fHER2, PR, ER, Ki-67 and Cytokeratin 5/6 Expression in Benign Feline Mammary Lesions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Population
2.2. Mammary Tissue Collection and Histopathological Classification
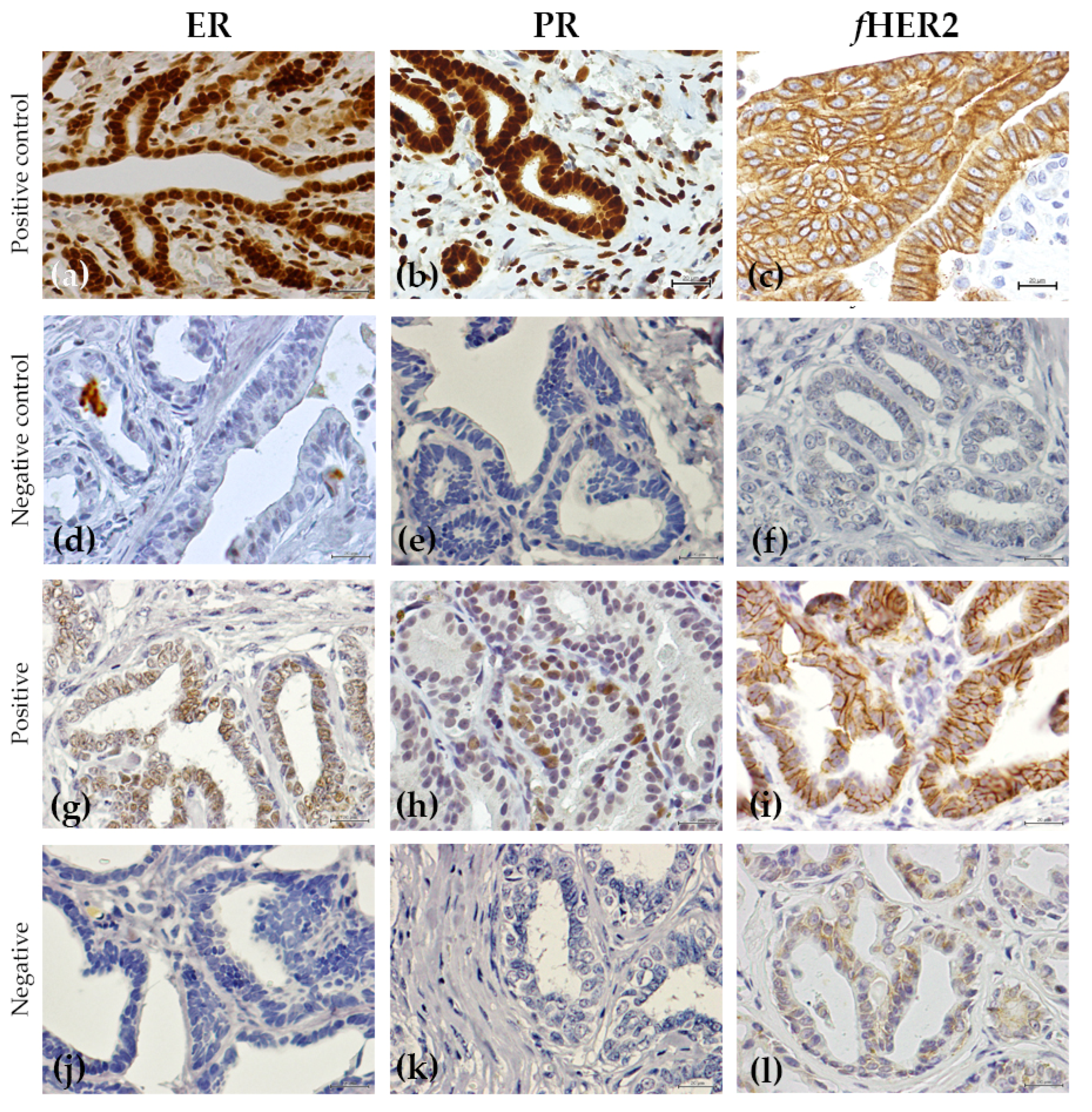
2.3. Immunohistochemistry Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinicopathological Features
3.2. Immunohistochemical Analysis
3.3. Reproductive Status and Hormone Exposition and Expression of the Hormone Receptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, M.W. Preneoplastic and precancerous lesions in rodents: Morphologic and molecular characteristics. J. Toxicol. Pathol. 2002, 15, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Gullino, P.M. Considerations of the preneoplastic lesions of the mammary gland. Am. J. Pathol. 1977, 89, 413–430. [Google Scholar] [PubMed]
- Lakhani, S.R. The transition from hyperplasia to invasive carcinoma of the breast. J. Pathol. 1999, 187, 272–278. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Ma, Y.; Zhang, G.J.; Liao, R.; Jiang, X.F.; Yan, X.X.; Bie, F.J.; Li, X.B.; Lv, Y.H. Eugenol alleviated breast precancerous lesions through HER2/PI3K-AKT pathway-induced cell apoptosis and S-phase arrest. Oncotarget 2017, 8, 56296–56310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K.U.; Worley, D.R.; Goldschmidt, M.H. Tumors of the mammary gland. In Withrow, MacEwen’s Editors. Small Animal Clinical Oncology, 5th ed.; Saunders Elsevier: Amsterdam, The Netherlands, 2013; pp. 538–556. [Google Scholar]
- Manuali, E.; Forte, C.; Vichi, G.; Genovese, D.A.; Mancini, D.; Pia de Leo, A.A.; Cavicchioli, L.; Pierucci, P.; Zappulli, V. Tumours in European shorthair cats: A retrospective study of 680 cases. J. Feline Med. Surg. 2020, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Marques, C.; Catarino, J.; Batista, M.R.; Catita, J.; Faísca, P. 2019 Cancer registry of feline tumours in Portugal. J. Feline Med. Surg. 2021, 23, 853. [Google Scholar]
- Egenvall, A.; Nødtvedt, A.; Häggström, J.; Ström Holst, B.; Möller, L.; Bonnet, B.N. Mortality of life-insured Swedish cats during 1999–2006: Age, breed, sex, and diagnosis. J. Vet. Inter. Med. 2009, 23, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Correia, J.; Peleteiro, C.; Ferreira, F. St Gallen molecular subtypes in feline mammary carcinoma and paired metastases—disease progression and clinical implications from a 3-year follow up study. Tumour Biol. 2016, 37, 4053–4064. [Google Scholar] [CrossRef]
- De Maria, R.; Olivero, M.; Iussich, S.; Nakaichi, M.; Murata, T.; Biolatti, B.; Di Renzo, M.F.; Flavia, M.; Renzo, D.; Di Renzo, M.F. Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer. Cancer Res. 2005, 65, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Bhardwaj, A.; Embury, M.D.; Singh, H.; Gunaratne, P.H.; Bedrosian, I.; Wang, J. Integrative analyses of multilevel omics reveal preneoplastic breast to possess a molecular landscape that is globally shared with invasive Basal-like breast cancer (Running title: Molecular landscape of Basal-like breast cancer progression). Cancers 2020, 12, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danforth, D.N. Genomic changes in normal breast tissue in women at normal risk or at high risk for Breast Cancer. Breast Cancer Basic Clin. Res. 2016, 10, 109–146. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Perle, M.A.; Inghirami, G.; Chan, W.; Delgado, Y.; Feiner, H. Amplification of Her-2/neu gene in Her-2/neu-overexpressing and nonexpressing breast carcinomas and their synchronous benign premalignant, and metastatic lesions detected by FISH in archival material. Mod. Pathol. 2002, 15, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feriancová, M.; Walter, I.; Singer, C.F.; Gazdarica, J.; Pohlodek, K. Expression of COX-2, p16, and Ki67 in the range from normal breast tissue to breast cancer. Neoplasm 2021, 68, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.A. Premalignant breast lesions: Role for biological markers in predicting progression to cancer. Eur. J. Cancer 1999, 35, 693–697. [Google Scholar] [CrossRef]
- Liang, J.; Shang, Y. Estrogen and Cancer. Annu. Rev. Physiol. 2013, 75, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Millanta, F.; Calandrella, M.; Bari, G.; Niccolini, M.; Vannozzi, I.; Poli, A. Comparison of steroid receptor expression in normal, dysplastic, and neoplastic canine and feline mammary tissues. Res. Vet. Sci. 2005, 79, 225–232. [Google Scholar] [CrossRef]
- Henderson, B.E.; Ross, R.; Bernstein, L. Estrogen as a cause of human cancer: The Richard and Hindau Rosenthal Foundation Award Lecture. Cancer Res. 1988, 48, 246–253. [Google Scholar] [PubMed]
- Lamb, C.A.; Fabris, V.T.; Lanari, C. Progesterone and breast. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Sneige, N. Molecular and biologic markers of premalignant lesions of Human Breast. Adv. Anat. Pathol. 2002, 9, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Joana Martin de las Mulas, J.; van Niel, M.; Millan, Y.; Blankenstein, M.A.; van Mil, F.; Misdorp, W. Immunohistochemical analysis of estrogen receptors in feline mammary gland benign and malignant lesions: Comparison with biochemical assay. Domest. Anim. Endocrinol. 2000, 18, 111–125. [Google Scholar] [CrossRef]
- Caliari, D.; Zappulli, V.; Rasotto, R.; Cardazzo, B.; Frassineti, F.; Goldschmidt, M.H.; Castagnaro, M. Triple-negative vimentin-positive heterogeneous feline mammary carcinomas as a potential comparative model for breast cancer. BMC Vet. Res. 2014, 10, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joana Martin de las Mulas, J.; van Niel, M.; Millán, Y.; Ordás, J.; Blankenstein, M.A.; Van Mil, F.; Misdorp, W. Progesterone receptors in normal, dysplastic and tumourous feline mammary glands. Comparison with oestrogen receptors status. Res. Vet. Sci. 2002, 72, 153–161. [Google Scholar] [CrossRef]
- Cesca, M.G.; Vian, L.; Cristóvão-Ferreira, S.; Pondé, N.; de Azambuja, E. HER2- positive Advanced Breast Cancer Treatment in 2020. Cancer Treat. Rev. Cancer Treat. Rev. 2020, 88, 102033. [Google Scholar] [CrossRef]
- Krishnamurti, U.; Silverman, J.F. HER2 in breast cancer: A review and update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Tsuda, H.; Hirohashi, S. Multiple developmental pathways of highly aggressive breast cancers disclosed by comparison of histological grades and c-erbB-2 expression patterns in both the non-invasive and invasive portions. Pathol. Int. 1998, 48, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Correia, J.; Rodrigues, P.; Simões, M.; de Matos, A.; Ferreira, F. Feline HER2 protein expression levels and gene status in Feline mammary carcinoma: Optimization of immunohistochemistry (IHC) and In Situ Hybridization (ISH) techniques. Microsc. Microanal. 2003, 19, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Soares, M.; Correia, J.; Adega, F.; Ferreira, F.; Chaves, R. Assessment of ERBB2 and TOP2α gene status and expression profile in feline mammary tumors: Findings and guidelines. Aging 2019, 11, 4688–4705. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Ribeiro, R.; Najmudin, S.; Gameiro, A.; Rodrigues, R.; Cardoso, F.; Ferreira, F. Serum HER2 levels are increased in cats with mammary carcinomas and predict tissue HER2 status. Oncotarget 2016, 7, 17314–17326. [Google Scholar] [CrossRef] [Green Version]
- Gameiro, A.; Nascimento, C.; Correia, J.; Fernando, F. HER2-targeted immunotherapy and combined protocols showed promising antiproliferative effects in Feline mammary carcinoma cell-based models. Cancers 2021, 13, 2007. [Google Scholar] [CrossRef]
- Ordás, J.; Millán, Y.; Dios, R.; Reymundo, C.; de las Mulas, J.M. Proto-oncogene HER-2 in normal, dysplastic and tumorous feline mammary glands: An immunohistochemical and chromogenic in situ hybridization study. BMC Cancer 2007, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Loh, S.F.; Cooper, C.; Selinger, C.I.; Barnes, E.H.; Chan, C.; Carmalt, H.; West, R.; Gluch, L.; Beith, J.M.; Caldon, C.E.; et al. Cell cycle marker expression in benign and malignant intraductal papillary lesions of the breast. J. Clin. Pathol. 2015, 68, 187–191. [Google Scholar] [CrossRef]
- Vougiouklakis, T.; Belovarac, B.J.; Lytle, A.; Chiriboga, L.; Ozerdem, U. The diagnostic utility of EZH2 H-score and Ki-67 index in non-invasive breast apocrine lesions. Pathol. Res. Pract. 2020, 216, 153041. [Google Scholar] [CrossRef] [PubMed]
- Rachna; Rai, M.K. The Er/Ki-67 proportion in breast tumours—An immunohistochemical study. J. Clin. Diagn. Res. 2016, 10, EC06–EC09. [Google Scholar]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; Kreipe, H.H.; et al. Assessment of Ki67 in Breast Cancer: Update recommendations from the international Ki67 in breast cancer working group. JNCI J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Ribeiro, R.; Carvalho, S.; Peleteiro, M.; Correia, J.; Ferreira, F. Ki-67 as a Prognostic factor in feline mammary carcinoma: What is the optimal cutoff value? Vet. Pathol. 2016, 53, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Alshareeda, A.T.; Soria, D.; Garibaldi, J.M.; Rakha, E.; Nolan, C.; Ellis, I.O.; Green, A.R. Characteristics of basal cytokeratin expression in breast cancer. Breast Cancer Res. Treat. 2013, 139, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, J.A.; Rogers, L.W.; Kyshtoobayeva, A.; Bloom, K. Fixation Time Does Not Affect the Expression of Estrogen Receptor. Am. J. Clin. Pathol. 2010, 133, 747–755. [Google Scholar] [CrossRef]
- Ibarra, J.A.; Rogers, L.W. Fixation Time Does Not Affect the Expression of HER2/neu A Pilot Study. Am. J. Clin. Pathol. 2010, 134, 594–596. [Google Scholar] [CrossRef]
- Sato, M.; Kojima, M.; Nagatsuma, A.K.; Nakamura, Y.; Saito, N.; Ochiai, A. Optimal fixation for total preanalytic phase evaluation in pathology laboratories. A comprehensive study including immunohistochemistry, DNA, and mRNA assays. Pathol. Int. 2014, 64, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Serrugs, J.L.; Kiupel, M. Mammary tumors. In Surgical Pathology of Tumors of Domestic Animals; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Washington, DC, USA, 2019; Volume 2, pp. 197–232. [Google Scholar]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.L.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American pathologists Clinical Practice guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [Green Version]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone receptor testing in Breast Cancer: ASCO/CAP Guideline update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef]
- Brunetti, B.; Asproni, P.; Beha, G.; Muscatello, L.V.; Millanta, F.; Poli, A.; Benazzi, C.; Sarli, G. Molecular phenotype in mammary tumours of queens: Correlation between primary tumour and lymph node metastasis. J. Comp. Pathol. 2013, 148, 206–213. [Google Scholar] [CrossRef]
- Millanta, F.; Calandrella, M.; Vannozzi, I.; Poli, A. Steroid hormone receptors in normal, dysplastic and neoplastic feline mammary tissues and their prognostic significance. Vet. Rec. 2006, 158, 821–824. [Google Scholar] [CrossRef]
- Lopez-Garcia, M.A.; Geyer, F.C.; Lacroix-Triki, M.; Marchió, C.; Reis-Filho, J.S. Breast cancer precursors revisited: Molecular features and progression pathways. Histopathology 2010, 57, 171–192. [Google Scholar] [CrossRef]
- Costa, A.; Zanini, V. Precancerous lesions of the breast. Nat. Clin. Pract. Oncol. 2008, 5, 700–704. [Google Scholar] [CrossRef]
- Lange, C.A.; Yee, D. Progesterone and breast cancer. Women’s Health 2008, 4, 151–162. [Google Scholar] [CrossRef] [Green Version]
- Rasotto, R.; Caliari, D.; Castagnaro, M.; Zanetti, R.; Zappulli, V. An immunohistochemical study of HER-2 expression in Feline Mammary Tumours. J. Comp. Path. 2011, 144, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhou, B.; Meng, X.; Zhu, W.; Zuo, A.; Wang, X.; Jiang, R.; Yu, S. A model o spontaneous nouse mammary tumor for human estrogen receptor- and progesterone receptor-negative breast cancer. Int. J. Oncol. 2014, 45, 2241–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D.; Nico, B.; Ruggieri, S.; Tamma, R.; Simone, G.; Mangia, A. Angiogenesis and antiangiogenesis in Triple-negative breast cancer. Transl. Oncol. 2016, 9, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, C.; Gameiro, A.; Ferreira, J.; Correia, J.; Ferreira, F. Diagnostic value of VEGF-A, VEGFR-1 and VEGFR-2 in feline mammary carcinoma. Cancers 2021, 13, 117. [Google Scholar] [CrossRef]
- Zhou, C.-J.; Zhang, Q.-H.; Zhang, T.-G.; Sun, S.-Z.; Li, H.; Wang, Y.; Liu, Z.-Y. Expression of ER, Ki-67 and cyclin D1 in the pre-cancerous breast of Chinese patients. Pathol. Oncol. Res. 2009, 15, 153–158. [Google Scholar] [CrossRef]
- Nofech-Mozes, S.; Holloway, C.; Hanna, W. The role of cytokeratin 5/6 as an adjunct diagnostic tool in breast core needle biopsies. Int. J. Surg. Pathol. 2008, 16, 399–406. [Google Scholar] [CrossRef]

| HER2 Score | ||
| 0 | No staining or membrane staining that is incomplete, weak and in ≤10% of lesion cells. | |
| 1+ | Incomplete membrane staining that is weak in >10% of lesion cells. | |
| 2+ | Weak to moderate complete membrane staining observed in >10% of lesion cells. | |
| 3+ | Circumferential membrane staining that is complete, intense and in >10% of lesion cells. | |
| ER/PR Score | ||
| Negative | Nuclear staining in <1% of lesions cells. | |
| Positive | Nuclear staining in ≥1% of lesions cells. | |
| Score for the percentage of positive lesion cells | |
| 0 | No staining. |
| 1 | <1% of nuclear staining. |
| 2 | 1–10% of nuclear staining. |
| 3 | 10–33% of nuclear staining. |
| 4 | 33–66% of nuclear staining. |
| 5 | >66% of nuclear staining. |
| Score for average intensity of staining | |
| 0 | None |
| 1 | Weak |
| 2 | Average |
| 3 | Strong |
| Allred score = summation of both scores (0–8) | |
| Features | Number of Animals (%) |
|---|---|
| Breed | |
| European Shorthair | 20 (74.1%) |
| Persian | 4 (14.8%) |
| Siamese | 2 (7.4%) |
| Norwegian Forest Cat | 1 (3.7%) |
| Spayed | |
| Yes | 8 (29.6%) |
| No | 19 (70.4%) |
| Contraceptive use | |
| Yes | 15 (57.7%) |
| No Information not available | 11 (42.3%) 1 |
| Presence of concomitant malignant tumors | |
| Yes | 16 (59.3%) |
| No | 11 (40.7%) |
| Histopathological Group | Histopathological Classification | n (%) | Lesion Size Mean ± SEM (cm) |
|---|---|---|---|
| Mammary hyperplasia and dysplasia | 41 (87.2%) | 1.77 ± 0.37 | |
| Duct ectasia | 23 (48.9%) | 1.44 ± 0.46 a | |
| Fibroadenomatous change | 8 (17%) | 2.79 ± 0.68 b | |
| Epitheliosis | 4 (8.5%) | 0.25 ± 0.9 a | |
| Lobular hyperplasia (adenosis) | 6 (12.7%) | 1.22 ± 0.52 ab | |
| Benign neoplasia | |||
| Simple adenoma | 6 (12.8%) | 1.75 ± 1.08 ab |
| Protein | Total (%) | Benign Non-Neoplastic Lesions (%) | Benign Tumors (%) |
|---|---|---|---|
| ER status | |||
| Positive | 43 (91.5%) | 39 (95.1%) | 4 (66.7%) |
| Negative | 4 (8.5%) | 2 (4.9%) | 2 (33.3%) |
| PR status | |||
| Positive | 17 (36.2%) | 16 (39%) | 1 (16.7%) |
| Negative | 30 (63.8%) | 25 (61%) | 5 (83.3%) |
| fHER2 status | |||
| Positive | 16 (35.6%) | 14 (35.9%) | 2 (33.3%) |
| Negative | 29 (64.4%) | 25 (64.1%) | 4 (66.7%) |
| Undetermined * | 2 | 2 | 0 |
| Ki-67 index | |||
| mean (max-min) | 12.9% (0–52%) | 13.2% (0–52%) | 10.6% (4–29%) |
| High | 10 (21.3%) | 9 (30%) | 1 (16.7%) |
| Low | 37 (78.7%) | 32 (70%) | 5 (83.3%) |
| CK5/6 status | |||
| Positive | 11 (23.4%) | 9 (30%) | 2 (33.3%) |
| Negative | 36 (76.6%) | 32 (70%) | 4 (66.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, M.; Correia, A.N.; Batista, M.R.; Correia, J.; Ferreira, F. fHER2, PR, ER, Ki-67 and Cytokeratin 5/6 Expression in Benign Feline Mammary Lesions. Animals 2022, 12, 1599. https://doi.org/10.3390/ani12131599
Soares M, Correia AN, Batista MR, Correia J, Ferreira F. fHER2, PR, ER, Ki-67 and Cytokeratin 5/6 Expression in Benign Feline Mammary Lesions. Animals. 2022; 12(13):1599. https://doi.org/10.3390/ani12131599
Chicago/Turabian StyleSoares, Maria, Assunção N. Correia, Mariana R. Batista, Jorge Correia, and Fernando Ferreira. 2022. "fHER2, PR, ER, Ki-67 and Cytokeratin 5/6 Expression in Benign Feline Mammary Lesions" Animals 12, no. 13: 1599. https://doi.org/10.3390/ani12131599







