Otolith Weight as an Estimator of the Age of Seriola lalandi Valenciennes, 1833 (Carangidae), in the Southeastern Pacific
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
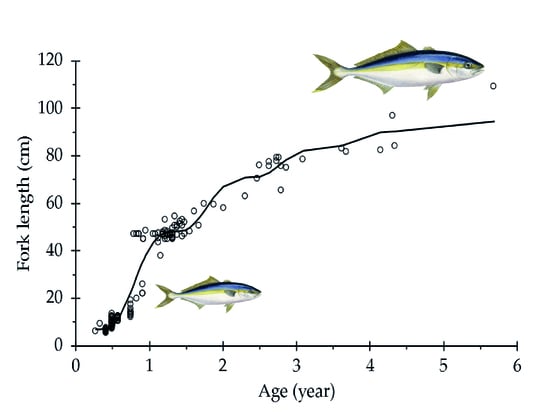
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ihssen, P.E.; Booke, H.E.; Casselman, J.M.; Mcglade, J.M.; Payne, N.R. Stock identification: Materials and methods. Can. J. Fish. Aquat. Sci. 1981, 38, 1838–1855. [Google Scholar] [CrossRef]
- Lombardi-Carlson, L.A.; Andrews, A.H. Age estimation and lead-radium dating of golden tilefish, Lopholatilus chamaeleonticeps. Environ. Biol. Fish. 2015, 98, 1787–1801. [Google Scholar] [CrossRef]
- Hanson, S.D.; Stafford, C.P. Modeling Otolith Weight using Fish Age and Length: Applications to Age Determination. Trans. Am. Fish. Soc. 2017, 146, 778–790. [Google Scholar] [CrossRef]
- Radford, D.S.; Lackmann, A.R.; Moody-Carpender, C.J.; Colombo, R.E. Comparison of Four Hard Structures Including Otoliths for Estimating Age in Blue Suckers. Trans. Am. Fish. Soc. 2021, 150, 514–527. [Google Scholar] [CrossRef]
- Lepak, M.J.; Cathcart, N.C.; Hooten, B.M. Otolith Weight as a Predictor of Age in Kokanee Salmon from Four Colorado Reservoirs. Can. J. Fish. Aquat. Sci. 2012, 69, 1569–1575. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Nazir, A.; Banday, U.Z. Utility of otolith weight to estimate age of Labeo bata (Actinopterygii: Cypriniformes: Cyprinidae) inhabiting the Ganga River. Acta Ichthyol. Piscat. 2018, 48, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.C.; Campana, S.E. Inferring age from otolith measurements: A review and a new approach. Can. J. Fish. Aquat. Sc. 2004, 61, 1269–1284. [Google Scholar] [CrossRef]
- Wilson, C.D.; Boehlert, G.W. The effects of different otolith aging techniques on estimates of growth and mortality for the splitnose rockfish, Sebastes diploproa, and canary rockfish, S. pinniger. Calif. Fish Game 1990, 76, 146–160. [Google Scholar]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Britton, J.R.; Blackburn, R. Application and utility of using otolith weights in the ageing of three flatfish species. Fish. Res. 2014, 154, 147–151. [Google Scholar] [CrossRef]
- D‘Iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famuari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spano, N.; Capillo, G. Otolith analysis higlight morpho-functional differences of three species of Mullet (Mugilidae) from transitional waters. Sustainability 2022, 14, 398. [Google Scholar] [CrossRef]
- Parisi-Baradad, V.; Manjabacas, A.; Lombarte, A.; Olivella, R.; Chic, O.; Piera, J.; Garcia-Ladona, E. Identification of teleost fishes using an otolith online database-AFOROV. Fis. Res. 2010, 105, 13–20. [Google Scholar] [CrossRef]
- Mahé, K.; Evano, H.; Mille, T.; Muths, D.; Bourjea, J. Otolith shape as a valuable tool to evaluate the stock structure of swordfish in the Indian Ocean. Afr. J. Mar. Sci. 2016, 38, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Ashford, J.; Serra, R.; Saavedra, J.; Letelier, J. Otolith chemistry indicates large-scale connectivity in Chilean jack mackerel (Trachurus murphyi), a highly mobile species in the Southern Pacific Ocean. Fish. Res. 2011, 107, 291–299. [Google Scholar] [CrossRef]
- Beamish, R.J. Differences in the age of Pacific hake (Merluccius productus) using whole otoliths and sections of otoliths. J. Fish. Res. Board Can. 1979, 36, 141–151. [Google Scholar] [CrossRef]
- Pawson, M.G. Using otolith weight to age fish. J. Fish Biol. 1990, 36, 521–531. [Google Scholar] [CrossRef]
- Fletcher, W.J. A test of the relationship between otolith weight and age for the pilchard Sardinops neopilchardus. Can. J. Fish. Aquat. Sc. 1991, 48, 35–38. [Google Scholar] [CrossRef]
- Worthington, D.G.; Fowler, A.J.; Doherty, P.J. Determining the most efficient method of age determination for estimating the age structure of a fish population. Can. J. Fish. Aquat. Sc. 1995, 52, 2320–2326. [Google Scholar] [CrossRef]
- Fowler, A.J.; Doherty, P.J. Validation of annual growth increments in the otoliths of two species of Damselfish from the southern Great Barrier Reef. Can. J. Fish. Aquat. Sci. 1992, 43, 1057–1068. [Google Scholar] [CrossRef]
- Pacheco, C.; Bustamante, C.; Araya, M. Mass-effect: Understanding the relationship between age and otolith weight in fishes. Fish Fish. 2021, 22, 623–633. [Google Scholar] [CrossRef]
- Cardinale, M.; Arrhenius, F.; Johnsson, B. Potential use of otolith weight for the determination of age-structure of Baltic cod (Gadus morhua) and plaice (Pleuronectes platessa). Fish. Res. 2000, 45, 239–252. [Google Scholar] [CrossRef]
- Araya, M.; Cubillos, L.A.; Guzmán, M.; Peñailillo, J.; Sepúlveda, A. Evidence of a relationship between age and otolith weight in the Chilean jack mackerel, Trachurus symmetricus murphyi (Nichols). Fish. Res. 2001, 51, 17–26. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Soofiani, N.M.; Keivany, Y.; Taghavi-Motlagh, S.A. Use of otolith length and weight in age estimations of the kingsoldier bream, Argyrops spinifer, in the Persian Gulf. Iran J. Ichthyol. 2014, 1, 1–6. [Google Scholar]
- Nazir, A.; Khan, M.A. Using otolith weight to predict the age of different stocks of Sperata aor (Siluriformes: Bagridae) from the River Ganga. Rev. Biol. Trop. 2019, 67, 534–540. [Google Scholar] [CrossRef]
- Pino, C.A.; Cubillos, L.A.; Araya, M.; Sepulveda, A. Otolith weight as an estimator of age in the Patagonian grenadier, Macruronus magellanicus, in central-south Chile. Fish. Res. 2004, 66, 145–156. [Google Scholar] [CrossRef]
- Fletcher, W.J. Application of the otolith weight—Age relationship for the pilchard, Sardinops sagax neopilchardus. Can. J. Fish. Aquat. Sci. 1995, 52, 657–664. [Google Scholar] [CrossRef]
- Mitani, F.; Sato, T. Studies on the growth and age of the yellowtail, Seriola quinqueradiata T. & S.; found in Japan and the adjacent region–II. Estimation of age and growth from the opercula bone. Bull. Japan Soc. Sci. Fish. 1959, 24, 803–808. (In Japanese) [Google Scholar]
- Baxter, J.L. A study of the yellowtail Seriola dorsalis (GILL). State of Californea Department of fish and Game. Fish. Bull. 1960, 110, 1–96. [Google Scholar]
- Nishioka, J.; Inoue, H.; Kawagishi, M.; Iizuka, S.; Sinoda, M. On results of measurement of vertebral centrum by means of replica method. Bull. Kyoto Inst. Ocean. Fish. Sci. 1985, 9, 5–10. [Google Scholar]
- Gillanders, B.M.; Ferrell, D.J.; Andrew, N.L. Ageing methods for yellowtail kingfish, Seriola lalandi, and results from age and size based growth models. Fish. Bull. 1999, 97, 812–827. [Google Scholar]
- Thompson, B.; Beasley, M.; Wilson, C. Age distribution and growth of greater amberjack, Seriola dumerili from the north-central Gulf of Mexico. Fish. Bull. 1999, 97, 362–371. [Google Scholar]
- Stewart, J.; Ferrell, D.; van der Walt, B. Sizes and ages in commercial landings with estimates of growth, mortality and yield per recruit of yellowtail kingfish (Seriola lalandi) from New South Wales, Australia. Mar. Freshw. Res. 2004, 55, 489–497. [Google Scholar] [CrossRef]
- Shiraishi, T.; Ohshimo, S.; Yukami, R. Age, growth and reproductive characterisitics of gold striped amberjack Seriola lalandi in the waters off western Kyushu, Japan. N. Z. J. Mar. Freshwater Res. 2010, 44, 117–127. [Google Scholar] [CrossRef]
- Dunn, K. The Diet, Reproductive Biology, Age and Growth of Yellowtail, Seriola lalandi, in South Africa. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014; 106p. Available online: https://open.uct.ac.za/handle/11427/6254 (accessed on 9 March 2020).
- Sepulveda, F.A.; González, M.T. Spatio-temporal patterns of genetic variations in populations of yellowtail kingfish Seriola lalandi from the south-eastern Pacific Ocean and potential implications for its fishery management. J. Fish Biol. 2017, 90, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Zar, H. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; 944p. [Google Scholar]
- McKenzie, J.S.; Watson, M.; Francis, T.; Malcolm, Ó.M.; Poortenaar, C.C.; Holdsworth, J. Age, Growth, Maturity and Natural Mortality of New Zealand Kingfish (Seriola lalandi lalandi). New Zealand Fisheries Assessment Report 2014/03. 38p. Available online: https://docs.niwa.co.nz/library/public/FAR-2014-03.pdf (accessed on 4 January 2022).
- Ricker, W.E. Growth rates and models. In Fish Physiology, III, Bioenergetics and Growth; Hoar, W.S., Randall, D.J., Brett, J.R., Eds.; Academic Press: New York, NY, USA, 1979; pp. 677–743. [Google Scholar]
- Somers, L.F. On a seasonally oscillating growth function. Fishbyte 1988, 6, 8–11. [Google Scholar]
- Ogle, D.H. Introductory Fisheries Analyses with R; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; 327p. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 4 January 2022).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer-Verlag: New York, NY, USA, 2002; 488p. [Google Scholar]
- Liu, Y.; Zhang, C.; Xu, B.; Xue, Y.; Ren, Y.; Chen, Y. Accounting for Seasonal Growth in Per-Recruit Analyses: A Case Study of Four Commercial Fish in Coastal China Seas. Front. Mar. Sci. 2021, 8, 567240. [Google Scholar] [CrossRef]
- Osei, I.K.; Yankson, K.; Obodai, E.A.; Okyere, I. Implications of overlooked seasonal growth dynamics in tropical fisheries assessment: A test case of an oyster (Crassostrea tulipa) fishery in the Densu Delta, Ghana. Fish. Res. 2021, 244, 106118. [Google Scholar] [CrossRef]
- Holdsworth, J.C.; McKenzie, J.R.; Walsh, C.; Bian, R.; Maolagáin, C.O. Catch-at-Age of Yellowtail Kingfish (Seriola lalandi) Caught by New Zealand Recreational Fishers 2014–15. New Zealand Fisheries Assessment Report 2016/45. Available online: https://www.mpi.govt.nz/dmsdocument/14725/direct (accessed on 4 January 2022).
- Schnute, J. A versatile growth model with statistically stable parameters. Can. J. Fish. Aquat. Sci. 1981, 38, 1128–1140. [Google Scholar] [CrossRef]

| Model | Parameter | Value | SE | p-Value | AIC |
|---|---|---|---|---|---|
| vBGF | L∞ | 94.18 | 2.799 | <0.0001 | 602.08 |
| K | 0.71 | 0.044 | <0.0001 | ||
| t0 | 0.34 | 0.012 | <0.0001 | ||
| GZ | L∞ | 81.99 | 1.691 | <0.0001 | 630.11 |
| G | 1.71 | 0.074 | <0.0001 | ||
| to | 0.93 | 0.021 | <0.0001 | ||
| SvBGF | L∞ | 98.58 | 2.981 | <0.0001 | 533.87 |
| K | 0.59 | 0.04 | <0.0001 | ||
| t0 | 0.07 | 0.039 | 0.086 | ||
| C | 0.97 | 0.131 | <0.0001 | ||
| ts | 0.84 | 0.016 | <0.0001 | ||
| LG | L∞ | 77.76 | 1.589 | <0.0001 | 673.42 |
| G | 2.86 | 0.123 | <0.0001 | ||
| to | 1.13 | 0.023 | <0.0001 |
| L∞ | K | t0 | Age | Method | Loc | Ref | Notes |
|---|---|---|---|---|---|---|---|
| 110.8 | 0.309 | −0.588 | 1–8 | Vertebra read (17th) | Japan | [33] | |
| 125.2 | 0.189 | −0.735 | 1–6 | Length frequency | Australia | [30] | |
| * | * | * | 1–9 | Otoliths and scales | Australia | [30] | |
| * | * | * | 1–9 | Vertebra read | Australia | [30] | |
| 141.9 | 0.13 | 1–11 | Tag | New Zealand | [37] | ||
| 140.6 | 0.096 | −1.339 | 4–23 | Otolith read | New Zealand | [37] | |
| 106.4 | 0.173 | −2.75 | 1–8 | Otolith read | South Africa | [34] | |
| 116.4 | 0.247 | −0.708 | 1–29 | Otolith read | Northland Gulf/NZ | [45] | Male |
| 131.06 | 0.172 | −0.156 | 1–29 | Otolith read | Northland Gulf/NZ | [45] | Female |
| 120.3 | 0.184 | −1.316 | 1–29 | Otolith read | Bay of Plenty/NZ | [45] | Male |
| 129.6 | 0.173 | −1.074 | 1–29 | Otolith read | Bay of Plenty/NZ | [45] | Female |
| 98.58 | 0.59 | 0.07 | 0.8–5.7 | Otolith weight | Northern Chile | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndjamba, T.S.I.; Araya, M.; Oliva, M.E. Otolith Weight as an Estimator of the Age of Seriola lalandi Valenciennes, 1833 (Carangidae), in the Southeastern Pacific. Animals 2022, 12, 1640. https://doi.org/10.3390/ani12131640
Ndjamba TSI, Araya M, Oliva ME. Otolith Weight as an Estimator of the Age of Seriola lalandi Valenciennes, 1833 (Carangidae), in the Southeastern Pacific. Animals. 2022; 12(13):1640. https://doi.org/10.3390/ani12131640
Chicago/Turabian StyleNdjamba, Tchimanda Simeão Imbo, Miguel Araya, and Marcelo Enrique Oliva. 2022. "Otolith Weight as an Estimator of the Age of Seriola lalandi Valenciennes, 1833 (Carangidae), in the Southeastern Pacific" Animals 12, no. 13: 1640. https://doi.org/10.3390/ani12131640
APA StyleNdjamba, T. S. I., Araya, M., & Oliva, M. E. (2022). Otolith Weight as an Estimator of the Age of Seriola lalandi Valenciennes, 1833 (Carangidae), in the Southeastern Pacific. Animals, 12(13), 1640. https://doi.org/10.3390/ani12131640