The Digestive System of the Arctocephalus australis in Comparison to the Dog as a Land-Carnivore Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
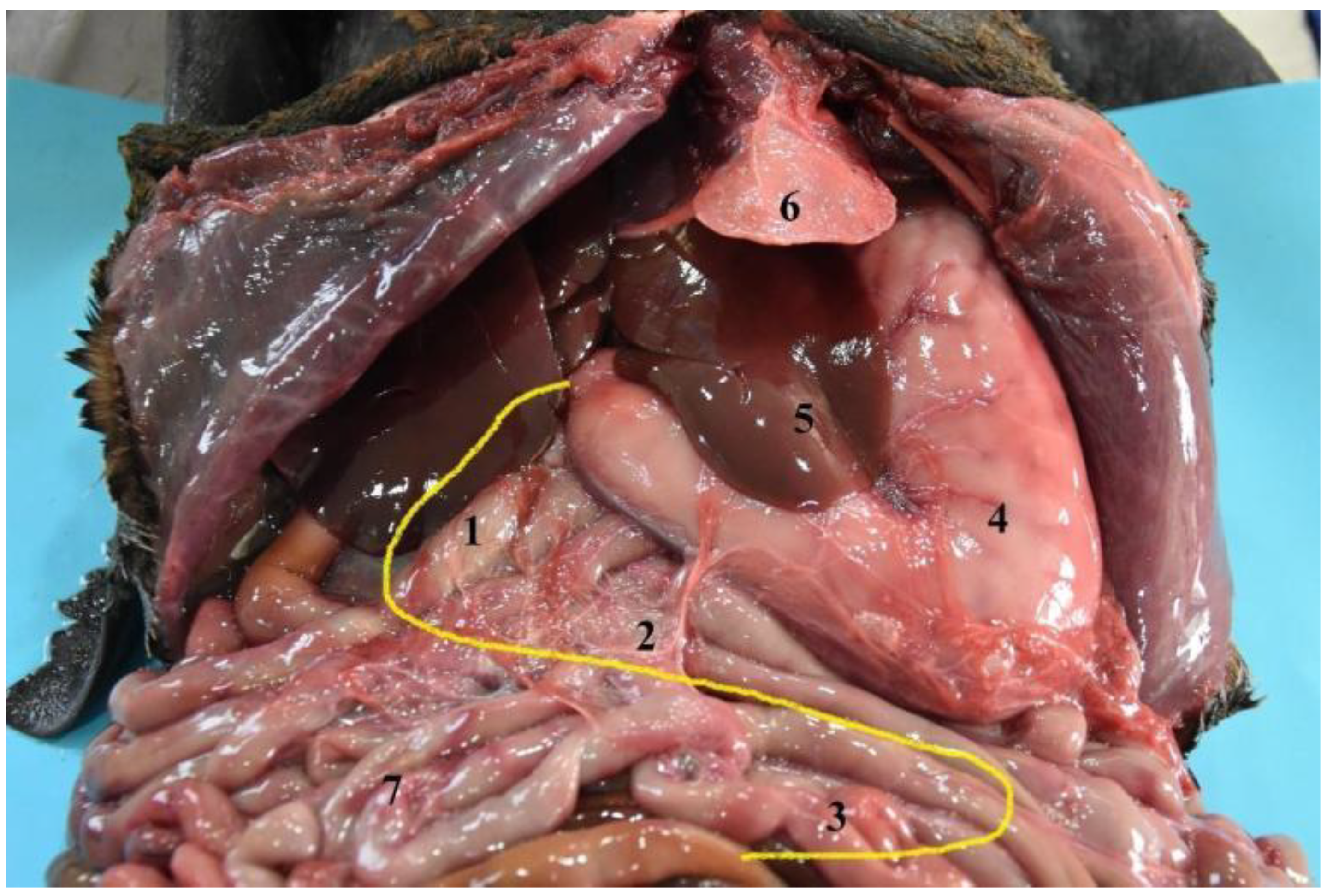
3. Results
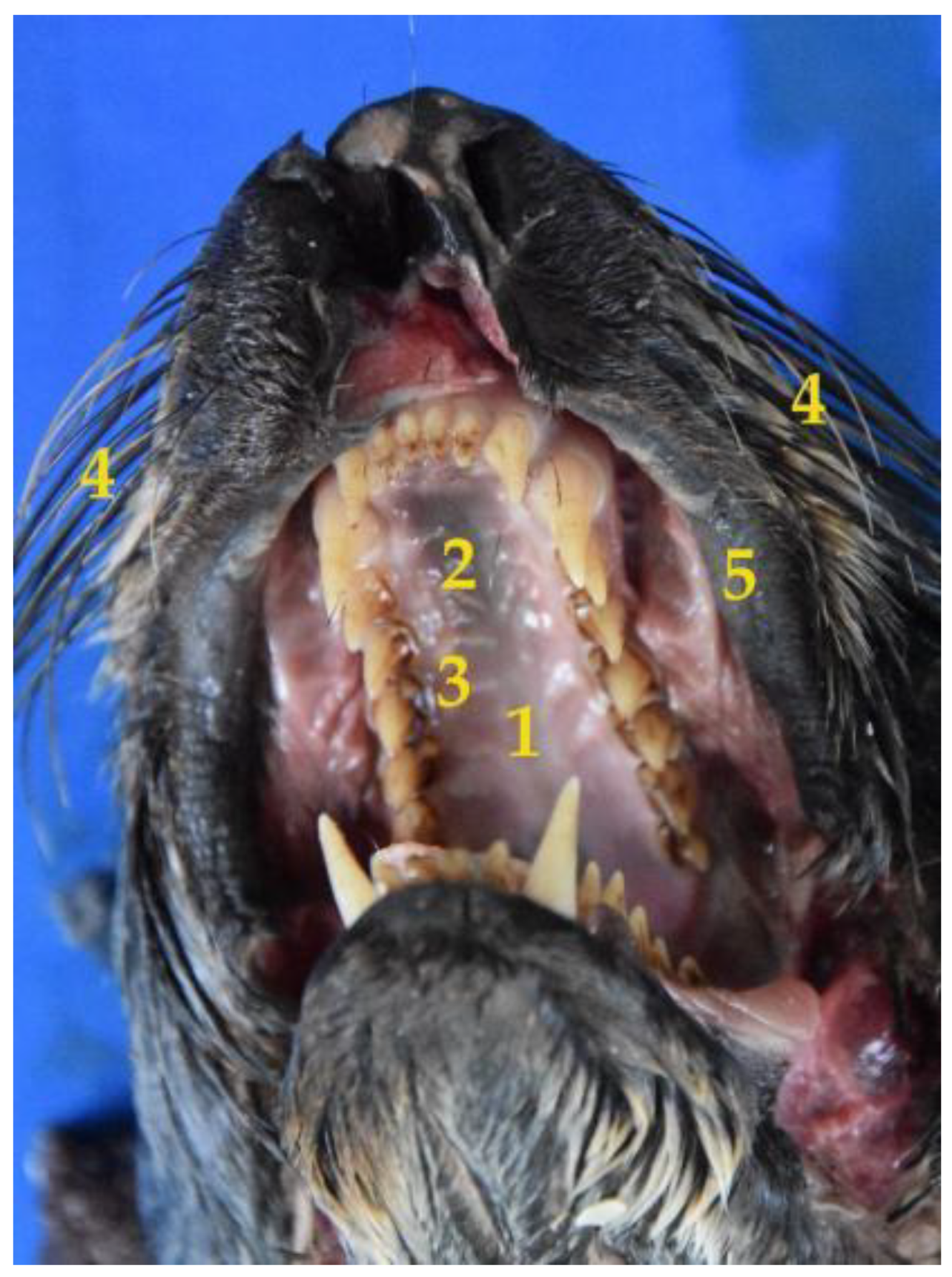
3.1. Oral Cavity
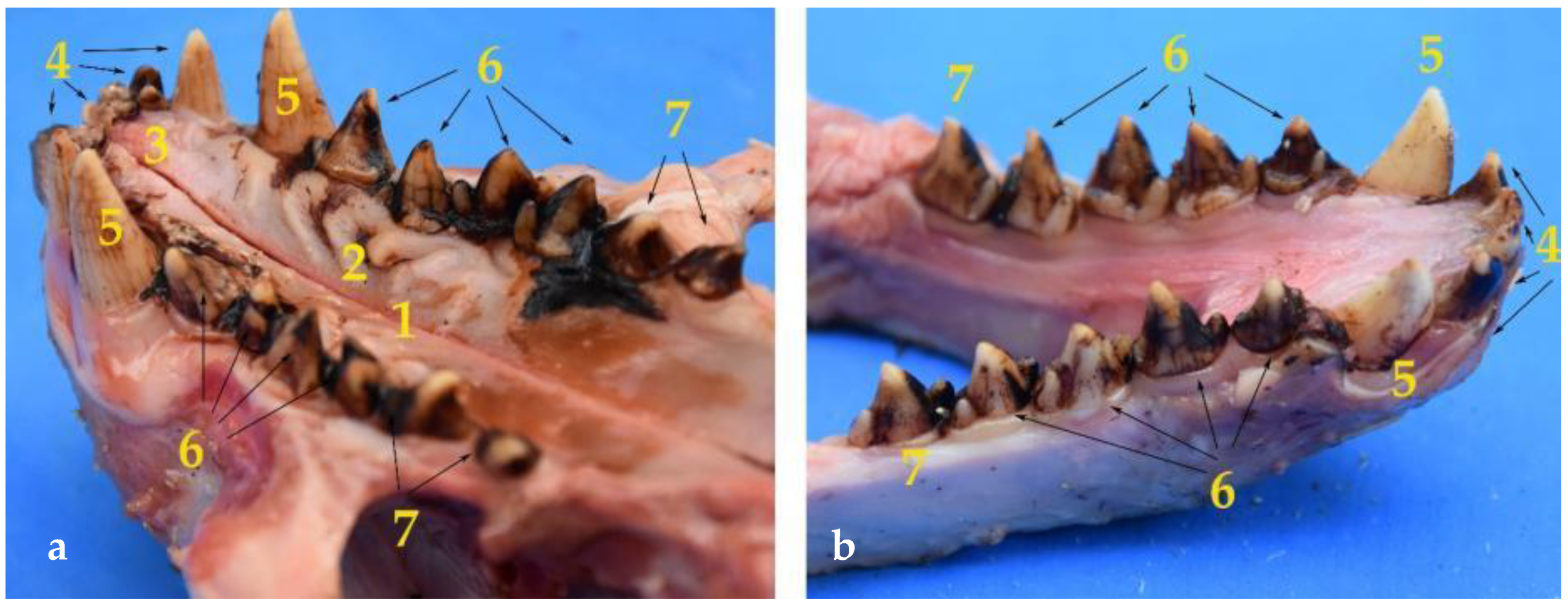
Teeth
3.2. Pharynx and Esophagus
3.3. Stomach
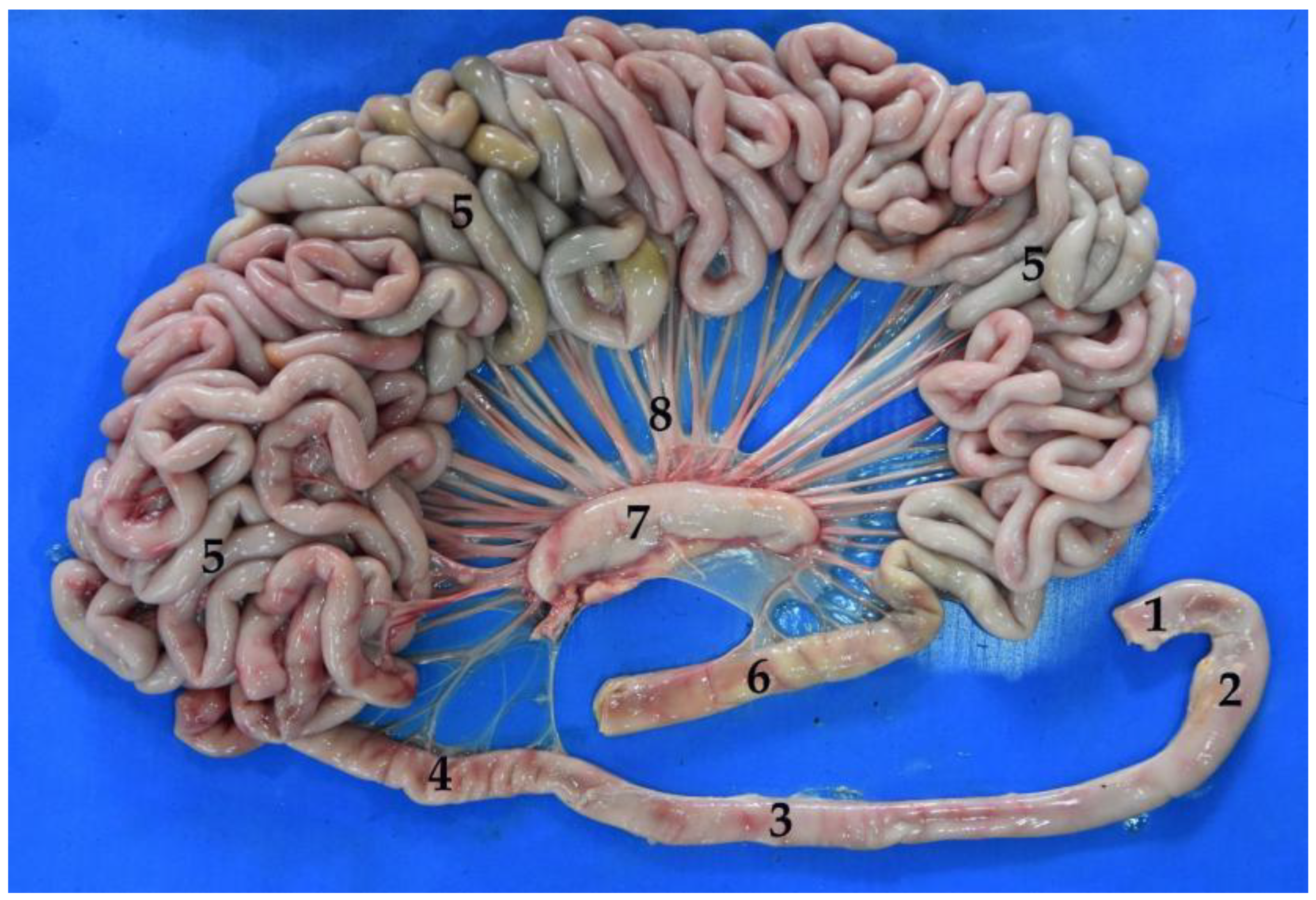
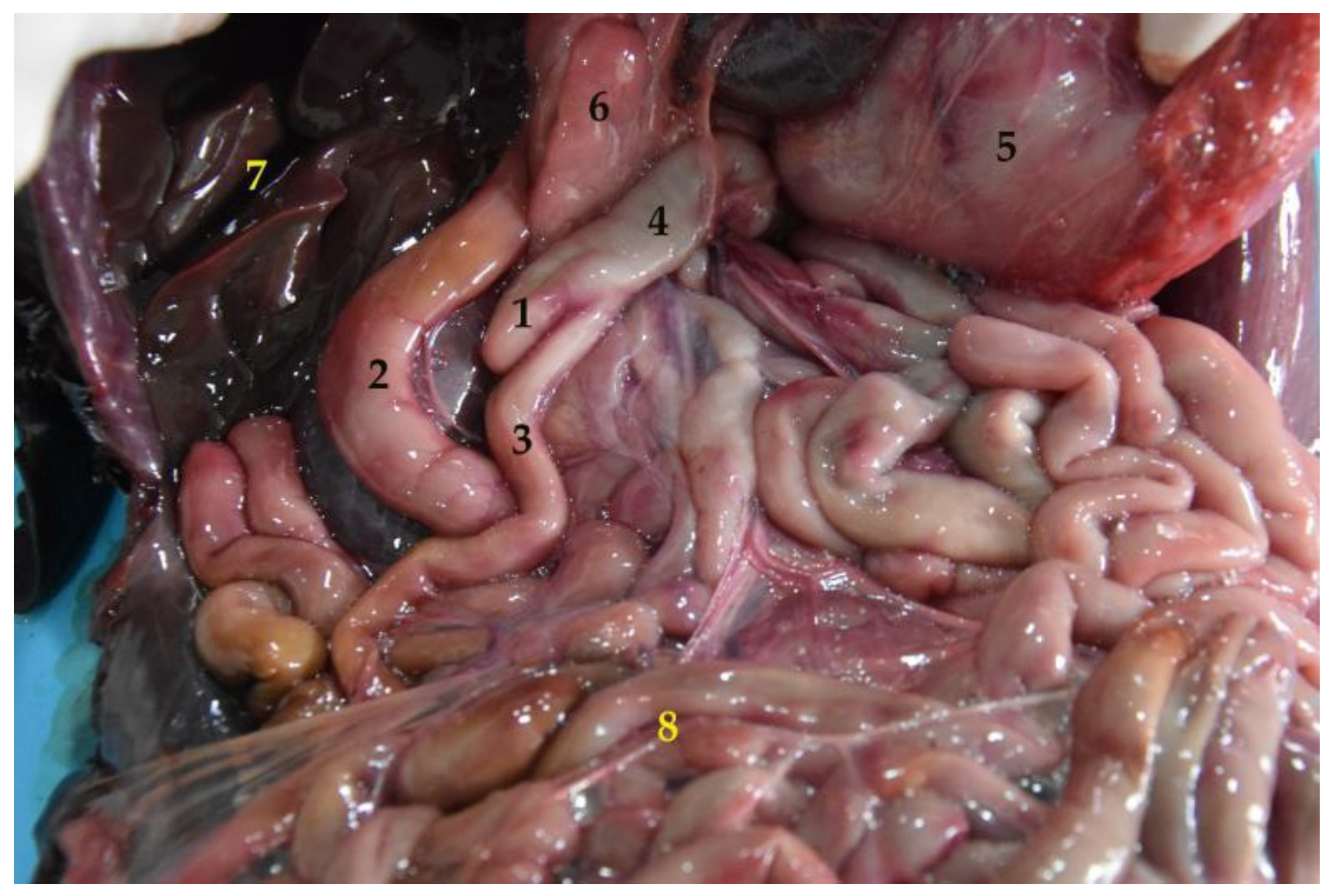
3.4. Small Intestine
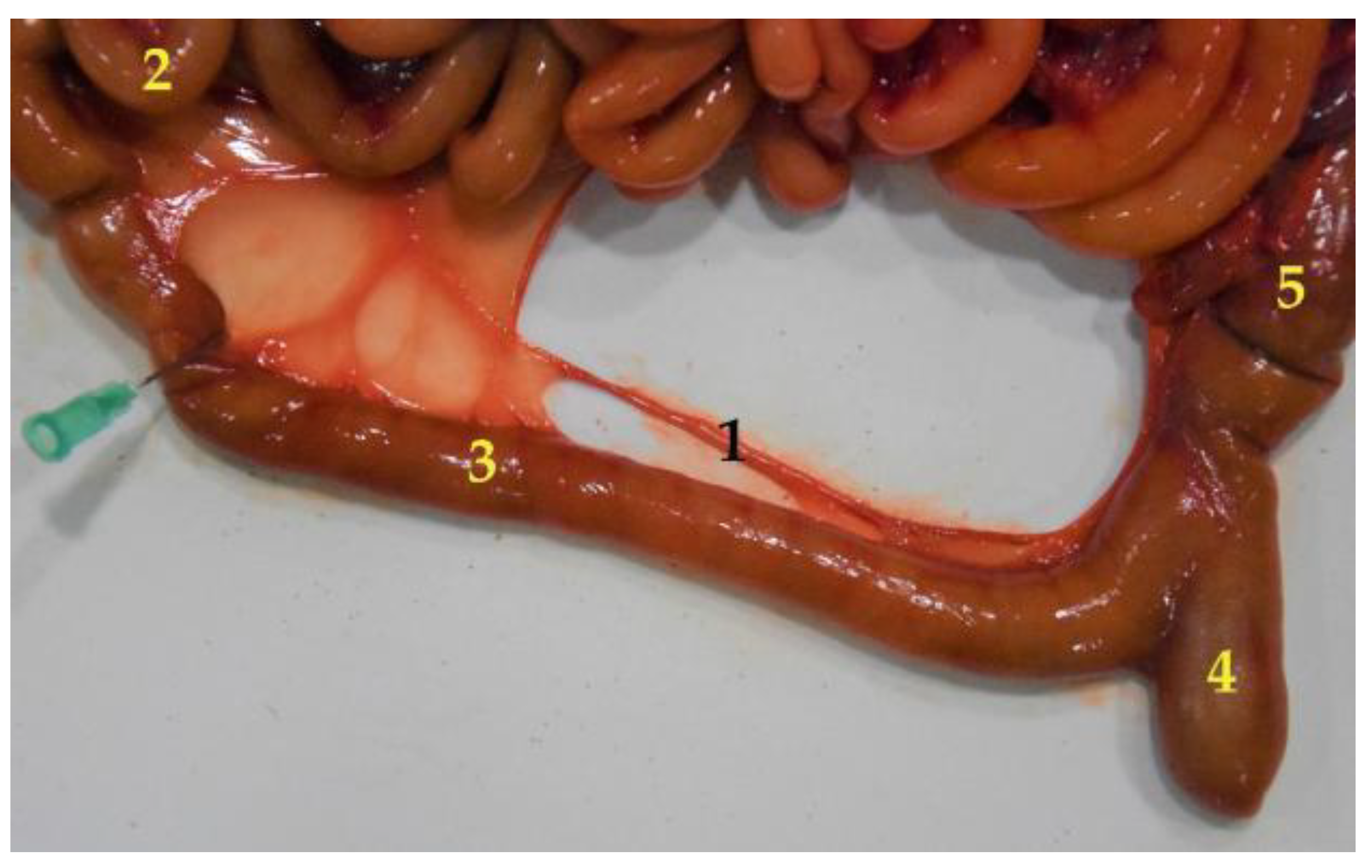
3.4.1. Duodenum and Pancreas
3.4.2. Jejunum
3.4.3. Ileum
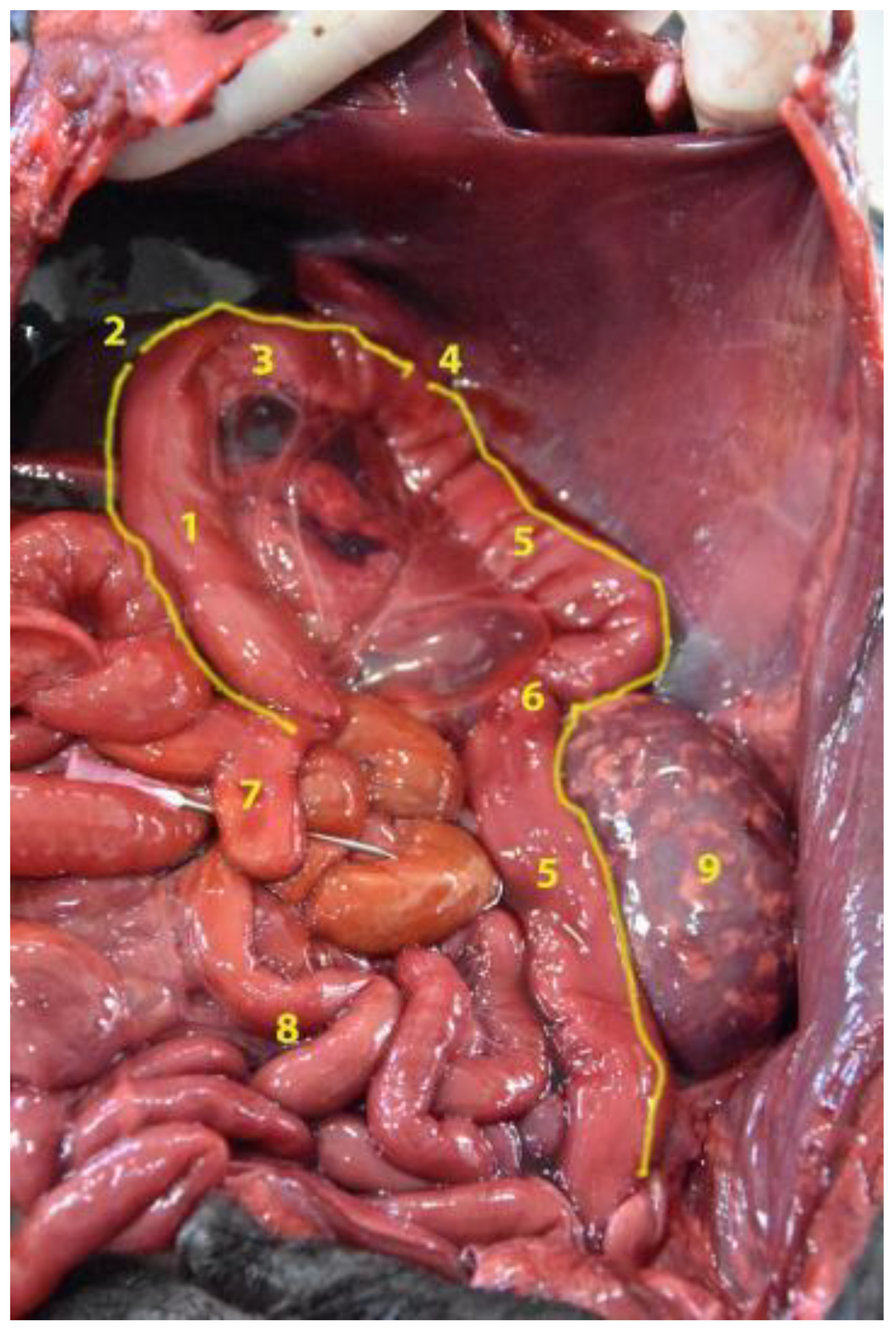
3.5. Large Intestine
3.5.1. Cecum
3.5.2. Colon
3.5.3. Rectum
3.6. Liver
3.7. Gallbladder
3.8. Radiology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, S.A.; Karina, C.; Loureiro, J.D.; Ruoppolo, V. Rescue and Attention of Pinnipeds on the Beach, 1st ed.; Fundación Mundo Marino: San Clemente, Argentina, 2015; pp. 11–46. [Google Scholar]
- English, A.W. Structural correlates of forelimb function in fur seals and sea lions. J. Morphol. 1977, 151, 325–352. [Google Scholar] [CrossRef] [PubMed]
- Noren, S.R.; Williams, T.M.; Pabst, D.A.; McLellan, W.A.; Dearolf, J.L. The development of diving in marine endotherms: Preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. J. Comp. Physiol. B 2001, 171, 127–134. [Google Scholar] [CrossRef]
- Dehnhardt, G.; Mauck, B.; Hanke, W.; Bleckmann, H. Hydrodynamic trail-following in harbour seals (Phoca Vitulina). Science 2001, 293, 102–104. [Google Scholar] [CrossRef]
- Reidenberg, J.S. Anatomical adaptations of aquatic mammals. Anat. Rec. 2007, 290, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.M. Osmoregulation in marine mammals. J. Exp. Biol. 2001, 204, 1831–1844. [Google Scholar] [CrossRef]
- Arbiza, J.; Blanc, A.; Castro-Ramos, M.; Katz, H.; Ponce de León, A.; Clara, M. Uruguayan Pinnipeds (Arctocephalus australis and Otaria flavescens): Evidence of Influenza Virus and Mycobacterium pinnipedii Infections. In New Approaches to the Study of Marine Mammals; Romero, A., Keith, E.O., Eds.; InTech: Rijeka, Croatia, 2012; ISBN 9789535108443. [Google Scholar]
- Churchill, M.; Clementz, M.T.; Kohno, N. Predictive equations for the estimation of body size in seals and sea lions (Carnivora: Pinnipedia). J. Anat. 2014, 225, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.; Pérez, W.; Bielli, A.; Chavez, R. Histomorphology of prepuberal ovaries in the South American fur seal (Arctocephalus australis Zimmerman, 1783). Folia Morphol. 2009, 68, 277–286. [Google Scholar]
- Le Bas, A.E. Renal handling of water, urea and electrolytes in wild South America fur seal (Arctocephalus australis). LAJAM 2003, 2, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Lord, E. Olfactory Discrimination of Aliphatic 2-Ketones and 1-Alcohols in South African fur Seals (Arctocephalus pusillus pusillus). Master’s Thesis, Linköpings Universitet, Linköpings, Sweden, 2009. [Google Scholar]
- Phillips, A.V. Behavioral cues used in reunions between mother and pup south american fur seals (Arctocephalus australis). J. Mammal. 2003, 84, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Seguel, M.; Pavés, H.; Paredes, E.; Schlatter, R. Causes of mortality in South American fur seal pups (Arctophoca australis gracilis) at Guafo Island, southern Chile (2004–2008). Mar. Mam. Sci. 2013, 29, 36–47. [Google Scholar] [CrossRef]
- Silva, R.Z.; Pereira, J., Jr.; Cousin, J.C. Histological patterns of the intestinal attachment of Corynosoma australe (Acanthocephala: Polymorphidae) in Arctocephalus australis (Mammalia: Pinnipedia). J. Parasit. Dis. 2013, 38, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szteren, D.; Naya, D.E.; Arim, M. Overlap between pinniped summer diet and artisanal fishery catches in Uruguay. LAJAM 2004, 3, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Drabeck, C.M. Some anatomical aspects of the cardiovascular system of antarctic seals and their possible functional significance in diving. J. Morphol. 1975, 145, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Pérez, W.; Katz, H.; Lima, M. Gross heart anatomy of Arctocephalus australis (Zimmerman, 1783). Anat. Sci. Int. 2008, 83, 6–10. [Google Scholar] [CrossRef]
- Stewardson, C.L.; Hemsley, S.; Meyer, M.A.; Canfield, P.J.; Maindonald, J.H. Gross and microscopic visceral anatomy of the male Cape fur seal, Arctocephalus pusillus pusillus (Pinnipedia: Otariidae), with reference to organ size and growth. J. Anat. 1999, 195, 235–255. [Google Scholar] [CrossRef]
- Ponganis, P. Diving Physiology of Marine Mammals and Seabirds; Cambridge University Press: Cambridge, UK, 2015; pp. 162–170. [Google Scholar] [CrossRef]
- Pauly, D.; Trites, A.W.; Capuli, E.; Christensen, V. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 1998, 55, 467–481. [Google Scholar] [CrossRef]
- Boyd, I.L.; Arnould, J.P.Y.; Barton, T.; Croxall, J.P. Foraging behaviour of Antarctic fur seals during periods of contrasting prey abundance. J. Anim. Ecol. 1994, 63, 703–713. [Google Scholar] [CrossRef]
- Harvey, J.T. Assesment of errors associated with harbor seal (Phoca vitulina) fecal sampling. J. Zool. 1989, 219, 101–111. [Google Scholar] [CrossRef]
- Reid, K.; Arnould, J.P.Y. The diet of Antarctic fur seals Arctocephalus gazella during the breeding season at South Georgia. Polar Biol. 1996, 16, 105–114. [Google Scholar] [CrossRef]
- Naya, D.; Arim, M.; Vargas, R. Diet of South American fur seals (Arctocephalus australis) in Isla de Lobos, Uruguay. Mar. Mamm. Sci. 2002, 18, 734–745. [Google Scholar] [CrossRef]
- Climent, S.; Sarasa, M.; Muniesa, P.; Latorre, R. Manual of Anatomy and Embryology of Domestic Animals; Acribia: Zaragoza, Spain, 2005; pp. 73–249. [Google Scholar]
- Getty, R.; Sisson, S.; Grossman, J.D. Anatomy of Domestic Animals, 5th ed.; Salvat Editores: Barcelona, Spain, 1982; Volume 2, pp. 1688–1709. [Google Scholar]
- Lavigne, D.M.; Innes, S.; Worthy, G.A.J.; Kovacs, K.M.; Schmitz, O.J.; Hickie, J.P. Metabolic rates of seals and whales. Can. J. Zool. 1986, 64, 279–284. [Google Scholar] [CrossRef]
- Gallivan, G.J.; Ronald, K. Temperature regulation in freely diving harp seals (Phoca groenlandica). Can. J. Zool. 1979, 57, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- De Sordi, N.; Bombardi, C.; Chiocchetti, R.; Clavenzani, P.; Trerè, C.; Canova, M.; Grandis, A. A new method of producing casts for anatomical studies. Anat. Sci. Int. 2014, 89, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Villar Arias, S.; Pérez, W. Morphology of the lingual Surface of South American fur seal (Arctocephalus australis) and sea lion (Otaria flavescens). Microsc. Res. Tech. 2015, 78, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Colin, E.H.; Peter, P.E. Small Animal Dentistry, 1st ed.; Mosby-Year Book: Maryland Heights, MI, USA, 1993; pp. 20–28. [Google Scholar]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Anatomía Veterinaria, 2nd ed.; McGraw-Hill Interamericana: Madrid, Spain, 1996; pp. 141–145. [Google Scholar]





















| Age | Length | Diameter (cm) | Weight | ||||
|---|---|---|---|---|---|---|---|
| ID | Group | Sex | (cm) | Base | Body | Tip | (g) |
| M7319 | A | ♀ | 16 | 14 | 12 | 6 | 135 |
| M99 | A | ♀ | 14 | 13 | 12 | 15 | 130 |
| M104 | J | ♂ | 11 | 8.5 | 8 | 4 | 86 |
| M100 | J | ♀ | 9.5 | 7.3 | 6.5 | 3 | 62.5 |
| M74 | J | ♂ | 10 | 8 | 6.5 | 4.5 | 50 |
| M83 | J | ♂ | 10.2 | 8.5 | 8 | 4.5 | 65 |
| M82 | J | ♀ | 9 | 10 | 7.8 | 5 | 58.4 |
| M70 | J | ♂ | 8.7 | 8 | 6 | 3 | 54 |
| M79 | J | ♂ | 10.5 | 9.2 | 7 | 4 | 71 |
| M97 | J | ♀ | 7 | 9 | 7.2 | 5 | 53.7 |
| M84 | J | ♀ | 7.6 | 7.5 | 6 | 4.5 | 46 |
| Mean | A | 15 | 13.5 | 12 | 5.5 | 132.5 | |
| Mean | J | 9.2 | 8.4 | 7 | 4.1 | 60.7 | |
| Age | Length | Diameter | Weight | ||
|---|---|---|---|---|---|
| ID | Group | Sex | (cm) | (cm) | (g) |
| M7319 | A | ♀ | 63 | 4.5 | 210 |
| M99 | A | ♀ | 59 | 4.4 | 175 |
| M104 | J | ♂ | 46 | 3.5 | 75 |
| M100 | J | ♀ | 34 | 2.5 | 45 |
| M74 | J | ♂ | 38 | 2.7 | 52.6 |
| M83 | J | ♂ | 33 | 2.5 | 55 |
| M82 | J | ♀ | 36 | 2.4 | 48 |
| M70 | J | ♂ | 38 | 2.7 | 43 |
| M79 | J | ♂ | 37 | 2.3 | 55 |
| M97 | J | ♀ | 32 | 2 | 39.6 |
| M84 | J | ♀ | 31 | 2.4 | 40 |
| Mean | A | 61 | 4.4 | 192.5 | |
| Mean | J | 36.1 | 2.5 | 50.3 | |
| Age | Length 1 | Curvature (cm) | Diameter (cm) | Weight | ||||
|---|---|---|---|---|---|---|---|---|
| ID | Group | Sex | (cm) | Major | Minor | Left Part | Pyloric Part | (g) |
| M7319 | A | ♀ | 60 (38 + 22) | 68 | 42 | 31 | 17 | 380 |
| M99 | A | ♀ | 47 (29 + 18) | 56 | 41 | 29 | 15 | 330 |
| M104 | J | ♂ | 32 (19 + 13) | 39 | 21 | 22 | 10 | 300 |
| M100 | J | ♀ | 27 (17 + 10) | 31 | 20 | 18.5 | 7.5 | 200 |
| M74 | J | ♂ | 28 (17 + 11) | 35 | 17 | 20.5 | 11 | 164 |
| M83 | J | ♂ | 29 (17 + 12) | 32 | 21 | 17 | 10 | 190 |
| M82 | J | ♀ | 33 (21 + 12) | 42 | 21 | 23.5 | 13 | 285 |
| M70 | J | ♂ | 32 (19 + 13) | 37 | 22 | 17 | 10 | 191 |
| M79 | J | ♂ | 33 (20 + 13) | 40 | 19 | 21 | 11 | 210 |
| M97 | J | ♀ | 37 (23 + 14) | 46 | 24 | 26 | 14 | 186 |
| M84 | J | ♀ | 32 (21 + 11) | 41 | 21 | 19 | 12 | 190 |
| Mean | A | 53.5 | 62 | 41.5 | 30 | 16 | 355 | |
| Mean | J | 31.4 | 38.1 | 20.6 | 20.5 | 10.9 | 212.8 | |
| Age | Length | Diameter (cm) | Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Group | Sex | D (cm) | J (m) | I (cm) | D | J | I | D | J | I |
| M7319 | A | ♀ | 43 | 20 | 16 | 5.5 | 3.7 | 4 | 25 | 1585 | 10 |
| M99 | A | ♀ | 40 | 29 | 14 | 5 | 3.2 | 3.6 | 21 | 2015 | 12 |
| M104 | J | ♂ | 26 | 15 | 10 | 4.1 | 3.4 | 2.9 | 16 | 655 | 6.6 |
| M100 | J | ♀ | 19 | 13 | 15 | 3.5 | 2.4 | 3 | 13 | 410 | 8.1 |
| M74 | J | ♂ | 22 | 12 | 9.5 | 3.3 | 2 | 3.2 | 12.5 | 490 | 6 |
| M83 | J | ♂ | 36 | 13.5 | 6.1 | 4.8 | 2.5 | 2.7 | 15 | 405 | 5 |
| M82 | J | ♀ | 25 | 14.4 | 7 | 4.5 | 2.6 | 3 | 19 | 680 | 4.9 |
| M70 | J | ♂ | 27 | 13.5 | 8 | 4.2 | 2.4 | 2.3 | 12.8 | 525 | 4.3 |
| M79 | J | ♂ | 26 | 14 | 8 | 3.7 | 2.6 | 3.2 | 17 | 615 | 4.7 |
| M97 | J | ♀ | 22 | 12.5 | 9 | 2.3 | 2.2 | 2.4 | 11.5 | 765 | 3.8 |
| M84 | J | ♀ | 25 | 12.5 | 8 | 3.5 | 2.5 | 2.6 | 10 | 405 | 3.8 |
| Mean | A | 41.5 | 24.5 | 15 | 5.2 | 3.4 | 3.8 | 23 | 1800 | 11 | |
| Mean | J | 25.3 | 13.3 | 8.9 | 3.8 | 2.5 | 2.8 | 14 | 550 | 5.2 | |
| Age | Length (cm) | Diameter (cm) | Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Group | Sex | Cecum | Colon | Rectum | Cecum | Colon | Rectum | Cecum | Colon | Rectum |
| M7319 | A | ♀ | 10 | 116 | 10 | 6 | 8 | 6 | 13 | 230 | 20 |
| M99 | A | ♀ | 7 | 113 | 9 | 4 | 7 | 7 | 6 | 235 | 35 |
| M104 | J | ♂ | 1.8 | 59 | 8.5 | 3.5 | 5.5 | 5.5 | 3.1 | 62 | 18 |
| M100 | J | ♀ | 2.2 | 67 | 8 | 4 | 5 | 4.7 | 2.2 | 46 | 11 |
| M74 | J | ♂ | 4.3 | 64 | 8 | 4.1 | 5.2 | 4.5 | 2.2 | 68 | 16.2 |
| M83 | J | ♂ | 3.5 | 70 | 5.5 | 3.2 | 6 | 4 | 1.4 | 55 | 15 |
| M82 | J | ♀ | 4 | 83 | 9 | 4 | 6.5 | 5.5 | 2 | 90 | 14 |
| M70 | J | ♂ | 1.6 | 59 | 8 | 3.2 | 4.7 | 4.6 | 1.3 | 52 | 14.3 |
| M79 | J | ♂ | 3 | 69 | 9 | 4 | 6 | 5 | 1.8 | 80 | 9.3 |
| M97 | J | ♀ | 2.4 | 48 | 8 | 3.3 | 4 | 4.9 | 2.8 | 54 | 11.6 |
| M84 | J | ♀ | 2.6 | 56 | 7 | 3 | 4.5 | 4.5 | 1.5 | 47.5 | 15.8 |
| Mean | A | 8.5 | 114.5 | 9.5 | 5 | 7.5 | 6.5 | 9.5 | 232.5 | 27.5 | |
| Mean | J | 2.8 | 63.8 | 7.8 | 3.5 | 5.2 | 4.8 | 2 | 61.6 | 13.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Orti, R.; Tostado-Marcos, C.; Loureiro, J.-P.; Molpeceres-Diego, I.; Tendillo-Domínguez, E.; Santos-Álvarez, I.; Pérez-Lloret, P.; González-Soriano, J. The Digestive System of the Arctocephalus australis in Comparison to the Dog as a Land-Carnivore Model. Animals 2022, 12, 1634. https://doi.org/10.3390/ani12131634
Martín-Orti R, Tostado-Marcos C, Loureiro J-P, Molpeceres-Diego I, Tendillo-Domínguez E, Santos-Álvarez I, Pérez-Lloret P, González-Soriano J. The Digestive System of the Arctocephalus australis in Comparison to the Dog as a Land-Carnivore Model. Animals. 2022; 12(13):1634. https://doi.org/10.3390/ani12131634
Chicago/Turabian StyleMartín-Orti, Rosario, Carlos Tostado-Marcos, Juan-Pablo Loureiro, Ignacio Molpeceres-Diego, Enrique Tendillo-Domínguez, Inmaculada Santos-Álvarez, Pilar Pérez-Lloret, and Juncal González-Soriano. 2022. "The Digestive System of the Arctocephalus australis in Comparison to the Dog as a Land-Carnivore Model" Animals 12, no. 13: 1634. https://doi.org/10.3390/ani12131634
APA StyleMartín-Orti, R., Tostado-Marcos, C., Loureiro, J.-P., Molpeceres-Diego, I., Tendillo-Domínguez, E., Santos-Álvarez, I., Pérez-Lloret, P., & González-Soriano, J. (2022). The Digestive System of the Arctocephalus australis in Comparison to the Dog as a Land-Carnivore Model. Animals, 12(13), 1634. https://doi.org/10.3390/ani12131634






