; Lievens, S.; Bombeke, J.; Gao, B.; Jeong, Y.; McGrath, T.J.; Covaci, A. Occurrence, patterns, and sources of hazardous organic chemicals in edible insects and insect-based food from the Japanese market. Food Chem. Toxicol. 2021, 154, 112311. [Google Scholar] [CrossRef]
- Hernández, R.B.; de Souza-Pinto, N.C.; Kleinjans, J.; van Herwijnen, M.; Piepers, J.; Moteshareie, H.; Burnside, D.; Golshani, A. Manganese-induced neurotoxicity through impairment of cross-talk pathways in human neuroblastoma cell line SH-SY5Y differentiated with retinoic acid. Toxics 2021, 9, 348. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; van Broekhoven, S.; den Hartog-Jager, C.F.; Gaspari, M.; de Jong, G.A.H.; Wichers, H.J.; van Hoffen, E.; Houben, G.F.; Knulst, A.C. House dust mite (Der p 10) and crustacean allergic patients may react to food containing yellow mealworm proteins. Food Chem. Toxicol. 2014, 65, 364–373. [Google Scholar] [CrossRef]
- Schlüter, O.; Rumpold, B.; Holzhauser, T.; Roth, A.; Vogel, R.F.; Quasigroch, W.; Vogel, S.; Heinz, V.; Jäger, H.; Bandick, N.; et al. Safety aspects of the production of foods and food ingredients from insects. Mol. Nutr. Food Res. 2017, 61, 1600520. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Zrimšek, P.; Kotnik, T.; Leonardi, A.; Križaj, I.; Jakovac Strajn, B. Insect protein-based diet as potential risk of allergy in dogs. Animals 2021, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Ekop, E.A.; Udoh, A.I.; Akpan, P.E. Proximate and anti-nutrient composition of four edible insects in Akwa Ibom State. Nigeria. World, J. Appl. Sci. Technol. 2010, 2, 224–231. [Google Scholar]
- Musundire, R.; Zvidzai, C.J.; Chidewe, C.; Samende, B.K.; Manditsera, F.A. Nutrient and anti-nutrient composition of Henicus whellani (Orthoptera: Stenopelmatidae), an edible ground cricket, in south-eastern Zimbabwe. Int. J. Trop. Insect Sci. 2014, 34, 223–231. [Google Scholar] [CrossRef]
- Kunatsa, Y.; Chidewe, C.; Zvidzai, C.J. Phytochemical and anti-nutrient composite from selected marginalized Zimbabwean edible insects and vegetables. J. Agric. Food Res. 2020, 2, 100027. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Van Rooijen, C.; Bosch, G.; van der Poel, A.F.B.; Wierenga, P.A.; Alexander, L.; Hendriks, W.H. The Maillard reaction and pet food processing: Effects on nutritive value and pet health. Nutr. Res. Rev. 2013, 26, 130–148. [Google Scholar] [CrossRef]
- Ferrer Llagostera, P.; Kallas, Z.; Reig, L.; Amores de Gea, D. The use of insect meal as a sustainable feeding alternative in aquaculture: Current situation, Spanish consumers’ perceptions and willingness to pay. J. Clean. Prod. 2019, 229, 10–21. [Google Scholar] [CrossRef]
- Mancuso, T.; Pippinato, L.; Gasco, L. The european insects sector and its role in the provision of green proteins in feed supply. Qual. Access Success 2019, 20, 374–381. [Google Scholar]
- Veldkamp, T.; van Duinkerken, G.; van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; van Boekel, M.A.J.S. Insects as a Sustainable Feed Ingredient in Pig and Poultry Diets—A Feasibility Study; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Bosch, G.; van Zanten, H.H.E.; Zamprogna, A.; Veenenbos, M.; Meijer, N.P.; van der Fels-Klerx, H.J.; van Loon, J.J.A. Conversion of organic resources by black soldier fly larvae: Legislation, efficiency and environmental impact. J. Clean. Prod. 2019, 222, 355–363. [Google Scholar] [CrossRef]
- Higa, J.E.; Ruby, M.B.; Rozin, P. Americans’ acceptance of black soldier fly larvae as food for themselves, their dogs, and farmed animals. Food Qual. Prefer. 2021, 90, 104119. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.E.; Williams, D.L. The feeding of live food to exotic pets: Issues of welfare and ethics. J. Exot. Pet Med. 2014, 23, 244–249. [Google Scholar] [CrossRef]
- Donoghue, S. Nutrition of pet amphibians and reptiles. Semin. Avian Exot. Pet Med. 1998, 7, 148–153. [Google Scholar] [CrossRef]
- Finke, M.D.; Oonincx, D. Insects as food for insectivores. In Mass Production of Beneficial Organisms; Elsevier: Amsterdam, The Netherlands, 2014; pp. 583–616. ISBN 9780123914538. [Google Scholar]
- Ferrie, G.M.; Alford, V.C.; Atkinson, J.; Baitchman, E.; Barber, D.; Blaner, W.S.; Crawshaw, G.; Daneault, A.; Dierenfeld, E.; Finke, M.; et al. Nutrition and health in amphibian husbandry. Zoo Biol. 2014, 33, 485–501. [Google Scholar] [CrossRef] [Green Version]
- Powers, L.V.; Perpiñán, D. Basic anatomy, physiology, and husbandry of ferrets. In Ferrets, Rabbits, and Rodents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–12. [Google Scholar]
- Smith, A.J. Husbandry and nutrition of hedgehogs. Vet. Clin. North Am. Exot. Anim. Pract. 1999, 2, 127–141. [Google Scholar] [CrossRef]
- Doss, G.A.; Carpenter, J.W. African pygmy hedgehogs. In Ferrets, Rabbits, and Rodents; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–415. [Google Scholar]
- Boyer, T.H.; Boyer, D.M. Turtles, tortoises, and terrapins. In Reptile Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2006; pp. 696–704. ISBN 9780721693279. [Google Scholar]
- Mott, R.; Pellett, S.; Hedley, J. Prevalence and risk factors for dental disease in captive Central bearded dragons (Pogona vitticeps) in the United Kingdom. J. Exot. Pet Med. 2021, 36, 1–7. [Google Scholar] [CrossRef]
- Jensen, N.H.; Lieberoth, A. We will eat disgusting foods together—Evidence of the normative basis of Western entomophagy-disgust from an insect tasting. Food Qual. Prefer. 2019, 72, 109–115. [Google Scholar] [CrossRef] [Green Version]
- British Veterinary Association (BVA). Grubs up! Can UK Pet Lovers Get to Grips with Insects in Pet Food? 2019. Available online: https://www.bva.co.uk/news-and-blog/blog-article/grubs-up-can-uk-pet-lovers-get-to-grips-with-insects-in-pet-food/ (accessed on 26 April 2022).
- Gałęcki, R.; Sokół, R. A parasitological evaluation of edible insects and their role in the transmission of parasitic diseases to humans and animals. PLoS ONE 2019, 14, e0219303. [Google Scholar] [CrossRef] [Green Version]
- Lähteenmäki-Uutela, A.; Grmelová, N.; Hénault-Ethier, L.; Deschamps, M.H.; Vandenberg, G.W.; Zhao, A.; Zhang, Y.; Yang, B.; Nemane, V. Insects as food and feed: Laws of the European union, United States, Canada, Mexico, Australia, and China. Eur. Food Feed Law Rev. 2017, 12, 22–36. [Google Scholar]
- Lähteenmäki-Uutela, A.; Hénault-Ethier, L.; Marimuthu, S.B.; Talibov, S.; Allen, R.N.; Nemane, V.; Vandenberg, G.W.; Józefiak, D. The impact of the insect regulatory system on the insect marketing system. J. Insects Food Feed 2018, 4, 187–198. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will yellow mealworm become a source of safe proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of dried yellow mealworm (Tenebrio Molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed (IPIFF). Insects as Novel Foods—An Overview. 2022. Available online: https://ipiff.org/insects-novel-food-eu-legislation-2/#:~:text=Following%20the%201st%20EFSA%20opinion,in%20the%20IPIFF%20Press%20Release (accessed on 23 May 2021).
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, Y.H.; Lee, J.W. Insect feed for animals under the Hazard Analysis and Critical Control Points (HACCP) regulations. Entomol. Res. 2016, 46, 2–4. [Google Scholar] [CrossRef]
- Han, R.; Shin, J.T.; Kim, J.; Choi, Y.S.; Kim, Y.W. An overview of the South Korean edible insect food industry: Challenges and future pricing/promotion strategies. Entomol. Res. 2017, 47, 141–151. [Google Scholar] [CrossRef]
- Servicio Agrícola y Ganadero. Resolución 6612 Exenta. 2018; pp. 1–35. Available online: http://bcn.cl/2n56b (accessed on 28 June 2021).
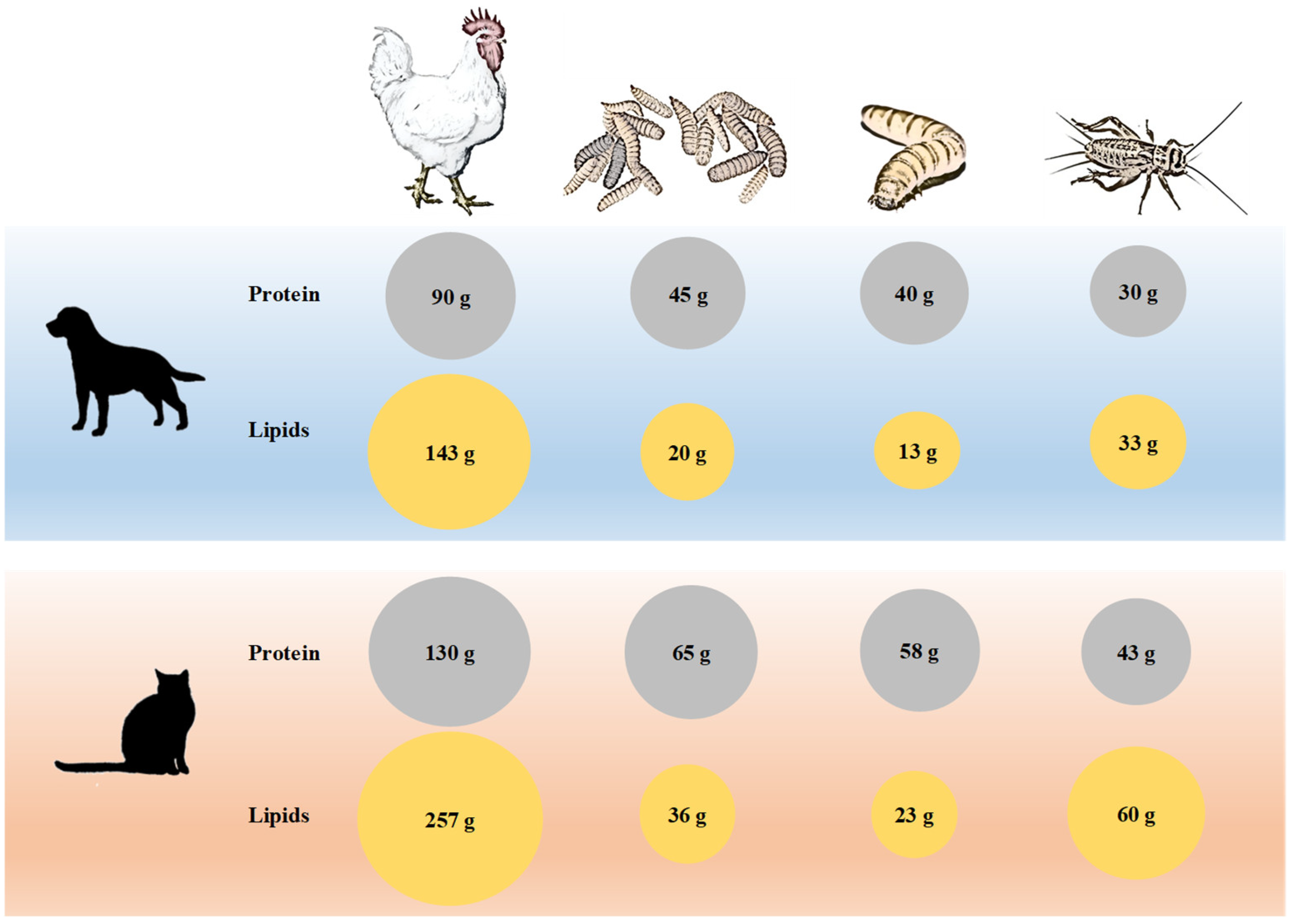

| Properties | Black Soldier Fly | Mealworm | Cricket | References |
|---|---|---|---|---|
 | ||||
| Crude protein (%) | 41–43 | 48–57 | 58–69 | [18] |
| Main amino acids | 1. Aspartic acid 2. Glutamic acid 3.Valine | 1. Glutamic acid 2. Leucine 3. Aspartic acid | 1. Glutamic acid 2. Leucine 3. Alanine | [18] |
| Lipids (%) | 17–34 | 32–40 | 11–23 | [18] |
| Main fatty acids | 1. Lauric acid 2. Oleic acid 3. Palmitic acid | 1. Oleic acid 2. Linoleic Acid 3. Palmitic acid | 1. Linoleic acid 2. Oleic Acid 3. Palmitic acid | [18] |
| Crude fiber (%) | 4–10 | 2–5 | 6–8 | [18,36,37,38,39,40] |
| Ash (%) | 15–27 | 2–4 | 3–8 | [18] |
| Gross energy (MJ/kg) | 20–24 | 26–27 | 20–22 | [18,38,41,42] |
| Calcium (g/kg) | 58–93 | 1–5 | 5–15 | [18] |
| Phosphorus (g/kg) | 5–13 | 4–11 | 7–8 | [18] |
| Company | Country | Feed Format | Insect | Pets |
|---|---|---|---|---|
| Activa care | Germany | Pellet | BSFL | Cats |
| Bug Bakes | United Kingdom | Pellet | Mealworm | Dogs |
| Buggy Bix | Australia | Treats | BSFL and mealworm | Dogs |
| Bugimine OÜ’s | Estonia | LL and DL | Mealworm | Exotic pets |
| Bugsforpets | Netherlands | Pellet | BSFL | Dogs |
| Catit nuna | Canada | Pellet, treats | BSFL and mealworm | Cats |
| Circular Pet | Chile | Pellet | BSFL | Dogs |
| Schecker | Germany | Pellet, treats | BSFL | Dogs |
| Eat small | Germany | Pellet, treats | BSFL and Mealworm | Dogs |
| EntoBento | United States | Treats | Cricket | Dogs |
| Entoma | France | Pellet, treats | BSFL and mealworm | Dogs and cats |
| Entomo Farms | Canada | Treats | Cricket | Dogs |
| Entovet | France | Pellet | BSFL and mealworm | Dogs and cats |
| Exo Terra | Canada | DI | Mealworm, cricket | Exotic pets |
| Green petfood | Germany | Pellet | Mealworm | Dogs and cats |
| Grubbets | United States | DL | BSFL | Exotic pets |
| Hexafly | Ireland | LL and DL | BSFL | Exotic pets |
| I love my cat | Germany | Pellet, wet food | BSFL | Cats |
| IBERinsect | Spain | LL, meal | Mealworm | Exotic pets |
| Invopets | Australia | Treats | Cricket | Dogs |
| Jiminy’s | United States | Pellet, treats | Cricket | Dogs |
| Lovebug | United Kingdom | Pellet | BSFL | Cats |
| Megalarva | Indonesia | DL | BSFL | Exotic pets |
| Mera | Germany | Pellet | BSFL | Dogs |
| Mjamjam | Germany | Wet food | Mealworm | Dogs and cats |
| Naturale for pets | Chile | DL | BSFL | Exotic pets |
| Nestlé Purina | Switzerland | Pellet | BSFL | Dogs and cats |
| PetZeba | Switzerland | Pellet | BSFL | Dogs |
| Protix | Netherlands | Wet food | BSFL | Dogs and cats |
| Sanimed | Netherlands | Pellet | Mealworm | Dogs |
| Tenetrio | Germany | Treats | Mealworm | Dogs |
| Tierliebhaber | Germany | Pellet | BSFL | Dogs |
| Tomojo | France | Pellet, treats | BSFL | Dogs and cats |
| Trovet | Netherlands | Pellet, treats | BSFL | Dogs and cats |
| Vet-concept | Germany | Pellet, treats | BSFL | Dogs and cats |
| Virbac | United Kingdom | Pellet | Mealworm | Dogs |
| Wilder Harrier | Canada | Pellet | BSFL | Dogs |
| Yora | United Kingdom | Pellet, treats | BSFL | Dogs |
| Zoo Med’s | United States | DL and LL | Cricket, silkworm | Exotic pets |
| Authors | Insects | Insect Processing | Conclusions |
|---|---|---|---|
| Bosch et al. [46] | HP, AHC, ML, LML, MWL, BSFL, BSFP, SSR, DHC, AC | Meal from lyophilized insects | In vitro study: the insects had good protein quality indices (high digestibility). Other aspects, such as product safety and pet owner perception are important for the use of insects as an alternative protein source in dog and cat feed. |
| Bosch et al. [21] | BSFL, HP, ML | Freeze-dried | In vitro study: the protein quality of the insects was high, and the undigested fractions were partially fermented by the microbiota of dogs. Mealworm larvae were the most fermentable. |
| Böhm et al. [33] | ML | Meal | In vivo study: insect protein-based diet is an interesting alternative for dogs with food allergies. |
| Kierończyk et al. [85] | ML, ATC, BSFL, ATHC | Air-dried | In vivo study: the smell that emanated from various species of insects was attractive to dogs and could even be a future replacement for flavoring agents. |
| Paßlack and Zentek [86] | BSFL | Meal | In vivo study: diets based on BSFL were well tolerated and accepted by most cats. The apparent digestibility of the crude protein and amino acids was moderate. It is recommended that an adequate safety margin be considered when formulating insect protein-based diets for cats to prevent nutrient deficiencies. |
| Jarett et al. [22] | ATHC | Meal | In vivo study: diets containing cricket generate a diversity of microorganisms in the gut microbiota, similar to a healthy balanced diet. These results indicate that crickets could become a nutritious and healthy ingredient for dogs. |
| Lei et al. [23] | BSFL | Defatted meal | In vivo study: supplementing the diet with BSFL may be beneficial to the nutrition and health of beagle dogs due to its high protein quality and anti-inflammatory and antioxidant capacity. |
| Feng et al. [87] | ML | Hydrolyzed larvae | In vivo study: mealworm showed lower palatability as dog food compared to hydrolyzed chicken liver but could be improved by the addition of key palatable volatile compounds. |
| Hong et al. [81] | HM | Meal | In vivo study: HM could be used as an alternative protein source in growing dogs without adverse effects. In addition, its use could reduce oxidative damage in growing dogs. |
| Hu et al. [88] | SC, MC, MWL | Meal | In vivo study: the insect meals tested had no negative effects on macronutrient digestibility, fecal characteristics and metabolites, or overall health of adult cats. |
| Kazimierska et al. [56] | BSFL | Meal | In vitro study: dog foods with insect protein exceeded the legal limit for manganese content. |
| Kilburn et al. [89] | ATHC | Meal | In vivo study: cricket is a highly acceptable ingredient for inclusion in the diet of dogs. |
| Kröger et al. [39] | BSFL | Meal | In vivo study: BSFL-based feed was well tolerated by dogs. This would indicate that it can be considered as an alternative protein source for dog nutrition. |
| Freel et al. [90] | BSFL | Meal and fat | In vivo study: food ingredients based on BSFL are well tolerated by dogs and their consumption has no negative physiological impact and could be safely included in dog diets. |
| Penazzi et al. [91] | BSFL | Meal | In vivo and in vitro study: digestibility analysis of BSFL-based food as sole source of protein showed promising results because it presented similar values as a meat-based diet. |
| Topic | Advantages | Disadvantages | References |
|---|---|---|---|
| Sustainability | Insect farming requires less land use and water consumption and has a lower carbon footprint compared to chicken, pork or beef production. | Insufficient studies have been conducted to determine the economic and social sustainability of insects. There is a lack of studies on large-scale industrial insect production. | [68,69] |
| Rentability | Insects have a high feed conversion efficiency (45–55%) compared to other production animals such as chicken (33%), which is the most commonly used animal protein in dog food. | Currently, the cost of insect meal is high (USD 2–10/kg). It is necessary to industrialize insect production to lower costs. | [68,118,119,120] |
| Circular economy | Insects are capable of feeding on organic wastes, thus contributing to waste management by transforming them into a high-quality food source. | The use of waste as a feed substrate, such as animal waste and feces, can result in food safety risks. | [105,121] |
| Nutrition | Insects have a high nutritional value, being a good source of highly digestible proteins, lipids and minerals. They also have a high energetic contribution. | Insect nutritional composition is highly variable and depends on many factors (species, diet and life cycle stage are among the most important). Insects may contain amounts of manganese that are excessive for the nutrition of dogs and cats. | [18,56,60,62] |
| Health | The use of insects in pet food would not generate negative health effects in dogs and cats. Insects have functional effects as antioxidants, anti-inflammatories and antimicrobials; however, this has not yet been studied in pets. | The inclusion of insects in pet diets may be associated with microbial, chemical, toxicological and allergenic risks. Although there are no reports of problems associated with these contaminants in pets, further food safety research is needed. | [17,22,23,33,39,81,86,88,89,90,98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, F.; Villanueva, V.; Durán, E.; Campos, F.; Avendaño, C.; Sánchez, M.; Domingoz-Araujo, C.; Valenzuela, C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals 2022, 12, 1450. https://doi.org/10.3390/ani12111450
Valdés F, Villanueva V, Durán E, Campos F, Avendaño C, Sánchez M, Domingoz-Araujo C, Valenzuela C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals. 2022; 12(11):1450. https://doi.org/10.3390/ani12111450
Chicago/Turabian StyleValdés, Fabrizzio, Valeria Villanueva, Emerson Durán, Francisca Campos, Constanza Avendaño, Manuel Sánchez, Chaneta Domingoz-Araujo, and Carolina Valenzuela. 2022. "Insects as Feed for Companion and Exotic Pets: A Current Trend" Animals 12, no. 11: 1450. https://doi.org/10.3390/ani12111450
APA StyleValdés, F., Villanueva, V., Durán, E., Campos, F., Avendaño, C., Sánchez, M., Domingoz-Araujo, C., & Valenzuela, C. (2022). Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals, 12(11), 1450. https://doi.org/10.3390/ani12111450








