3.1. Fermentation Quality of Stylosanthes Silage
The fermentation quality-related dynamics of high-moisture content
Stylosanthes silage are shown in
Table 1. Throughout the fermentation process, the pH value of each treatment group showed a significant decreasing trend (
p < 0.05). On the 45th day, the pH value of the LP + xg treatment group was the lowest.
During the entire fermentation process, the lactic acid content of each treatment group showed a significant increasing trend (p < 0.05). Fifteen days before fermentation, the lactic acid content of each treatment group increased rapidly, stabilized after the 15th day of fermentation, and peaked on the 45th day of fermentation. During the early stage of silage fermentation, the lactic acid content of each treatment group was similar.
The acetic acid content of each treatment group increased significantly during the entire silage fermentation process (p < 0.05); it increased rapidly in each treatment group 7 days before fermentation and stabilized after 7 days of fermentation. On the 45th day of fermentation, the acetic acid levels of the treatment groups xg and LP + xg were significantly lower than those of the control group and LP treatment group (p < 0.05), while the acetic acid content of the xg treatment group was the lowest.
With the increase in silage fermentation time, the propionic acid content in each treatment group showed a significant increase (p < 0.05); at the same fermentation time, there was no significant difference in the propionic acid content between treatment groups (p > 0.05). No butyric acid was detected during the entire process of silage fermentation.
Within days 1–45 of fermentation, the ammonia nitrogen content for each treatment group showed a significant increasing trend (p < 0.05) with an increase in the fermentation time. At the beginning of the fermentation process, there was no significant difference in the ammonia nitrogen content between treatment groups (p > 0.05). Subsequently, the ammonia nitrogen content in the control group became significantly higher than that in the three treatment groups (p < 0.05).
The fermentation quality dynamics of
Stylosanthes silage with low moisture contents are shown in
Table 2. During the first 1–7 days of fermentation, the pH value of each treatment group decreased gradually. On the 45th day, the pH value of the control was significantly higher than that of the other three treatment groups (
p < 0.05), and the pH value of the LP treatment group was significantly higher than that of xg and LP + xg (
p < 0.05). There was no significant difference in the pH value between the LP, LP + xg, and control groups, as compared to that of the xg (
p > 0.05) group; the values were 5.06, 4.95, 4.64, and 4.58, respectively.
During the first 15 days of fermentation, with an increase in the fermentation time, the lactic acid content of each treatment group increased significantly (p < 0.05); it tended to be stable between the 15th to 45th day of fermentation, and there was hardly any change in the lactic acid content.
From the 1st to the 45th day of the fermentation process, the acetic acid content in the control group did not change significantly (p > 0.05) with an increase in the silage fermentation time. On the 45th day of fermentation, the acetic acid content in the control group was significantly higher than that in the three additive treatment groups (p < 0.05), and there was no significant difference between the three additive treatment groups (p > 0.05). The acetic acid content in the LP + xg treatment group was the lowest.
From the 1st to the 45th day of fermentation, with an increase in the silage fermentation time, the propionic acid content in each treatment group showed a significant increase (p < 0.05). On the 45th day of fermentation, the propionic acid content of the control group was the highest, and that of the LP + xg treatment group was the lowest. No butyric acid was detected during the entire process of silage fermentation.
Within days 1–45 of fermentation, the ammonia nitrogen content in each treatment group showed a significant increasing trend (p < 0.05) with an increase in the fermentation time. At the initial fermentation stage, there was no significant difference in the ammonia nitrogen content between the treatment groups (p > 0.05). Subsequently, the ammonia nitrogen content in the control group became significantly higher than that in the three additive treatment groups (p < 0.05). There was no significant difference among the treatment groups (p > 0.05).
The effects of different factors and the interactions between them on the fermentation quality of
Stylosanthes silage are shown in
Table 3. Moisture content had a very significant effect on the pH value and the content of NH
3-N, lactic acid, acetic acid, and propionic acid (
p < 0.01). Treatment had a very significant effect on the pH value and the NH
3-N, lactic acid, and acetic acid content (
p < 0.01), but had no significant effect on the propionic acid content (
p > 0.05). Ensiling time had a very significant effect on the pH value and the NH
3-N, lactic acid, acetic acid, and propionic acid content (
p < 0.01). The interaction between moisture content and treatment as well as the interaction between treatment and ensiling time had a very significant effect on the pH value and the NH
3-N, lactic acid, and acetic acid (
p < 0.01) content, but had no significant effect on the propionic acid content (
p > 0.05). The interaction between the moisture content and ensiling time had a very significant effect on the pH value and the NH
3-N, lactic acid, acetic acid, and propionic acid (
p < 0.01) content. The interactions among these three processing factors had a very significant effect on the pH value and the NH
3-N, lactic acid, and acetic acid (
p < 0.01) content, but had no significant effect on the propionic acid (
p > 0.05) content.
3.2. Chemical Compositions of Stylosanthes Silages
The chemical composition of
Stylosanthes before the silage process is shown in
Table 4.
Table 5 shows the effects of additives on nutrient quality dynamics in high-moisture content
Stylosanthes silage. The DM content in
Stylosanthes silage exhibited a relatively stable changing trend during the entire fermentation process. At the initial stage of silage fermentation, there was no significant difference in the DM content of
Stylosanthes silage between treatment groups (
p > 0.05).
The CP content in Stylosanthes silage exhibited a relatively stable changing trend during the entire fermentation process, and no significant difference in the extent of change was observed (p > 0.05). At the same silage fermentation time, there was no significant difference in the CP content between the treatment groups (p > 0.05).
During days 1–45 of silage fermentation, the WSC content in each treatment group showed a significant decreasing trend (p < 0.05). In the first 15 days of silage fermentation, with an increase in the silage fermentation time, the WSC content in each treatment group decreased rapidly and began to stabilize after 15 days of fermentation. During the entire fermentation process, the WSC content of the xg and LP + xg treatment groups decreased to a lesser extent. At a later stage of fermentation, the WSC content of the xg treatment group was significantly higher than that of the LP + xg (p < 0.05) and LP groups (p < 0.05). The WSC content in the treatment groups was significantly higher than that in the control group (p < 0.05)
During days 1–45 of silage fermentation, with an increase in the fermentation time, the NDF content in each treatment group showed a significant decreasing trend (p < 0.05). On the 45th day of fermentation, the NDF levels in each treatment group were the lowest. Of these, the NDF content of the xg treatment group was the lowest and was significantly lower than that of the three other treatment groups (p < 0.05).
During the silage fermentation process, for 1–15 days, the ADF content of each treatment group showed a significant decreasing trend (p < 0.05) with an increase in the fermentation time. During the entire fermentation process, the ADF content of the xg and LP + xg treatment groups was lower than that of the control group and the LP treatment group. By the 45th day of silage fermentation, the ADF content in the xg treatment group was significantly lower than that in the three other treatment groups (p < 0.05).
Table 6 shows the effects of the additives on the nutrient quality dynamics of
Stylosanthes silage with low moisture contents. The DM content of
Stylosanthes silage in each treatment group gradually increased with an increase in the silage fermentation time. In the early stage of silage fermentation, the difference in the DM content between the treatment groups was not significant (
p > 0.05). At day 45 of silage fermentation, the DM content in the LP, xg, and LP + xg additive treatment groups was significantly lower than that of the control group (
p < 0.05).
The CP content in Stylosanthes silage exhibited a relatively stable changing trend during the entire fermentation process, and no significant differences were observed in the changes (p > 0.05). At the same silage fermentation time, there was no significant difference in the CP content among the treatment groups (p > 0.05).
During the process of silage fermentation for 1–15 days, it was observed that the WSC content of each treatment group decreased rapidly with an increase in the silage fermentation time, and began to stabilize after 15 days of fermentation. By the 45th day of fermentation, the WSC content of the xg treatment group was significantly higher than that of the other three groups (p < 0.05).
During the process of silage fermentation for 1–45 days, it was observed that the change in the NDF content in each treatment group showed a significant decreasing trend (p < 0.05) with an increase in the fermentation time. By the 45th day of fermentation, the NDF content was the lowest, and was significantly lower than that of the control group and the LP treatment group.
With an increase in the silage fermentation time, the trend of changes observed for the ADF content in the LP treatment group and control group was relatively stable, while those in the xg and LP + xg treatment groups decreased significantly (p < 0.05). By the 45th day of fermentation, the ADF content in the LP + xg treatment group was the lowest, and was significantly lower than that in the LP treatment group and control group (p < 0.05).
The effects of different factors and the interactions between them on the chemical compositions of
Stylosanthes silage are shown in
Table 7. Moisture content had a very significant effect on the DM, CP, WSC, NDF, and ADF (
p < 0.01) content. Treatment had a very significant effect on the DM, WSC, NDF, and ADF (
p < 0.01) content, but had no significant effect on the CP content (
p > 0.05). Ensiling time had a very significant effect on the DM, WSC, NDF, and ADF (
p < 0.01) content, and this had a significant effect on the CP content (
p < 0.05). The interaction between moisture content and treatment as well as the interaction between moisture content and ensiling time had a very significant effect on the DM, CP, WSC, NDF, and ADF (
p < 0.01) content. The interaction between treatment and ensiling time had a very significant effect on the CP, WSC, NDF, and ADF (
p < 0.01) content, and a significant effect on the DM content (
p < 0.05). The interactions among these three processing factors had a very significant effect on the DM, CP, WSC, NDF, and ADF (
p < 0.01) content.
3.3. Effects of Moisture Content and Additives on the In Vitro Digestibility of Stylosanthes Silage
It can be seen from
Table 8 that the effect of moisture content on the in vitro DM digestibility of
Stylosanthes silage for 48 h was not significant (
p > 0.05). The effect of additives on the in vitro DM digestibility of
Stylosanthes silage for 48 h was extremely significant (
p < 0.01). The interaction between the moisture content and additives resulted in no significant differences in the 48 h in vitro DM digestibility of
Stylosanthes silage (
p > 0.05).
Among the four treatment groups, the 48 h in vitro DM digestibility of xg Stylosanthes silage was the highest, i.e., 67.59% and 68.57%; these values were significantly higher than those of the other three treatment groups (p < 0.05). The second-highest value was observed for the LP + xg treatment group, and was significantly higher than that observed with LP (p < 0.05). The values for the three additive treatment groups were significantly higher than that for the control group (p < 0.05).
It can be seen from
Table 9 that the effect of moisture content on the 48 h in vitro digestibility of neutral detergent fiber of
Stylosanthes silage was not significant (
p > 0.05). The effect of additives on the 48 h in vitro neutral detergent fiber digestibility of
Stylosanthes silage was extremely significant (
p < 0.01). The interaction between the moisture content and additives resulted in no significant differences in the 48 h in vitro neutral detergent fiber digestibility of
Stylosanthes silage (
p > 0.05).
Among the four treatment groups, the 48 h in vitro neutral detergent fiber digestibility of xg Stylosanthes silage was the highest, i.e., 38.31% and 38.93%. These values were significantly higher than those observed for the three other treatment groups (p < 0.05). The second-highest values were observed for the LP + xg treatment group, and were significantly higher than that observed for LP (p < 0.05). The 48 h in vitro neutral detergent fiber digestibility of the control group was the lowest, i.e., 27.01% and 27.49%.
3.4. Effects of Moisture Content and Additives on the In Vitro Gas Production by Stylosanthes Silage
It can be seen from
Table 10 that additive treatment can significantly increase the in vitro gas production by
Stylosanthes silage (
p < 0.01). The effect of the addition of xg was the most obvious, and the addition of LP + xg resulted in the second-most notable effect. The effect of moisture content on the in vitro gas production of
Stylosanthes silage was not significant (
p > 0.05). The interaction of moisture content and additives resulted in no significant differences in the in vitro gas production by
Stylosanthes silage (
p > 0.05).
The effects of additives on in vitro gas production in the rumen of high-moisture content
Stylosanthes silage are shown in
Figure 1. In the initial stage, the additives did not have a significant effect on in vitro gas production by
Stylosanthes silage (
p > 0.05). At 4 h and 6 h, the in vitro gas production by the xg treatment group was significantly higher than that of the three other groups (
p < 0.05). There was no significant difference in the in vitro gas production between the LP treatment group and the control group (
p > 0.05). By 36 h, the in vitro gas production had gradually stabilized in
Stylosanthes silage. The in vitro gas production in each group was the highest at 48 h. The xg treatment group exhibited the highest in vitro gas production, followed by the LP + xg treatment group and the LP treatment group. The in vitro gas production in the three treatment groups was significantly higher than that of the control group (
p <0.05).
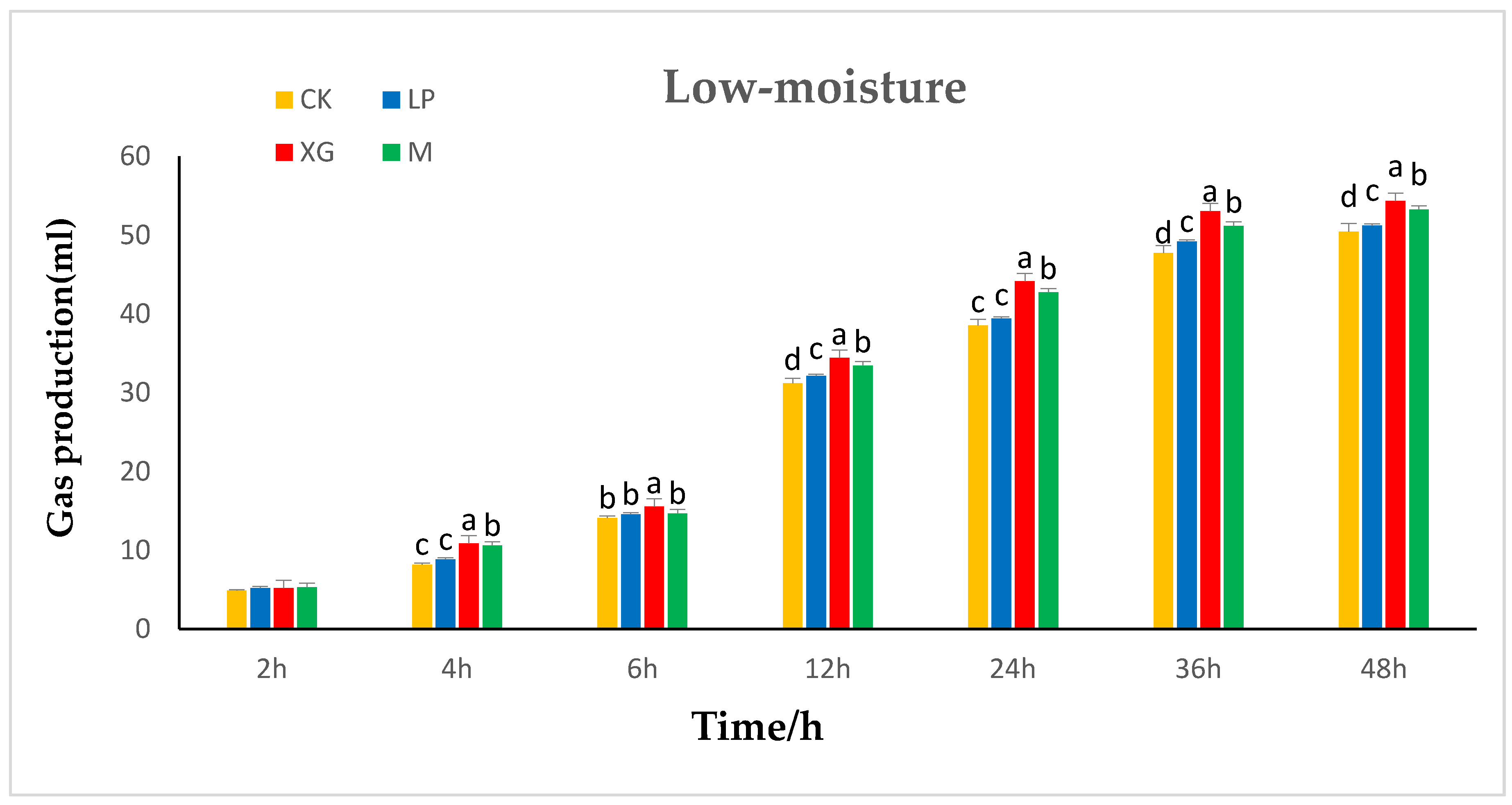
The effects of additives on in vitro gas production in the rumen of low-moisture content
Stylosanthes silage are shown in
Figure 2. At 2 h, the additives did not have a significant effect on in vitro gas production by
Stylosanthes silage (
p > 0.05). From 4 h to 12 h, the in vitro gas production in the xg and LP + xg treatment groups was significantly higher than that in the LP treatment group and control group (
p < 0.05). There was no significant difference in in vitro gas production between the LP treatment group and the control group (
p > 0.05). From 24 h to 48 h, the in vitro gas production in the xg treatment group was the highest, followed by the LP + xg treatment group and the LP treatment group. The in vitro gas production in the three treatment groups was significantly higher than that of the control group (
p < 0.05). From 36 h to 48 h, there was hardly any difference in the in vitro gas production among the groups, and it gradually stabilized and reached the highest value.