Frequency of Semen Collection Affects Ram Sperm Cryoresistance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Collection and Sample Processing
2.3. Sperm Motility and Kinetic Parameters
2.4. Multiparametric Flow Cytometry Analyses
2.5. MiOXSYS® Analysis
2.6. SOD Assay
2.7. GPX Assay
2.8. Statistical Analysis
3. Results
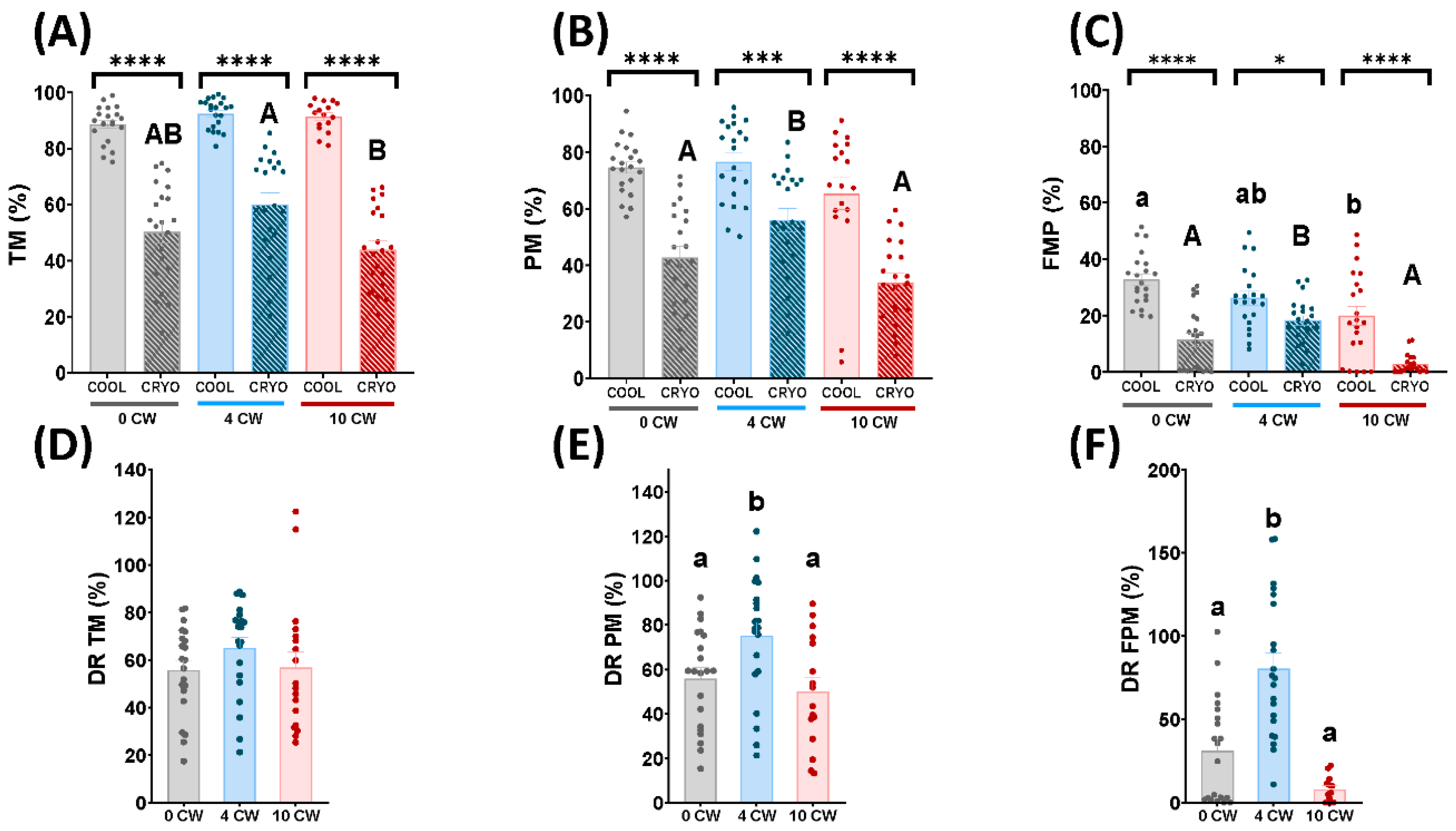
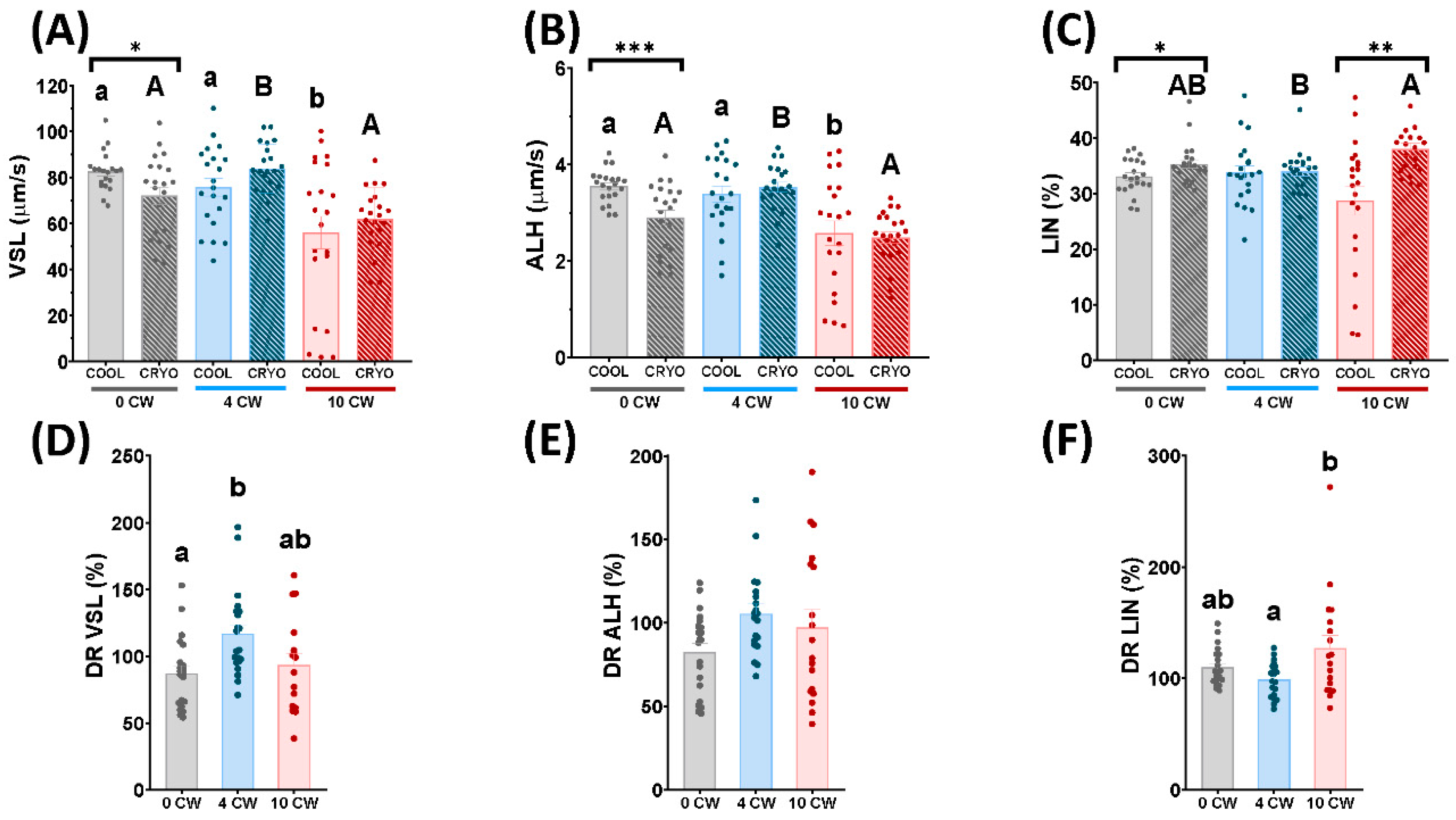
3.1. Sperm Motility and Kinetics Parameters
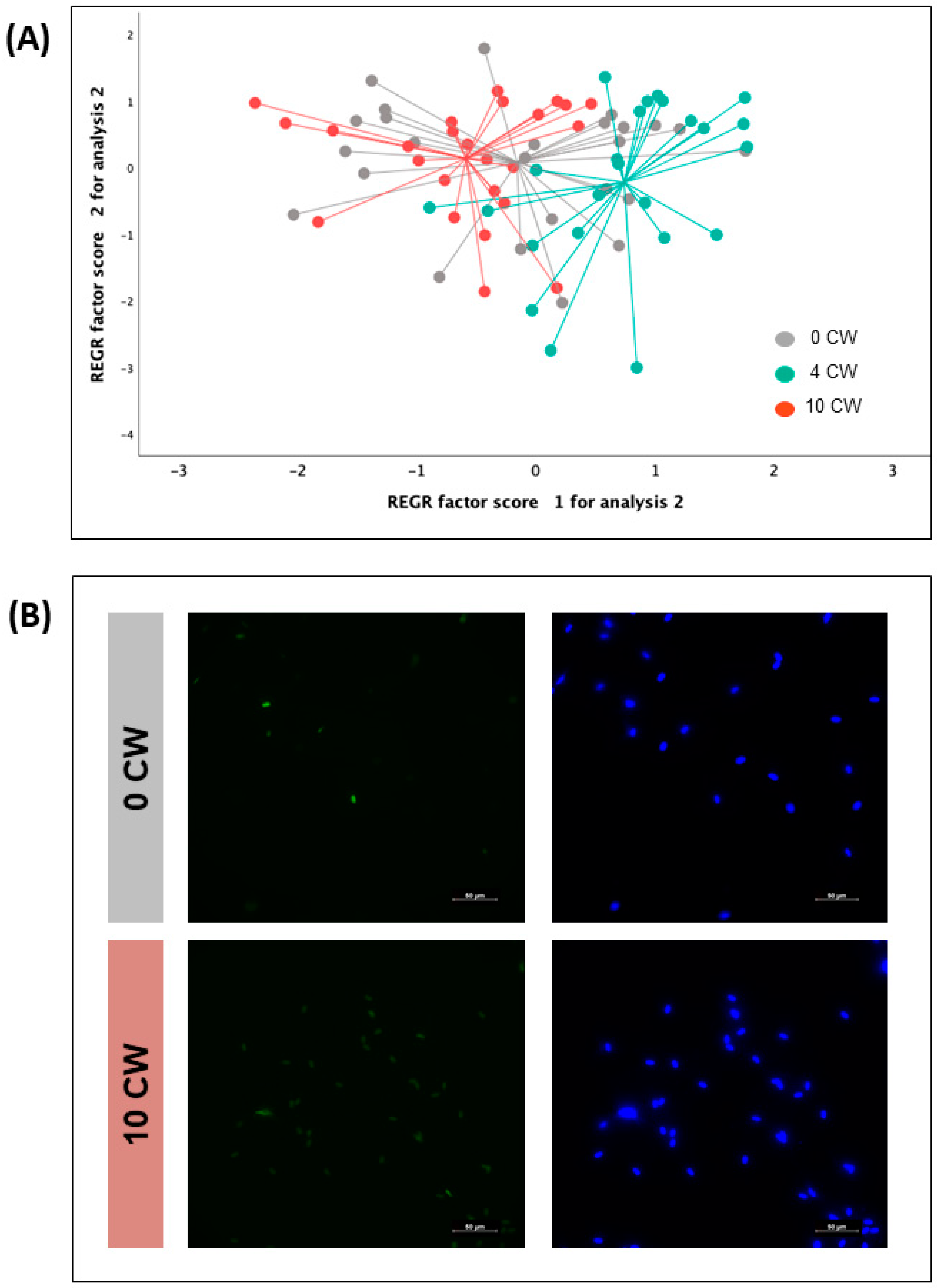
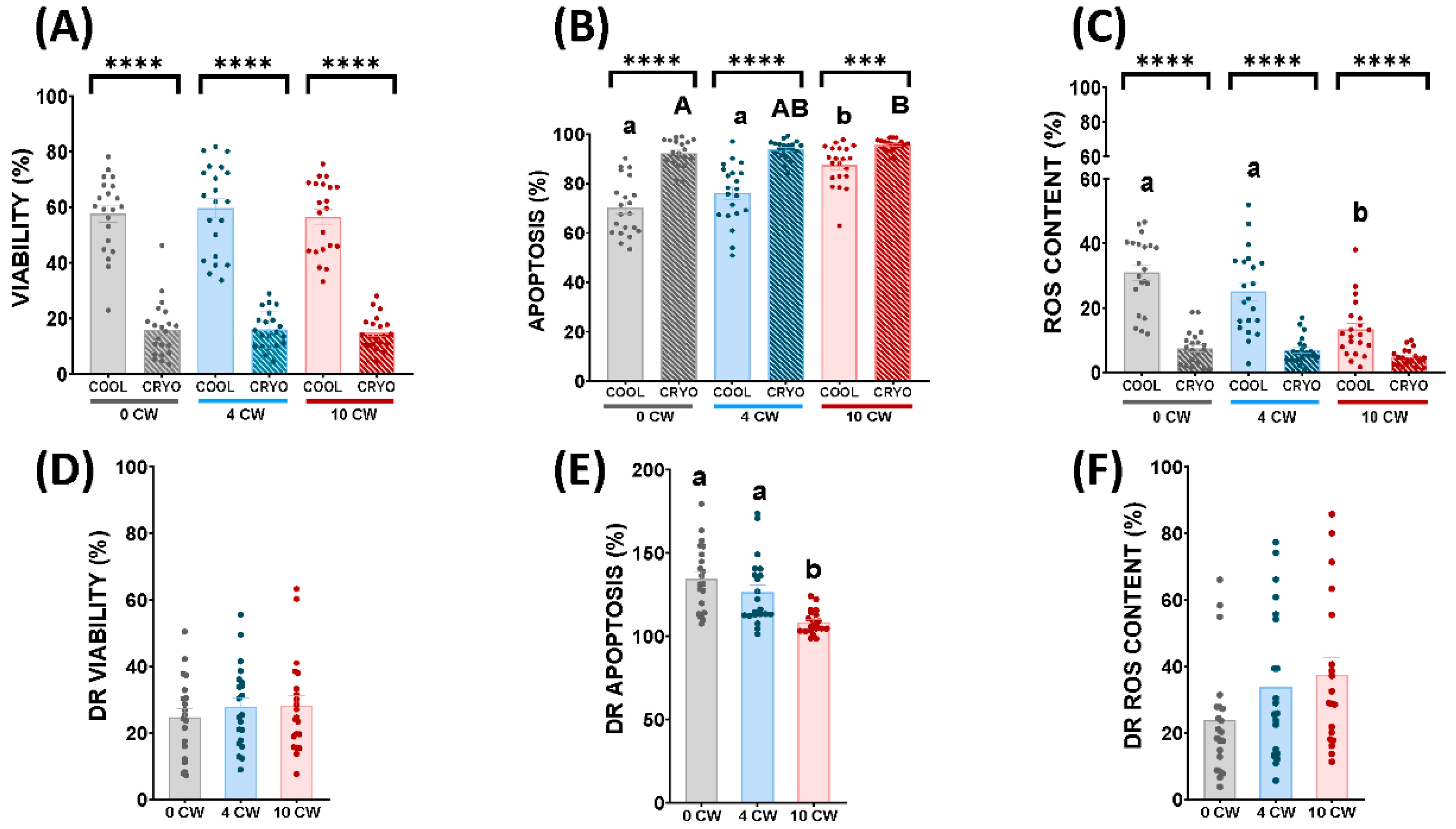
3.2. Flow Cytometry Analyses
3.3. Redox Analyses: Oxidation-Reduction Potential, SOD and GPX Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Nagata, M.P.B.; Egashira, J.; Katafuchi, N.; Endo, K.; Ogata, K.; Yamanaka, K.; Yamanouchi, T.; Matsuda, H.; Hashiyada, Y.; Yamashita, K. Bovine Sperm Selection Procedure Prior to Cryopreservation for Improvement of Post-Thawed Semen Quality and Fertility. J. Anim. Sci. Biotechnol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.; Anel-Lopez, L.; Boixo, J.C.; Chamorro, C.; Neila-Montero, M.; Montes-Garrido, R.; de Paz, P.; Anel, L. Current Challenges in Sheep Artificial Insemination: A Particular Insight. Reprod. Domest. Anim. 2019, 54, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Anel, L.; Alvarez, M.; Martinez-Pastor, F.; Garcia-Macias, V.; Anel, E.; de Paz, P. Improvement Strategies in Ovine Artificial Insemination. Reprod. Domest. Anim. 2006, 41, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Pini, T.; Leahy, T.; de Graaf, S.P. Sublethal Sperm Freezing Damage: Manifestations and Solutions. Theriogenology 2018, 118, 172–181. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef]
- He, Y.; Wang, K.; Zhao, X.; Zhang, Y.; Ma, Y.; Hu, J. Differential Proteome Association Study of Freeze-Thaw Damage in Ram Sperm. Cryobiology 2016, 72, 60–68. [Google Scholar] [CrossRef]
- Peris, S.I.; Bilodeau, J.F.; Dufour, M.B.J. Impact of Cryopreservation and Reactive Oxygen Species on DNA Integrity, Lipid Peroxidation, and Functional Parameters in Ram Sperm. Mol. Reprod. Dev. 2007, 74, 878–892. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Cesari, A.; Garde, J.J.; Villar, M.; Soler, A.J. Cryopreservation of Ram Sperm Alters the Dynamic Changes Associated with in Vitro Capacitation. Theriogenology 2020, 145, 100–108. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative Stress and Male Reproductive Health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Battut, I.B.; Kempfer, A.; Lemasson, N.; Chevrier, L.; Camugli, S. Prediction of the Fertility of Stallion Frozen-Thawed Semen Using a Combination of Computer-Assisted Motility Analysis, Microscopical Observation and Flow Cytometry. Theriogenology 2017, 97, 186–200. [Google Scholar] [CrossRef]
- Pabón, D.; Meseguer, M.; Sevillano, G.; Cobo, A.; Romero, J.L.; Remohí, J.; de los Santos, M.J. A New System of Sperm Cryopreservation: Evaluation of Survival, Motility, DNA Oxidation, and Mitochondrial Activity. Andrology 2019, 7, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; O’Flaherty, C.; Ortiz Rodríguez, J.M.; Martín Cano, F.E.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ferrusola, C.O. Redox Regulation and Oxidative Stress: The Particular Case of the Stallion Spermatozoa. Antioxidants 2019, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colagar, A.H.; Karimi, F.; Jorsaraei, S.G.A. Correlation of Sperm Parameters with Semen Lipid Peroxidation and Total Antioxidants Levels in Astheno- and Oligoasheno- Teratospermic Men. Iran. Red Crescent Med. J. 2013, 15, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Badade, Z.; More, K.; Narshetty, J.; Badade, V.; Yadav, B. Human Seminal Oxidative Stress: Correlation with Antioxidants and Sperm Quality Parameters. Ann. Biol. Res. 2011, 2, 351–359. [Google Scholar]
- Huang, C.; Cao, X.; Pang, D.; Li, C.; Luo, Q.; Zou, Y.; Feng, B.; Li, L.; Cheng, A.; Chen, Z. Is Male Infertility Associated with Increased Oxidative Stress in Seminal Plasma? A-Meta Analysis. Oncotarget 2018, 9, 24494–24513. [Google Scholar] [CrossRef] [Green Version]
- Kasimanickam, R.; Pelzer, K.D.; Kasimanickam, V.; Swecker, W.S.; Thatcher, C.D. Association of Classical Semen Parameters, Sperm DNA Fragmentation Index, Lipid Peroxidation and Antioxidant Enzymatic Activity of Semen in Ram-Lambs. Theriogenology 2006, 65, 1407–1421. [Google Scholar] [CrossRef]
- Marti, E.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Effect of the Cryopreservation Process on the Activity and Immunolocalization of Antioxidant Enzymes in Ram Spermatozoa. J. Androl. 2008, 29, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous Lipid Peroxidation and Production of Hydrogen Peroxide and Superoxide in Human Spermatozoa Superoxide Dismutase as Major Enzyme Protectant against Oxygen Toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Cassani, P.; Beconi, M.T.; O’Flaherty, C. Relationship between Total Superoxide Dismutase Activity with Lipid Peroxidation, Dynamics and Morphological Parameters in Canine Semen. Anim. Reprod. Sci. 2005, 86, 163–173. [Google Scholar] [CrossRef]
- Murawski, M.; Saczko, J.; Marcinkowska, A.; Chwiłkowska, A.; Grybós, M.; Banaś, T. Evaluation of Superoxide Dismutase Activity and Its Impact on Semen Quality Parameters of Infertile Men. Folia Histochem. Cytobiol. 2007, 45, 123–126. [Google Scholar] [CrossRef]
- Maier, C.M.; Chan, P.H. Role of Superoxide Dismutases in Oxidative Damage and Neurodegenerative Disorders. Neuroscientist 2002, 8, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.T.K. Glutathione Peroxidase Isolated from Plasma Reduces Phospholipid Hydroperoxides. Arch. Biochem. Biophys. 1993, 305, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Cofré-Narbona, E.J.; Peralta-Troncoso, O.A.; Urquieta-Mangiola, B.E.; Raggi-Saini, L.A.; Benavides-Aguila, N.; Parraguez-Gamboa, V.H. Improvement of Antioxidant Status and Semen Quality by Oral Supplementation with Vitamins c and e in Rams. Rev. Cient. La Fac. Ciencias Vet. La Univ. Del Zulia 2016, 26, 156–163. [Google Scholar]
- Lecewicz, M.; Hering, D.M.; Kamiński, S.; Majewska, A.; Kordan, W. Selected Qualitative and Biochemical Parameters of Cryopreserved Semen of Holstein-Friesian (HF) AI Bulls. Pol. J. Vet. Sci. 2015, 18, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Papas, M.; Catalán, J.; Fernandez-Fuertes, B.; Arroyo, L.; Bassols, A.; Miró, J.; Yeste, M. Specific Activity of Superoxide Dismutase in Stallion Seminal Plasma Is Related to Sperm Cryotolerance. Antioxidants 2019, 8, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Bui, A.D. Oxidation-Reduction Potential as a New Marker for Oxidative Stress: Correlation to Male Infertility. Investig. Clin. Urol. 2017, 58, 385–399. [Google Scholar] [CrossRef]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic Application of Oxidation-Reduction Potential Assay for Measurement of Oxidative Stress: Clinical Utility in Male Factor Infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.; Sharafi, M.; Hosseini, S.M.; Ostadhosseini, S.; Hajian, M.; Hosseini, L.; Abedi, P.; Nili, N.; Rahmani, H.R.; Nasr-Esfahani, M.H. In Vitro Comparison of Egg Yolk-Based and Soybean Lecithin-Based Extenders for Cryopreservation of Ram Semen. Theriogenology 2010, 73, 480–487. [Google Scholar] [CrossRef]
- Alcay, S.; Ustuner, B.; Nur, Z. Effects of Low Molecular Weight Cryoprotectants on the Post-Thaw Ram Sperm Quality and Fertilizing Ability. Small Rumin. Res. 2016, 136, 59–64. [Google Scholar] [CrossRef]
- Riesco, M.F.; Alvarez, M.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Boixo, J.C.; de Paz, P.; Anel, L. Multiparametric Study of Antioxidant Effect on Ram Sperm Cryopreservation—from Field Trials to Research Bench. Animals 2021, 11, 283. [Google Scholar] [CrossRef]
- Allai, L.; Benmoula, A.; Marciane da Silva, M.; Nasser, B.; El Amiri, B. Supplementation of Ram Semen Extender to Improve Seminal Quality and Fertility Rate. Anim. Reprod. Sci. 2018, 192, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Anel, L.; De Paz, P.; Álvarez, M.; Chamorro, C.A.; Boixo, J.C.; Manso, A.; González, M.; Kaabi, M.; Anel, E. Field and in Vitro Assay of Three Methods for Freezing Ram Semen. Theriogenology 2003, 60, 1293–1308. [Google Scholar] [CrossRef]
- Söderquist, L.; Madrid-Bury, N.; Rodriguez-Martinez, H. Assessment of Ram Sperm Membrane Integrity Following Different Thawing Procedures. Theriogenology 1997, 48, 1115–1125. [Google Scholar] [CrossRef]
- Ayad, B.M.; Gerhard Van der, H.; Du Plessis, S.S. Revisiting the Relationship between the Ejaculatory Abstinence Period and Semen Characteristics. Int. J. Fertil. Steril. 2018, 11, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ayad, B.M.; Van der Horst, G.; du Plessis, S.S. Short Abstinence: A Potential Strategy for the Improvement of Sperm Quality. Middle East Fertil. Soc. J. 2018, 23, 37–43. [Google Scholar] [CrossRef]
- Kaya, A.; Aksoy, M.; Tekeli, T. Influence of Ejaculation Frequency on Sperm Characteristics, Ionic Composition and Enzymatic Activity of Seminal Plasma in Rams. Small Rumin. Res. 2002, 44, 153–158. [Google Scholar] [CrossRef]
- Frangež, R.; Gider, T.; Kosec, M. Frequency of Boar Ejaculate Collection and Its Influence on Semen Quality, Pregnancy Rate and Litter Size. Acta Vet. Brno 2005, 74, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Ollero, M.; Muiño-Blanco, T.; López-Pérez, M.J.; Cebrián-Pérez, J.A. Viability of Ram Spermatozoa in Relation to the Abstinence Period and Successive Ejaculations. Int. J. Androl. 1996, 19, 287–292. [Google Scholar] [CrossRef]
- Sieme, H.; Katila, T.; Klug, E. Effect of Semen Collection Practices on Sperm Characteristics before and after Storage and on Fertility of Stallions. Theriogenology 2004, 61, 769–784. [Google Scholar] [CrossRef]
- Álvarez, M.; Chamorro, C.A.; Kaabi, M.; Anel-López, L.; Boixo, J.C.; Anel, E.; Anel, L.; de Paz, P. Design and “in Vivo” Evaluation of Two Adapted Catheters for Intrauterine Transcervical Insemination in Sheep. Anim. Reprod. Sci. 2012, 131, 153–159. [Google Scholar] [CrossRef]
- Yeste, M. Sperm Cryopreservation Update: Cryodamage, Markers, and Factors Affecting the Sperm Freezability in Pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Toker, M.B.; Alcay, S.; Gokce, E.; Ustuner, B. Cryopreservation of Ram Semen with Antioxidant Supplemented Soybean Lecithin-Based Extenders and Impacts on Incubation Resilience. Cryobiology 2016, 72, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; Álvarez, M.; Tamayo-Canul, J.; Anel, L.; de Paz, P.; Martínez-Pastor, F. Post-Thawing Quality and Incubation Resilience of Cryopreserved Ram Spermatozoa Are Affected by Antioxidant Supplementation and Choice of Extender. Theriogenology 2015, 83, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Torres, B.J.M.; Camargo, M.; Agarwal, A.; du Plessis, S.S.; Cadavid, Á.P.; Cardona Maya, W.D. Influence of Ejaculation Frequency on Seminal Parameters. Reprod. Biol. Endocrinol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pastor, F.; Garcia-Macias, V.; Alvarez, M.; Herraez, P.; Anel, L.; de Paz, P. Sperm Subpopulations in Iberian Red Deer Epididymal Sperm and Their Changes through the Cryopreservation Process. Biol. Reprod. 2005, 72, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Al-Bulushi, S.; Manjunatha, B.M.; Bathgate, R.; Rickard, J.P.; de Graaf, S.P. Effect of Semen Collection Frequency on the Semen Characteristics of Dromedary Camels. Anim. Reprod. Sci. 2018, 197, 145–153. [Google Scholar] [CrossRef]
- Ferré, L.B.; Malik, G.; Aller, J.F.; Alberio, R.H.; Fresno, C.; Kjelland, M.E. Llama (Lama Glama) Semen Collection via Thermo-Electric Artificial Vagina: Effect of Seasonality and Collection Interval on Ejaculate Characteristics. Small Rumin. Res. 2015, 133, 140–147. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Kafi, M.; Safdarian, M.; Hashemi, M. Seasonal Variation in Semen Characteristics, Scrotal Circumference and Libido of Persian Karakul Rams. Small Rumin. Res. 2004, 53, 133–139. [Google Scholar] [CrossRef]
- Plaza Davila, M.; Martin Muñoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; Balao da Silva C, C.; Peña, F.J.; Davila, M.P.; Muñoz, P.M.; Tapia, J.A.; Ferrusola, C.O.; et al. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, While the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef] [Green Version]
- Desai, N.R.; Mahfouz, R.; Sharma, R.; Gupta, S.; Agarwal, A. Reactive Oxygen Species Levels Are Independent of Sperm Concentration, Motility, and Abstinence in a Normal, Healthy, Proven Fertile Man: A Longitudinal Study. Fertil. Steril. 2010, 94, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.-L.L. Oxidation-Reduction Potential of Semen: What Is Its Role in the Treatment of Male Infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riesco, M.F.; Anel-Lopez, L.; Neila-Montero, M.; Palacin-Martinez, C.; Montes-Garrido, R.; Alvarez, M.; de Paz, P.; Anel, L. ProAKAP4 as Novel Molecular Marker of Sperm Quality in Ram: An Integrative Study in Fresh, Cooled and Cryopreserved Sperm. Biomolecules 2020, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodriguez, J.M.; da Silva, C.B.; Masot, J.; Redondo, E.; Gazquez, A.; Tapia, J.A.; Gil, C.; Ortega-Ferrusola, C.; Peña, F.J. Rosiglitazone in the Thawing Medium Improves Mitochondrial Function in Stallion Spermatozoa through Regulating Akt Phosphorylation and Reduction of Caspase 3. PLoS ONE 2019, 14, e0211994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, R.; Elsuity, M.; Henkel, R.; Agarwal, A. High Levels of Oxidation–Reduction Potential in Frozen-Thawed Human Semen Are Significantly Correlated with Poor Post-Thaw Sperm Quality. Andrologia 2020, 52, e13608. [Google Scholar] [CrossRef] [PubMed]
- Gürler, H.; Calisici, O.; Bollwein, H. Inter- and Intra-Individual Variability of Total Antioxidant Capacity of Bovine Seminal Plasma and Relationships with Sperm Quality before and after Cryopreservation. Anim. Reprod. Sci. 2015, 155, 99–105. [Google Scholar] [CrossRef]
- Papas, M.; Catalan, J.; Barranco, I.; Arroyo, L.; Bassols, A.; Yeste, M.; Miró, J. Total and Specific Activities of Superoxide Dismutase (SOD) in Seminal Plasma Are Related with the Cryotolerance of Jackass Spermatozoa. Cryobiology 2020, 92, 109–116. [Google Scholar] [CrossRef]
- Papas, M.; Arroyo, L.; Bassols, A.; Catalán, J.; Bonilla-Correal, S.; Gacem, S.; Yeste, M.; Miró, J. Activities of Antioxidant Seminal Plasma Enzymes (SOD, CAT, GPX and GSR) Are Higher in Jackasses than in Stallions and Are Correlated with Sperm Motility in Jackasses. Theriogenology 2019, 140, 180–187. [Google Scholar] [CrossRef]
- Baumber, J.; Ball, B.A. Determination of Glutathione Peroxidase and Superoxide Dismutase-like Activities in Equine Spermatozoa, Seminal Plasma, and Reproductive Tissues. Am. J. Vet. Res. 2005, 66, 1415–1419. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Kazeminejad, S.R.; Shahbazian, N.; Pourmehdi, M.; Jabbari, A. Oxidative Stress Status and Sperm DNA Fragmentation in Fertile and Infertile Men. Andrologia 2017, 49, e12762. [Google Scholar] [CrossRef]
- Waheed, M.M.; Gouda, E.M.; Khalifa, T.A.A. Impact of Seminal Plasma Superoxide Dismutase and Glutathione Peroxidase on Cryopreserved Buffalo Spermatozoa. Anim. Reprod. Sci. 2013, 142, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal Plasma Antioxidants Are Directly Involved in Boar Sperm Cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Gupta, S.; Du Plessis, S.; Sharma, R.; Esteves, S.C.; Cirenza, C.; Eliwa, J.; Al-Najjar, W.; Kumaresan, D.; Haroun, N.; et al. Abstinence Time and Its Impact on Basic and Advanced Semen Parameters. Urology 2016, 94, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Gagnon, C. A Human Seminal Plasma Protein Blocks the Motility of Human Spermatozoa. J. Urol. 1988, 140, 1045–1048. [Google Scholar] [CrossRef]
- Skandhan, K.P. Hypothesis: Epididymis Inhibits Sperm Motility inside Male Reproductive Tract. Med. Hypotheses 2004, 62, 146–150. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacin-Martinez, C.; Alvarez, M.; Montes-Garrido, R.; Neila-Montero, M.; Anel-Lopez, L.; de Paz, P.; Anel, L.; Riesco, M.F. Frequency of Semen Collection Affects Ram Sperm Cryoresistance. Animals 2022, 12, 1492. https://doi.org/10.3390/ani12121492
Palacin-Martinez C, Alvarez M, Montes-Garrido R, Neila-Montero M, Anel-Lopez L, de Paz P, Anel L, Riesco MF. Frequency of Semen Collection Affects Ram Sperm Cryoresistance. Animals. 2022; 12(12):1492. https://doi.org/10.3390/ani12121492
Chicago/Turabian StylePalacin-Martinez, Cristina, Mercedes Alvarez, Rafael Montes-Garrido, Marta Neila-Montero, Luis Anel-Lopez, Paulino de Paz, Luis Anel, and Marta F. Riesco. 2022. "Frequency of Semen Collection Affects Ram Sperm Cryoresistance" Animals 12, no. 12: 1492. https://doi.org/10.3390/ani12121492
APA StylePalacin-Martinez, C., Alvarez, M., Montes-Garrido, R., Neila-Montero, M., Anel-Lopez, L., de Paz, P., Anel, L., & Riesco, M. F. (2022). Frequency of Semen Collection Affects Ram Sperm Cryoresistance. Animals, 12(12), 1492. https://doi.org/10.3390/ani12121492





